Influence of Gender and Age of Brown Seaweed (Fucus vesiculosus) on Biochemical Activities of Its Aqueous Extracts
Abstract
:1. Introduction
2. Materials and Methods
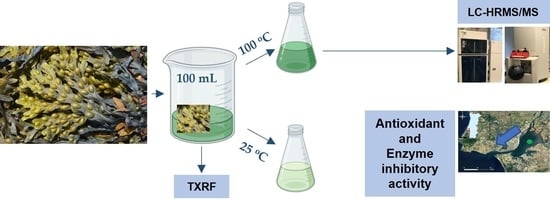
2.1. Seaweeds Material
2.2. Aqueous Extractions
2.3. Total Phenolic Content Analysis
2.4. Antioxidant Activity Determination
2.5. Chemical Analysis by HPLC-DAD and LC-HRMS-MS/MS
2.6. Acetylcholinesterase Activity
2.7. Heavy Metals Identification Essay
2.8. Statistical Analyses
3. Results and Discussion
3.1. Total Phenol Content
3.2. Antioxidant Activity Determination
3.3. Acetylcholinesterase Activity
3.4. Characterization of Compounds Present in F. vesiculosus
3.4.1. Characterization by High-Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD)
3.4.2. Compound Identification by LC/HRMS-MS
3.5. Elemental Analysis Using TXRF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houghton, P.J. The Role of Plants in Traditional Medicine. J. Altern. Complementary Med. 1995, 1, 131–143. [Google Scholar] [CrossRef]
- Cairrão, E.; Pereira, M.J.; Pastorinho, M.R.; Morgado, F.; Soares, A.M.V.M.; Guilhermino, L. Fucus Spp. as a Mercury Contamination Bioindicator in Costal Areas (Northwestern Portugal). Bull. Environ. Contam. Toxicol. 2007, 79, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Mar. Drugs 2017, 15, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, B.; Carreiras, J.; Feijão, E.; Reis-Santos, P.; Caçador, I.; Matos, A.R.; Fonseca, V.F. Fatty Acid Profiles of Estuarine Macroalgae Are Biomarkers of Anthropogenic Pressures: Development and Application of 07a Multivariate Pressure Index. Sci. Total Environ. 2021, 788, 147817. [Google Scholar] [CrossRef] [PubMed]
- Rupérez, P. Mineral Content of Edible Marine Seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential Antioxidant Capacity of Sulfated Polysaccharides from the Edible Marine Brown Seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins Are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant Activities of Phlorotannins Isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705–711. [Google Scholar] [CrossRef]
- Kurutas, E.B. The Importance of Antioxidants Which Play the Role in Cellular Response against Oxidative/Nitrosative Stress: Current State. Nutr. J. 2016, 15, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of Oxygen Radicals in DNA Damage and Cancer Incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef]
- Halliwell, B. Role of Free Radicals in the Neurodegenerative Diseases. Drugs Aging 2001, 18, 685–716. [Google Scholar] [CrossRef]
- Singh, R.; Sharad, S.; Kapur, S. Free Radicals and Oxidative Stress in Neurodegenerative Diseases: Relevance of Dietary Antioxidants. J. Indian Acad. Clin. Med. 2004, 5, 218–225. [Google Scholar]
- MacNee, W. Oxidative Stress and Lung Inflammation in Airways Disease. Eur. J. Pharmacol. 2001, 429, 195–207. [Google Scholar] [CrossRef]
- Hoshino, Y.; Mishima, M. Redox-Based Therapeutics for Lung Diseases. Antioxid. Redox Signal. 2008, 10, 701–704. [Google Scholar] [CrossRef]
- Sung, M.K.; Bae, S.C. Dietary Antioxidants and Rheumatoid Arthritis. In Bioactive Food as Interventions for Arthritis and Related Inflammatory Diseases, 1st ed.; Watson, R., Preedy, V., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 515–527. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.H.; Sekundo, W. Nutritional Supplementation to Prevent Cataract Formation. Dev. Ophthalmol. 