Comparison of Oxidative and Physical Stabilities of Conjugated Linoleic Acid Emulsions Stabilized by Glycosylated Whey Protein Hydrolysates via Two Pathways
Abstract
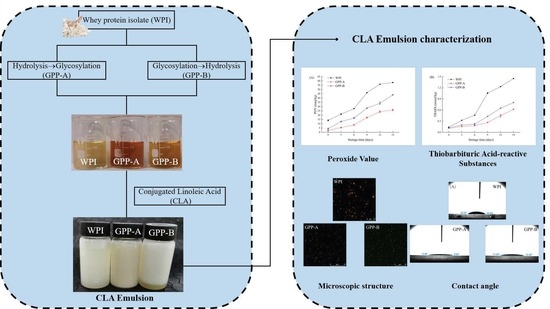
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Preparation of Two Glycosylated Hydrolysates
2.2.1. Preparation of Glycosylated Hydrolysate A
2.2.2. Preparation of Glycosylated Hydrolysate B
2.3. Preparation of Emulsions Stabilized by Two Glycosylated Hydrolysates
2.4. Browning Intensity
2.5. Free Amino Production
2.6. Antioxidant Property
2.7. Oxidative Stabilities
2.8. Mean Particle Size and ζ-Potential
2.9. Creaming Index
- HC was the height (cm) of the creaming volume of CLA emulsions;
- HS was the total height (cm) of emulsion samples.
2.10. Fraction of Interfacial Adsorption
2.11. Confocal Laser Scanning Microscopy
2.12. Contact Angle and Interfacial Tension
2.13. Statistical Analysis
3. Results
3.1. Comparison of Characterizations of Glycosylated Hydrolysates
3.2. Comparison of Oxidative Stability of Emulsions Stabilized by Glycosylated Hydrolysates
3.3. Comparison of Physical Stability of Emulsions Stabilized by Glycosylated Hydrolysates
3.4. Comparison of Fraction of Interfacial Adsorption of Emulsions Stabilized by Glycosylated Hydrolysates
3.5. Comparison of Microstructure of CLA Emulsions Stabilized by Glycosylated Hydrolysates
3.6. Comparison of Contact Angle of CLA Emulsions Stabilized by Glycosylated Hydrolysates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Decourcelle, N.; Sabourin, C.; Dauer, G.; Guérard, F. Effect of the Maillard reaction with xylose on the emulsifying properties of a shrimp hydrolysate (Pandalus borealis). Food Res. Int. 2010, 43, 2155–2160. [Google Scholar] [CrossRef]
- Szumaa, P.; Pacyna-Kuchta, A.; Wasik, A. Proteolysis of whey protein isolates in nanoemulsion systems: Impact of nanoemulsification and additional synthetic emulsifiers. Food Chem. 2021, 351, 129356. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, A.; Wang, X.; Xu, N.; Jiang, L. The radiation assisted-Maillard reaction comprehensively improves the freeze-thaw stability of soy protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2020, 103, 105684. [Google Scholar] [CrossRef]
- Raikos, V. Effect of heat treatment on milk protein functionality at emulsion interfaces. A review. Food Hydrocoll. 2010, 24, 259–265. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Sun, R.; Wang, M.; Wang, K.; Li, Y.; Shang, H.; Hou, J.; Jiang, Z. Lactobacillus plantarum 23-1 improves intestinal inflammation and barrier function through the TLR4/NF-κB signaling pathway in obese mice. Food Funct. 2022, 13, 5971–5989. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, X.; Wang, W.; Gu, L.; Hu, C.; Sun, H.; Xu, C.; Hou, J.; Jiang, Z. Lactobacillus paracasei 24 Attenuates Lipid Accumulation in High-Fat Diet-Induced Obese Mice by Regulating the Gut Microbiota. J. Agric. Food Chem. 2022, 70, 4631–4643. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Fu, Y.; Liu, F.; Liu, Z.; Ma, J.; Jiang, R.; Song, C.; Jiang, Z.; Hou, J. Purification and antimicrobial mechanism of a novel bacteriocin produced by Lactobacillus rhamnosus. LWT—Food Sci. Technol. 2021, 137, 110338. [Google Scholar] [CrossRef]
