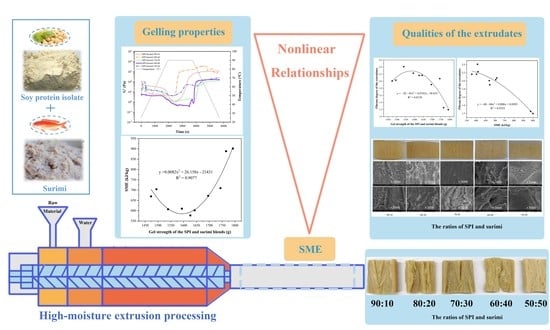
High-Moisture Extrusion of Mixed Proteins from Soy and Surimi: Effect of Protein Gelling Properties on the Product Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rheological Measurements
2.3. Protein Gelling Properties
2.4. High-Moisture Extrusion Experiments
2.5. Specific Mechanical Energy (SME)
2.6. Textural Properties of the Extrudates
2.7. Macro- and Microstructure Detection
2.8. Statistical Analysis
3. Results and Discussion
3.1. Rheological Properties of the SPI–surimi Blends
3.2. Heat-Induced Gelling Properties of the SPI–surimi Blends
3.3. Textural Properties of the SPI–surimi High-Moisture Extrudates
3.4. Macro- and Microstructure of the SPI–surimi High-Moisture Extrudates
3.5. Specific Mechanical Energy (SME) during the High-Moisture Extrusion Process
3.6. Effect of the Heat-Induced Gel Strength of the Blends on the SME
3.7. Effect of the Heat-Induced Gel Strength of the Blends on Textural Properties of the Extrudates
3.8. Effect of the SME on Textural Properties of the Extrudates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors nfluencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittendorfer, B.; Klein, S.; Fontana, L. A word of caution against excessive protein intake. Nat. Rev. Endocrinol. 2020, 16, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; Agricultural Development Economics Division, Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; ISSN 2521-1838. [Google Scholar]
- Ginn, J.; Lickel, B. A motivated defense of meat: Biased perceptions of meat’s environmental impact. J. Soc. Issues 2020, 76, 54–69. [Google Scholar] [CrossRef]
- Rust, N.A.; Ridding, L.; Ward, C.; Clark, B.; Kehoe, L.; Dora, M.; Whittingham, M.J.; McGowan, P.; Chaudhary, A.; Reynolds, C.J.; et al. How to transition to reduced-meat diets that benefit people and the planet. Sci. Total Environ. 2020, 718, 137208. [Google Scholar] [CrossRef] [PubMed]
- Rubio, N.R.; Xiang, N.; Kaplan, D.L. Plant-based and cell-based approaches to meat production. Nat. Commun. 2020, 11, 6276. [Google Scholar] [CrossRef]
- Bohrer, B.M. An investigation of the formulation and nutritional composition of modern meat analogue products. Food Sci. Hum. Wellness 2019, 8, 320–329. [Google Scholar] [CrossRef]
- McCann, T.H.; Guyon, L.; Fischer, P.; Day, L. Rheological properties and microstructure of soy-whey protein. Food Hydrocoll. 2018, 82, 434–441. [Google Scholar] [CrossRef]
- Borderías, A.J.; Tovar, C.A.; Domínguez-Timón, F.; Díaz, M.T.; Pedrosa, M.M.; Moreno, H.M. Characterization of healthier mixed surimi gels obtained through partial substitution of myofibrillar proteins by pea protein isolates. Food Hydrocoll. 2020, 107, 105976. [Google Scholar] [CrossRef]
- Jose, J.; Pouvreau, L.; Martin, A.H. Mixing whey and soy proteins: Consequences for the gel mechanical response and water holding. Food Hydrocoll. 2016, 60, 216–224. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Kitagawa, M.; Quéguiner, C. New protein texturization processes by extrusion cooking at high moisture levels. Food Rev. Int. 1992, 8, 235–275. [Google Scholar] [CrossRef]
- Thiébaud, M.; Dumay, E.; Cheftel, J.C. Influence of process variables on the characteristics of a high moisture fish soy protein mix texturized by extrusion cooking. LWT-Food Sci. Technol. 1996, 29, 526–535. [Google Scholar] [CrossRef]
- He, T.; Mo, B.; Huang, J.; Fan, D.; Zhang, W.; Wang, L.; Zhao, J.; Chen, W.; Zhang, H. Twin-screw extrusion of hairtail surimi and soy protein isolate blends. Food Sci. Technol. Res. 2014, 20, 517–527. [Google Scholar] [CrossRef] [Green Version]
- Osen, R.; Toelstede, S.; Eisner, P.; Schweiggert-Weisz, U. Effect of high moisture extrusion cooking on protein–protein interactions of pea (Pisum sativum L.) protein isolates. Int. J. Food Sci. Technol. 2015, 50, 1390–1396. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Faisal, S.; Wei, L.; Cao, C.; Yan, W.; Wang, Q. Converting peanut protein biomass waste into “double green” meat substitutes using a high-moisture extrusion process: A multiscale method to explore a process for forming a meat-like fibrous structure. J. Agric. Food Chem. 2019, 67, 10713–10725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Q.; Liu, L.; Zhang, Y.; He, N.; Wang, Q. High-moisture extrusion process of transglutaminase-modified peanut protein: Effect of transglutaminase on the mechanics of the process forming a fibrous structure. Food Hydrocoll. 2021, 112, 106346. [Google Scholar] [CrossRef]