2005, 38, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Amyloid β Deposition, Neurodegeneration, and Cognitive Decline in Sporadic Alzheimer’s Disease: A Prospective Cohort Study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Mabinya, L.V.; Olaniran, A.O.; Okoh, A.I. Chemical Characterization of Sulfated Polysaccharides from Gracilaria gracilis and Ulva lactuca and Their Radical Scavenging, Metal Chelating, and Cholinesterase Inhibitory Activities. Int. J. Food Prop. 2019, 22, 100–110. [Google Scholar] [CrossRef] [Green Version]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Aqueous–Ethanol Extracts of Some South African Seaweeds Inhibit Beta-Amyloid Aggregation, Cholinesterases, and Beta-Secretase Activities in Vitro. J. Food Biochem. 2019, 43, 1–10. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.K. Neuroprotective Properties of Chitosan and Its Derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T. The Interplay of Neurotransmitters in Alzheimer’s Disease. CNS Spectr. 2005, 10, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Brinza, L.; Nygård, C.A.; Dring, M.J.; Gavrilescu, M.; Benning, L.G. Bioresource Technology Cadmium Tolerance and Adsorption by the Marine Brown Alga Fucus vesiculosus from the Irish Sea and the Bothnian Sea. Bioresour. Technol. 2009, 100, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, C.; Rey-Asensio, A.; Carballeira, A. Interannual Changes in Δ15N Values in Fucus vesiculosus L. Mar. Pollut. Bull. 2014, 85, 141–145. [Google Scholar] [CrossRef]
- André, R.; Guedes, L.; Melo, R.; Ascens, L.; Pacheco, R.; Vaz, P.D.; Serralheiro, M.L. Effect of Food Preparations on In Vitro Bioactivities and Chemical Components of Fucus vesiculosus. Foods 2020, 9, 955. [Google Scholar] [CrossRef] [PubMed]
- Falé, P.L.; Ferreira, C.; Rodrigues, A.M.; Cleto, P.; Madeira, P.J.A.; Florêncio, M.H.; Frazão, F.N.; Serralheiro, M.L.M. Antioxidant and Anti-Acetylcholinesterase Activity of Commercially Available Medicinal Infusions after in vitro Gastrointestinal Digestion. J. Med. Plants Res. 2013, 7, 1370–1378. [Google Scholar] [CrossRef] [Green Version]
- Cruz de Carvalho, R.; Feijão, E.; Kletschkus, E.; Marques, J.C.; Reis-Santos, P.; Fonseca, V.F.; Papenbrock, J.; Caçador, I.; Duarte, B. Halophyte Bio-Optical Phenotyping: A Multivariate Photochemical Pressure Index (Multi-PPI) to Classify Salt Marsh Anthropogenic Pressures Levels. Ecol. Indic. 2020, 119, 106816. [Google Scholar] [CrossRef]
- Tuomi, J.; Ilvessalo, H.; Niemela, P.; Siren, S.; Jormalainen, V. Within-Plant Variation in Phenolic Content and Toughness of the Brown Alga Fucus vesiculosus L. Bot. Mar. 1989, 32, 505–510. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Ribeiro, A.R.; Patinha, C.; Silva, A.M.S.; Cardoso, S.M.; Costa, R. Water Extraction Kinetics of Bioactive Compounds of Fucus vesiculosus. Molecules 2019, 24, 3408. [Google Scholar] [CrossRef] [Green Version]
- Henriques, J.; Falé, P.L.; Pacheco, R.; Florêncio, M.H.; Serralheiro, M.L. Phenolic Compounds from Actinidia Deliciosa Leaves: Caco-2 Permeability, Enzyme Inhibitory Activity and Cell Protein Profile Studies. J. King Saud Univ. Sci. 2018, 30, 513–518. [Google Scholar] [CrossRef]
- André, P.; Villain, F. Free Radical Scavenging Properties of Mannitol and Its Role as a Constituent of Hyaluronic Acid Fillers: A Literature Review. Int. J. Cosmet. Sci. 2017, 39, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Ryan, E.M.; Duryee, M.J.; Hollins, A.; Dover, S.K.; Pirruccello, S.; Sayles, H.; Real, K.D.; Hunter, C.D.; Thiele, G.M.; Mikuls, T.R. Antioxidant Properties of Citric Acid Interfere with the Uricase-Based Measurement of Circulating Uric Acid. J. Pharm. Biomed. Anal. 2019, 164, 460–466. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, G.Y.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Antioxidant Activities, Phenolic Profiles, and Organic Acid Contents of Fruit Vinegars. Antioxidants 2019, 8, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant Properties of Phlorotannins from Brown Seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2017, 10, S2608–S2614. [Google Scholar] [CrossRef] [Green Version]