- Karbasi, M.; Askari, G. Modification of whey protein microgel particles with mono- oligo- and polysaccharides through the Maillard reaction: Effects on structural and techno-functional properties. Food Struct. 2021, 28, 100184. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, S.W.; Gong, J.; Guo, Q.; Wang, Q.; Hua, Y. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll. 2015, 48, 155–164. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, X.; Zhu, K.; Peng, W.; Zhou, H. Improvement of emulsifying properties of oat protein isolate-dextran conjugates by glycation. Carbohydr. Polym. 2015, 127, 168–175. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, F.; Zhao, M.; Lin, L.; Ning, Z.; Sun, B. Soy peptide nanoparticles by ultrasound-induced self-assembly of large peptide aggregates and their role on emulsion stability. Food Hydrocoll. 2018, 74, 62–71. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, Z.; Rao, J.; Chen, B. Gum Arabic-Mediated Synthesis of Glyco-pea Protein Hydrolysate via Maillard Reaction Improves Solubility, Flavor Profile, and Functionality of Plant Protein. J. Agric. Food Chem. 2019, 67, 10195–10206. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Zhao, J.; Yu, R.; Muhammad, A.; Abdul, Q.; Jiang, Z.; Qu, B. Glycosylated whey protein isolate enhances digestion behaviors and stabilities of conjugated linoleic acid oil in water emulsions. Food Chem. 2022, 383, 132403. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, Y.; Yu, H.; Mu, S.; Li, H.; Liu, X.; Zhang, M.; Jiang, Z.; Hou, J. Biological activities and in vitro digestion characteristics of glycosylated α -lactalbumin prepared by microwave heating: Impacts of ultrasonication. LWT—Food Sci. Technol. 2022, 158, 113141. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhao, P.P.; Li, J.Z.; Wang, X.D.; Hou, J.C.; Jiang, Z.M. Effects of ultrasound synergized with microwave on structure and functional properties of transglutaminase-crosslinked whey protein isolate. Ultrason. Sonochem. 2022, 83, 105935. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, T.; Gantumur, M.-A.; Qayum, A.; Bilawal, A.; Jiang, Z.; Wang, L. Non-covalent interaction and digestive characteristics between α-lactalbumin and safflower yellow: Impacts of microwave heating temperature. LWT—Food Sci. Technol. 2022, 159, 113206. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.; Zhao, J.; Sun, R.; Shang, H.; Sun, C.; Liu, L.; Hou, J.; Jiang, J. Physical and oxidative stability of astaxanthin microcapsules prepared with liposomes. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Tan, C.C.; Karim, A.A.; Uthumporn, U.; Ghazali, F.C. Effect of Thermal Treatment on the Physicochemical Properties of Emulsion Stabilized by Gelatin from Black Tilapia (Oreochromis mossambicus) Skin. Food Biophys. 2020, 15, 423–432. [Google Scholar] [CrossRef]
- Chen, H.; Lu, Y.; Yuan, F.; Gao, Y.; Mao, L. Effect of interfacial compositions on the physical properties of alginate-based emulsion gels and chemical stability of co-encapsulated bioactives. Food Hydrocoll. 2021, 111, 106389. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, J.; Zhao, X.; Sun, R.; Sun, C.; Hou, D.; Zhang, X.; Jiang, L.; Hou, J.; Jiang, Z. Oil bodies extracted from high-oil soybeans (Glycine max) exhibited higher oxidative and physical stability than oil bodies from high-protein soybeans. Food Funct. 2022, 13, 3271–3282. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, M.; Lyu, B.; Ma, J.; Li, Y. Preparation of amphiphilic Janus SiO2 particles and its application on polyacrylate emulsion–ScienceDirect. Colloids Surf. A—Physicochem. Eng. Asp. 2020, 607, 125295. [Google Scholar] [CrossRef]