- Wu, C.; Wang, T.; Ren, C.; Ma, W.; Wu, D.; Xu, X.; Wang, L.-S.; Du, M. Advancement of food-derived mixed protein systems: Interactions, aggregations, and functional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 627–651. [Google Scholar] [CrossRef]
- Luo, Y.; Kuwahara, R.; Kaneniwa, M.; Murata, Y.; Yokoyama, M. Effect of soy protein isolate on gel properties of Alaska pollock and common carp surimi at different setting conditions. J. Sci. Food Agric. 2004, 84, 663–671. [Google Scholar] [CrossRef]
- Park, J.W. Functional protein additives in surimi gels. J. Food Sci. 1994, 59, 525–527. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavares, G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocoll. 2019, 97, 105171. [Google Scholar] [CrossRef]
- Wang, Z.; Liang, J.; Jiang, L.; Li, Y.; Wang, J.; Zhang, H.; Li, D.; Han, F.; Li, Q.; Wang, R.; et al. Effect of the interaction between myofibrillar protein and heat-induced soy protein isolates on gel properties. CyTA-J. Food 2015, 13, 527–534. [Google Scholar] [CrossRef]
- Ramı́rez-Suárez, J.C.; Xiong, Y.L. Effect of transglutaminase-induced cross-linking on gelation of myofibrillar/soy protein mixtures. Meat Sci. 2003, 65, 899–907. [Google Scholar] [CrossRef]
- Guo, J.; Hu, L.; Yang, X.; Yu, S.; Liu, Y.; Jin, Y. Influence of soy protein isolate prepared by phosphate-assisted hydrothermal cooking on the gelation of myofibrillar protein. J. Am. Oil Chem. Soc. 2015, 92, 523–531. [Google Scholar] [CrossRef]
- Guo, Y.; Hu, H.; Wang, Q.; Liu, H. A novel process for peanut tofu gel: Its texture, microstructure and protein behavioral changes affected by processing conditions. LW-Food Sci. Technol. 2018, 96, 140–146. [Google Scholar] [CrossRef]
- Wang, X.; He, Z.; Zeng, M.; Qin, F.; Adhikari, B.; Chen, J. Effects of the size and content of protein aggregates on the rheological and structural properties of soy protein isolate emulsion gels induced by CaSO4. Food Chem. 2017, 221, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Li, Y.; Han, J.; Liu, Q.; Kong, B. Gelation and rheological properties of myofibrillar proteins influenced by the addition of soybean protein isolates subjected to an acidic pH treatment combined with a mild heating. Food Hydrocoll. 2017, 70, 269–276. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Zhu, S.; Wang, Q. Texturisation behaviour of peanut–soy bean/wheat protein mixtures during high moisture extrusion cooking. Int. J. Food Sci. Technol. 2018, 53, 2535–2541. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Faisal, S.; Wang, Q. A new insight into the high-moisture extrusion process of peanut protein: From the aspect of the orders and amount of energy input. J. Food Eng. 2020, 264, 109668. [Google Scholar] [CrossRef]
- Grisel, M.; Muller, G. Rheological Properties of the Schizophyllan−Borax System. Macromolecules 1998, 31, 4277–4281. [Google Scholar] [CrossRef]
- Peckham, M.; Knight, P.J. When a predicted coiled coil is really a single α-helix, in myosins and other proteins. Soft Matter 2009, 5, 2493–2503. [Google Scholar] [CrossRef]
- Rayment, I.; Rypniewski, W.R.; Schmidt-Bäse, K.; Smith, R.; Tomchick, D.R.; Benning, M.M.; Winkelmann, D.A.; Wesenberg, G.; Holden, H.M. Three-dimensional structure of myosin subfragment-1: A molecular motor. Science 1993, 261, 50–58. [Google Scholar] [CrossRef]
- Ahmed, J.; Ramaswamy, H.S.; Alli, I. Thermorheological Characteristics of Soybean Protein Isolate. J. Food Sci. 2006, 71, E158–E163. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.M.; Xiong, S.B.; Xie, B.J.; Liu, H.M. Studies on Fish and Pork Paste Gelation by Dynamic Rheology and Circular Dichroism. J. Food Sci. 2007, 72, E399–E403. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, F.; Yamagishi, T.; Iwabuchi, S. Molecular understanding of heat-induced phenomena of soybean protein. Food Rev. Int. 1991, 7, 283–322. [Google Scholar] [CrossRef]
- Egelandsdal, B.; Fretheim, K.; Samejima, K. Dynamic rheological measurements on heat-induced myosin gels: Effect of ionic strength, protein concentration and addition of adenosine triphosphate or pyrophosphate. J. Sci. Food Agric. 1986, 37, 915–926. [Google Scholar] [CrossRef]