- Hermund, D.B.; Plaza, M.; Turner, C.; Jónsdóttir, R.; Kristinsson, H.G.; Jacobsen, C.; Nielsen, K.F. Structure Dependent Antioxidant Capacity of Phlorotannins from Icelandic Fucus vesiculosus by UHPLC-DAD-ECD-QTOFMS. Food Chem. 2018, 240, 904–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical Constituents and Biological Activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef] [Green Version]
- Jang, C.; Yadav, D.K.; Subedi, L.; Venkatesan, R.; Venkanna, A.; Afzal, S.; Lee, E.; Yoo, J.; Ji, E.; Kim, S.Y.; et al. Identification of Novel Acetylcholinesterase Inhibitors Designed by Pharmacophore-Based Virtual Screening, Molecular Docking and Bioassay. Sci. Rep. 2018, 8, 14921. [Google Scholar] [CrossRef] [Green Version]
- Kannan, R.R.R.; Aderogba, M.A.; Ndhlala, A.R.; Stirk, W.A.; van Staden, J. Acetylcholinesterase Inhibitory Activity of Phlorotannins Isolated from the Brown Alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res. Int. 2013, 54, 1250–1254. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Chemical Composition and Potential Use of Fucus vesiculosus from Gulf of Riga. Energy Procedia 2016, 95, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Peinado, I.; Girón, J.; Koutsidis, G.; Ames, J.M. Chemical Composition, Antioxidant Activity and Sensory Evaluation of Five Different Species of Brown Edible Seaweeds. Food Res. Int. 2014, 66, 36–44. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Hu, J.; Yang, B.; Lin, X.; Zhou, X.; Yang, X.; Liu, Y. Chemical Composition of Seaweeds; Elsevier Inc.: Amsterdam, The Netherlands, 2015; ISBN 9780124186972. [Google Scholar]
- FDA. FDA Vitamins and Minerals Chart; U.S. Food Drug Administration: White Oak, MA, USA, 2016. [Google Scholar]
- Ross, A.C.; Taylor, C.L.; Yaktine, A.L.; Valle, H.B. Del DRI Calcium Vitamin D; National Academies Press: Washington, DC, USA, 2011; ISBN 9780309163941. [Google Scholar]
- National Academies Press. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Mangenese, Molybdenum, Nickel, Silicon, Vanadium and Zinc; National Academies Press: Washington, DC, USA, 2000; ISBN 0309511992. [Google Scholar]
- EFSA. European Food Safety Authority Dietary Reference Values for Nutrients Summary Report. EFSA Supporting Publ. 2017, 14, e15121E. [Google Scholar] [CrossRef] [Green Version]
- Zava, T.T.; Zava, D.T. Assessment of Japanese Iodine Intake Based on Seaweed Consumption in Japan: A Literature-Based Analysis. Thyroid Res. 2011, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bove, M.; Stansbury, J.E.; Romm, A. Endocrine Disorders and Adrenal Support. In Botanical Medicine for Women’s Health; Churchill Livingstone: London, UK, 2010; pp. 186–210. [Google Scholar] [CrossRef]
- Lefavi, R.G.; Anderson, R.A.; Keith, R.E.; Wilson, G.D.; McMillan, J.L.; Stone, M.H. Efficacy of Chromium Supplementation in Athletes: Emphasis on Anabolism. Int. J. Sport Nutr. 1992, 2, 111–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balk, E.; Tatsioni, A.; Lichtenstein, A. Effect of Chromium Supplementation on A Systematic Review of Randomized Controlled Trials. Diabetes Care 2007, 30, 2154–2163. [Google Scholar] [CrossRef] [Green Version]
- Pechova, A.; Pavlata, L. Chromium as an Essential Nutrient: A Review. Vet. Med. 2007, 52, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Wood, R.J. Manganese and Birth Outcome. Nutr. Rev. 2009, 67, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- FAO/WHO Expert Consultation Evaluation of Certain Food Additives. World Health Organ.-Tech. Rep. Ser. 1975, 960, 149–177.