- Karbasi, M.; Sánchez-Ferrer, A.; Adamcik, J.; Askari, G.; Madadlou, A.; Mezzenga, R. Covalent β-lactoglobulin-maltodextrin amyloid fibril conjugate prepared by the Maillard reaction. Food Chem. 2020, 342, 128388. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hu, J.; Wei, L.; Du, Y.; Shi, X.; Zhang, L. Antioxidant and antimicrobial activity of Maillard reaction products from xylan with chitosan/chitooligomer/glucosamine hydrochloride/taurine model systems. Food Chem. 2014, 148, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, J.; Kong, B.; Jia, N.; Li, P. Antioxidant capacity of maillard reaction products formed by a porcine plasma protein hydrolysate-sugar model system as related to chemical characteristics. Food Sci. Biotechnol. 2014, 23, 33–41. [Google Scholar] [CrossRef]
- Zhang, X.L.; Gao, H.; Wang, C.Y.; Qayum, A.; Mu, Z.S.; Gao, Z.L.; Jiang, Z.M. Characterization and comparison of the structure and antioxidant activity of glycosylated whey peptides from two pathways. Food Chem. 2018, 257, 279–288. [Google Scholar] [CrossRef]
- He, W.; Tian, L.; Fang, F.; Chen, D.; Federici, E.; Pan, S.; Jones, O.G. Limited hydrolysis and conjugation of zein with chitosan oligosaccharide by enzymatic reaction to improve functional properties. Food Chem. 2021, 348, 129035. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, F.G.; Zhang, X.M.; Lu, Z.L.; Guo, Y.; Wang, H.W. Controlled enzymatic hydrolysis on characteristic and antioxidant properties of soybean protein isolate-maltodextrin conjugates. Int. J. Food Prop. 2018, 21, 2239–2249. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.J.; Ji, H.; Chen, Y.; Zhang, Y.F.; Chen, Y. Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. Int. J. Biol. Macromol. 2020, 158, 1194–1203. [Google Scholar] [CrossRef]
- Jia, F.U.; Bai, W.D.; Liu, Y.R.; Wang, Q. Improving the Functional Properties of Chicken Protein in Maillard Reaction by Hydrolysis. Mod. Food Sci. Technol. 2016, 32, 186–195. [Google Scholar]
- Li, M.; Yu, R.; Fu, Y.X.; He, Y.T.; Zhao, P.P.; Jiang, Z.M.; Hou, J.C. Limited hydrolysis of glycosylated whey protein isolate ameliorates the oxidative and physical stabilities of conjugated linoleic acid oil-in-water emulsions. Food Chem. 2021, 362, 130212. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, C.; Abbas, S.; Eric, K.; Xia, S.; Zhang, X. Modified SPI improves the emulsion properties and oxidative stability of fish oil microcapsules. Food Hydrocoll. 2015, 51, 108–117. [Google Scholar] [CrossRef]
- Wang, W.; Wang, M.; Xu, C.; Liu, Z.; Gu, L.; Ma, J.; Jiang, L.; Jiang, Z.; Hou, J. Effects of Soybean Oil Body as a Milk Fat Substitute on Ice Cream: Physicochemical, Sensory and Digestive Properties. Foods 2022, 11, 1504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gao, Y.; Li, J.; Wang, K.; Ma, C.; Sun, D.; Hussain, M.A.; Qayum, A.; Hou, J. Consecutive pH-shift and ultrasound treatment modify the physicochemical properties of whey protein isolate. Int. Dairy J. 2022, 127, 105211. [Google Scholar] [CrossRef]
- Pan, Y.; Wu, Z.; Xie, Q.T.; Li, X.M.; Jin, Z.Y. Insight into the stabilization mechanism of emulsions stabilized by Maillard conjugates: Protein hydrolysates-dextrin with different degree of polymerization. Food Hydrocoll. 2020, 99, 105347. [Google Scholar] [CrossRef]