- Kamal, M.; Foukani, M.; Karoui, R. Rheological and physical properties of camel and cow milk gels enriched with phosphate and calcium during acid-induced gelation. J. Food Sci. Technol. 2017, 54, 439–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a phenomenological definition of the term ‘gel’. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, S.; Xiong, S.; Huang, Q. Steady, dynamic, and creep-recovery rheological properties of myofibrillar protein from grass carp muscle. Food Hydrocoll. 2016, 61, 48–56. [Google Scholar] [CrossRef]
- Firoozmand, H.; Rousseau, D. Microstructure and rheology design in protein–protein–polysaccharide composites. Food Hydrocoll. 2015, 50, 84–93. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Molecular forces involved in heat-induced pea protein gelation: Effects of various reagents on the rheological properties of salt-extracted pea protein gels. Food Hydrocoll. 2012, 28, 325–332. [Google Scholar] [CrossRef]
- Maurya, A.; Said, P. Extrusion processing on physical and chemical properties of protein rich products-an overview. J. Bioresour. Eng. Technol. 2014, 2, 61–67. [Google Scholar]
- Jafarpour, A.; Hajiduon, H.A.; Aie, M.R. A comparative study on effect of egg white, soy protein isolate and potato starch on functional properties of common carp (Cyprinus carpio) surimi gel. J. Food Process. Technol. 2012, 3, 190. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, S.; Zhang, B.; Drago, S.R.; Zhang, J. Relationships between the gelatinization of starches and the textural properties of extruded texturized soybean protein-starch systems. J. Food Eng. 2016, 174, 29–36. [Google Scholar] [CrossRef]
- Wu, C.; Yan, X.; Wang, T.; Ma, W.; Xu, X.; Du, M. A self-sorted gel network formed by heating a mixture of soy and cod proteins. Food Funct. 2019, 10, 5140–5151. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Qiao, F.; Yang, P.; Guo, F.; Huang, X.; Adhikari, B.; Chen, J. Roles of soluble and insoluble aggregates induced by soy protein processing in the gelation of myofibrillar protein. Int. J. Food Sci. Technol. 2016, 51, 480–489. [Google Scholar] [CrossRef]
- Akdogan, H. Pressure, torque, and energy responses of a twin screw extruder at high moisture contents. Food Res. Int. 1996, 29, 423–429. [Google Scholar] [CrossRef]
- Unlu, E.; Faller, J.F. RTD in twin-screw food extrusion. J. Food Eng. 2002, 53, 115–131. [Google Scholar] [CrossRef]







| SPI/Surimi | Hardness (N) | Chewiness (N) | Gel Strength (N) | Crosswise Strength (N) | Lengthwise Strength (N) | Fibrous Degree |
|---|---|---|---|---|---|---|
| 90:10 | 16.11 ± 0.42 b | 11.70 ± 0.39 b | 3.02 ± 0.11 b | 0.93 ± 0.04 a | 0.40 ± 0.03 ab | 2.33 ± 0.10 a |
| 80:20 | 17.14 ± 0.26 a | 13.71 ± 0.26 a | 3.50 ± 0.16 a | 0.97 ± 0.05 a | 0.45 ± 0.01 a | 2.16 ± 0.04 ab |
| 70:30 | 15.97 ± 0.75 b | 12.01 ± 0.40 b | 2.89 ± 0.09 b | 0.83 ± 0.02 b | 0.41 ± 0.04 a | 2.03 ± 0.17 b |
| 60:40 | 13.55 ± 0.19 c | 10.71 ± 0.03 c | 2.19 ± 0.09 c | 0.72 ± 0.01 c | 0.35 ± 0.02 b | 2.05 ± 0.11 b |
| 50:50 | 5.36 ± 0.12 d | 4.08 ± 0.12 d | 0.76 ± 0.05 d | 0.28 ± 0.01 d | 0.23 ± 0.02 c | 1.24 ± 0.07 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, J.; Chen, Q.; He, N.; Wang, Q. High-Moisture Extrusion of Mixed Proteins from Soy and Surimi: Effect of Protein Gelling Properties on the Product Quality. Foods 2022, 11, 1397. https://doi.org/10.3390/foods11101397
Zhang Y, Zhang J, Chen Q, He N, Wang Q. High-Moisture Extrusion of Mixed Proteins from Soy and Surimi: Effect of Protein Gelling Properties on the Product Quality. Foods. 2022; 11(10):1397. https://doi.org/10.3390/foods11101397
Chicago/Turabian StyleZhang, Yujie, Jinchuang Zhang, Qiongling Chen, Ning He, and Qiang Wang. 2022. "High-Moisture Extrusion of Mixed Proteins from Soy and Surimi: Effect of Protein Gelling Properties on the Product Quality" Foods 11, no. 10: 1397. https://doi.org/10.3390/foods11101397
APA StyleZhang, Y., Zhang, J., Chen, Q., He, N., & Wang, Q. (2022). High-Moisture Extrusion of Mixed Proteins from Soy and Surimi: Effect of Protein Gelling Properties on the Product Quality. Foods, 11(10), 1397. https://doi.org/10.3390/foods11101397