- World Health Organization. Preventing Disease through Healthy Environments: Exposure to Arsenic: A Major Public Health Concern. World Health Organization. 2019. Available online: https://apps.who.int/iris/handle/10665/329482 (accessed on 20 December 2021).
- (EFSA) European Food Safety Authority Scientific Opinion on the Risks to Public Health Related to the Presence of Nickel in Food and Drinking Water. EFSA J. 2015, 13, 4002. [CrossRef] [Green Version]
- Sandau, E.; Sandau, P.; Pulz, O.; Zimmermann, M. Heavy Metal Sorption by Marine Algae and Algal By-Products. Acta Biotechnol. 1996, 16, 103–119. [Google Scholar] [CrossRef]
- Sáez, C.A.; Lobos, M.G.; Macaya, E.C.; Oliva, D.; Quiroz, W.; Brown, M.T. Variation in Patterns of Metal Accumulation in Thallus Parts of Lessonia trabeculata (Laminariales; Phaeophyceae): Implications for Biomonitoring. PLoS ONE 2012, 7, e50170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caçador, I.; Vale, C.; Catarino, F. The Influence of Plants on Concentration and Fractionation of Zn, Pb, and Cu in Salt Marsh Sediments (Tagus Estuary, Portugal). J. Aquat. Ecosyst. Stress Recovery 1996, 5, 193–198. [Google Scholar] [CrossRef]
- Barreiro, R.; Picado, L.; Real, C. Biomonitoring Heavy Metals in Estuaries: A Field Comparison of Two Brown Algae Species Inhabiting Upper Estuarine Reaches. Environ. Monit. Assess. 2002, 75, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Barnett, B.E.; Ashcroft, C.R. Heavy Metals in Fucus vesiculosus in the Humber Estuary. Environ. Pollut. B Chem. Phys. 1985, 9, 193–213. [Google Scholar] [CrossRef]
- Foster, P. Concentrations and Concentration Factors of Heavy Metals in Brown Algae. Environ. Pollut. 1976, 10, 45–53. [Google Scholar] [CrossRef]
- Schoenwaelder, M.E.A. The Occurrence and Cellular Significance of Physodes in Brown Algae. Phycologia 2002, 41, 125–139. [Google Scholar] [CrossRef]









 ; 1000—
; 1000—  . n.d: not detected.
. n.d: not detected.
 ; 1000—
; 1000—  . n.d: not detected.
. n.d: not detected.| Intensities | |||||||
|---|---|---|---|---|---|---|---|
| Rt (min) | Proposed Compound | Accurate [M-H] − m/z (Error, ppm) | <20 cm | 20–25 cm | 25–30 cm | >30 cm | |
| 1.1 | Mannitol | 181.0719 (0.76) | 40,486 | 42,480 | 70,480 | 29,590 | M |
| 47,238 | 42,634 | 22,130 | 22,134 | F | |||
| 1.2 | Citric Acid | 191.0195 (−1.18) | 1,233,346 | 894,690 | 1,217,112 | 434,158 | M |
| 1,112,302 | 1,205,844 | 467,928 | 640,274 | F | |||
| 1.6 | Glycidyl compound | 551.1826 (−0.52) | 1112 | 1070 | 9630 | 1752 | M |
| 1764 | 2500 | 2164 | 2730 | F | |||
| 1.9 | Phloroglucinol derivative (phlorotannin of fucol type) | 497.0721 (−0.91) | 11,740 | 5686 | 6402 | 2212 | M |