- Ullah, S.F.; Khan, N.M.; Ali, F.; Ahmad, S.; Khan, Z.U.; Rehman, N.; Jan, A.K.; Muhammad, N. Effects of Maillard reaction on physicochemical and functional properties of walnut protein isolate. Food Sci. Biotechnol. 2019, 28, 1391–1399. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y. Active oxygen scavenging activity of egg-yolk protein hydrolysates and their effects on lipid oxidation in beef and tuna homogenates. Food Chem. 2006, 95, 243–249. [Google Scholar] [CrossRef]
- Nice, D.J.; Robinson, D.S. Inhibition of lipid autoxidation by bovine superoxide dismutase. Food Chem. 1992, 45, 99–103. [Google Scholar] [CrossRef]
- Amza, T.; Balla, A.; Tounkara, F.; Ma, L.; Zhou, H.M. Effect of hydrolysis time on nutritional, functional and antioxidant properties of protein hydrolysates prepared from gingerbread plum (Neocarya macrophylla) seeds. Int. Food Res. J. 2013, 20, 2081–2090. [Google Scholar]
- Haruna, M.H.; Wang, Y.Y.; Pang, J. Konjac glucomannan-based composite films fabricated in the presence of carnauba wax emulsion: Hydrophobicity, mechanical and microstructural properties evaluation. J. Food Sci. Technol. 2019, 56, 5138–5145. [Google Scholar] [CrossRef]
- Chen, S.; Chen, F.P.; Tang, C.H. Fabrication of Plant Globulin/Curcumin Nano-complexes and Their Impact on Physical and Oxidative Stability of Oil-in-water Pickering Emulsions. Mod. Food Sci. Technol. 2015, 31. [Google Scholar] [CrossRef]
- Zhang, J.B.; Wu, N.N.; Yang, X.Q.; He, X.T.; Wang, L.J. Improvement of emulsifying properties of Maillard reaction products from β-conglycinin and dextran using controlled enzymatic hydrolysis. Food Hydrocoll. 2012, 28, 301–312. [Google Scholar] [CrossRef]
- Saito, M.; Yin, L.J.; Kobayashi, I.; Nakajima, M. Preparation characteristics of monodispersed oil-in-water emulsions with large particles stabilized by proteins in straight-through microchannel emulsification. Food Hydrocoll. 2005, 19, 745–751. [Google Scholar] [CrossRef]
- Kinoshita, T.; Maruyama, S.; Matsumoto, Y. In Situ Wettability Characterization of Chemically Heterogeneous Surfaces Probed by Ionic Liquid Contact Angle in Vacuum: Pentacene on Single Crystal SrTiO3 (001). J. Phys. Chem. C 2018, 122, 8390–8395. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Li, J.; Huang, Y.; Gantumur, M.-A.; Bilawal, A.; Qayum, A.; Jiang, Z. Comparison of Oxidative and Physical Stabilities of Conjugated Linoleic Acid Emulsions Stabilized by Glycosylated Whey Protein Hydrolysates via Two Pathways. Foods 2022, 11, 1848. https://doi.org/10.3390/foods11131848
Li M, Li J, Huang Y, Gantumur M-A, Bilawal A, Qayum A, Jiang Z. Comparison of Oxidative and Physical Stabilities of Conjugated Linoleic Acid Emulsions Stabilized by Glycosylated Whey Protein Hydrolysates via Two Pathways. Foods. 2022; 11(13):1848. https://doi.org/10.3390/foods11131848
Chicago/Turabian StyleLi, Meng, Jinzhe Li, Yuxuan Huang, Munkh-Amgalan Gantumur, Akhunzada Bilawal, Abdul Qayum, and Zhanmei Jiang. 2022. "Comparison of Oxidative and Physical Stabilities of Conjugated Linoleic Acid Emulsions Stabilized by Glycosylated Whey Protein Hydrolysates via Two Pathways" Foods 11, no. 13: 1848. https://doi.org/10.3390/foods11131848
APA StyleLi, M., Li, J., Huang, Y., Gantumur, M. -A., Bilawal, A., Qayum, A., & Jiang, Z. (2022). Comparison of Oxidative and Physical Stabilities of Conjugated Linoleic Acid Emulsions Stabilized by Glycosylated Whey Protein Hydrolysates via Two Pathways. Foods, 11(13), 1848. https://doi.org/10.3390/foods11131848