| 3762 | 7770 | 4944 | 17,434 | F | |||
| 2 | Glycosidic derivative | 327.1315 (5.59) | 2218 | n.d | n.d | n.d | M |
| n.d | 1222 | n.d | n.d | F | |||
| 2.2 | 3,5,7-Trihydroxy-4-oxochromene-2-carboxylic acid | 237.0074 (14.03) | n.d | n.d | n.d | n.d | M |
| 14,472 | n.d | n.d | n.d | F | |||
| 2.4 | Vanillic acid sulfate | 246.9916 (−0.8) | 64,612 | 68,672 | 64,316 | 78,434 | M |
| 69,226 | 77,480 | 75,676 | 79,882 | F | |||
| 3.2 | Phloroglucinol glycosidic derivative | 277.0926 (−1.05) | 74,446 | 87,044 | 120,130 | 73,364 | M |
| 107,880 | 139,220 | 92,550 | 105,762 | F | |||
| 3.2 | Phloroglucinol glycosidic derivative | 555.1742 (−0.72) | n.d | n.d | n.d | 2600 | M |
| n.d | n.d | 3352 | 6576 | F | |||
| 4.5 | Tetrapeptide ProSerGlyPro | 355.1615 (−2.28) | n.d | n.d | n.d | n.d | M |
| n.d | 1906 | n.d | n.d | F | |||
| 5 | Butanedioic derivate | 175.0554 (−33.11) | n.d | n.d | 29,672 | n.d | M |
| n.d | n.d | n.d | n.d | F | |||
| 6.7 | Chroman-2,5-diol | 165.0554 (−1.93) | 87,114 | n.d | n.d | n.d | M |
| 55,278 | n.d | n.d | 4506 | F | |||
| F. vesiculosus Growth Stage per Gender Analyzed | ||||||
|---|---|---|---|---|---|---|
| <20 cm F | 20–25 cm M | 25–30 cm F | 25–30 cm M | >30 cm F | >30 cm M | |
| Na | 117.12 ± 13.22 | 185.57 ± 88.16 | 92.50 ± 11.85 | 165.84 ± 36.20 | 124.59 ± 42.27 | 158.73 ± 25.89 |
| Mg | 7.55 ± 1.03 | 8.62 ± 1.51 | 6.72 ± 2.78 | 10.57 ± 0.82 | 4.50 ± 0.60 | 9.54 ± 0.98 |
| K | 16.39 ± 14.30 | 13.38 ± 4.89 | 11.77 ± 3.38 | 16.10 ± 3.60 | 7.87 ± 2.92 | 12.91 ± 4.24 |
| Ca | 35.08 ± 20.20 | 26.63 ± 8.45 | 24.54 ± 3.88 | 31.00 ± 11.15 | 18.49 ± 3.41 | 26.14 ± 5.42 |
| V | 0.06 ± 0.02 | 0.09 ± 0.02 | 0.08 ± 0.04 | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.07 ± 0.02 |
| Cr | 0.05 ± 0.02 | 0.08 ± 0.01 | 0.05 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.10 ± 0.01 |
| Mn | 6.49 ± 1.66 | 10.91 ± 1.56 | 7.18 ± 0.63 | 11.64 ± 1.40 | 8.83 ± 0.70 | 10.97 ± 1.22 |
| Fe | 17.08 ± 4.21 | 24.70 ± 4.59 | 19.12 ± 2.56 | 28.68 ± 2.79 | 18.88 ± 4.33 | 28.27 ± 4.30 |
| Co | 0.14 ± 0.03 | 0.24 ± 0.06 | 0.18 ± 0.02 | 0.28 ± 0.05 | 0.20 ± 0.05 | 0.28 ± 0.05 |
| Ni | 0.08 ± 0.02 | 0.14 ± 0.02 | 0.09 ± 0.01 | 0.14 ± 0.02 | 0.11 ± 0.02 | 0.15 ± 0.01 |
| Cu | 0.26 ± 0.06 | 0.39 ± 0.07 | 0.23 ± 0.02 | 0.39 ± 0.07 | 0.30 ± 0.06 | 0.35 ± 0.03 |
| Zn | 2.79 ± 0.57 | 5.57 ± 0.47 | 3.18 ± 0.35 | 4.88 ± 0.60 | 3.44 ± 0.66 | 4.59 ± 0.27 |
| As | 0.21 ± 0.13 | 0.37 ± 0.19 | 0.23 ± 0.05 | 0.34 ± 0.07 | 0.29 ± 0.07 | 0.22 ± 0.02 |
| Se | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.01 |
| Br | 2.55 ± 0.23 | 3.65 ± 0.85 | 2.71 ± 0.052 | 3.35 ± 0.52 | 2.69 ± 0.54 | 3.92 ± 1.19 |
| Sr | 19.22 ± 1.32 | 31.66 ± 6.60 | 20.60 ± 3.42 | 32.79 ± 11.28 | 22.47 ± 6.66 | 25.07 ± 2.15 |
| Cd | n.d | n.d | n.d | n.d | n.d | n.d |
| I | 0.99 ± 0.36 | 1.05 ± 0.36 | 1.13 ± 0.33 | 1.22 ± 0.38 | 0.74 ± 0.20 | 1.06 ± 0.10 |
| Hg | 0 | 0 | 0 | 0 | 0 | 0 |
| Pb | 0.74 ± 0.05 | 1.20 ± 0.40 | 0.72 ± 0.11 | 1.02 ± 0.22 | 0.71 ± 0.22 | 0.97 ± 0.15 |
| Mineral Recommended Daily Intake (RDI) vs. F. vesiculosus Mineral Composition | ||||
|---|---|---|---|---|
| FDA [43] | National Academy [44,45] | EFSA [46] | >30 cm M | |
| Na | 2400 mg | 2300 mg | 2000 mg | 158.73 mg |
| Mg | 400 mg | M: 420 mg; W: 380 mg | M: 350 mg; W: 300 mg | 9.54 mg |
| K | 3500 mg | 4700 mg | 3500 mg | 12.91 mg |
| Ca | 1000 mg | 1000 mg | 1000 mg | 26.14 mg |
| Cr | 120 µg | M: 35 µg; W: 25 µg | n.a. | 100 µg |
| Mn | 2 mg | M: 2.3 mg; W: 1.8 mg | 3 mg | 10.97 mg |
| Fe | 18 mg | M: 8 mg; W: 18 mg | M: 11 µg; W: 16 µg | 28.27 mg |
| Cu | 2 mg | 900 µg | M: 1.6 mg; W: 1.5 mg | 35 µg |
| Zn | 15 mg | M: 11 mg; W: 8 mg | M:16.3 mg; W:12.7 mg | 4.59 mg |
| Se | 70 µg | 55 µg | 70 µg | 10 µg |
| I | 150 µg | 150 µg | 150 µg | 1.06 mg |
| Heavy Metals Tolerable Daily Intake (TDI) [45,54,55,56] vs. F. vesiculosus Mineral Composition | ||
|---|---|---|
| >30 cm M | ||
| Ni | 2.8 µg/kg b.w. | 150 µg |
| As | 3 µg/kg b.w. | 220 µg |
| Pb | 25 µg/kg b.w. | 970 µg |
| Fe | 45 mg/day UL | 28.27 mg |
| Zn | 40 mg/day UL | 4.59 mg |
| Cu | 10 mg/day UL | 35 µg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, D.; André, R.; Ressaissi, A.; Duarte, B.; Melo, R.; Serralheiro, M.L. Influence of Gender and Age of Brown Seaweed (Fucus vesiculosus) on Biochemical Activities of Its Aqueous Extracts. Foods 2022, 11, 39. https://doi.org/10.3390/foods11010039
Nunes D, André R, Ressaissi A, Duarte B, Melo R, Serralheiro ML. Influence of Gender and Age of Brown Seaweed (Fucus vesiculosus) on Biochemical Activities of Its Aqueous Extracts. Foods. 2022; 11(1):39. https://doi.org/10.3390/foods11010039
Chicago/Turabian StyleNunes, Diogo, Rebeca André, Asma Ressaissi, Bernardo Duarte, Ricardo Melo, and Maria Luísa Serralheiro. 2022. "Influence of Gender and Age of Brown Seaweed (Fucus vesiculosus) on Biochemical Activities of Its Aqueous Extracts" Foods 11, no. 1: 39. https://doi.org/10.3390/foods11010039
APA StyleNunes, D., André, R., Ressaissi, A., Duarte, B., Melo, R., & Serralheiro, M. L. (2022). Influence of Gender and Age of Brown Seaweed (Fucus vesiculosus) on Biochemical Activities of Its Aqueous Extracts. Foods, 11(1), 39. https://doi.org/10.3390/foods11010039