Beneficial Effects of Sodium Nitroprusside on the Aroma, Flavors, and Anthocyanin Accumulation in Blood Orange Fruits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blood Orange Fruits and Sodium Nitroprusside (SNP) Treatments
2.2. Determination of Physical Traits of the Fruits
2.3. Determination of Flavor Compounds
2.4. Determination of Sugars and VC
2.5. Determination of Organic Acids and Amino Acids
2.6. Mineral Element Determination
2.7. Cellulose, Hemicellulose, and Pectin Determination
2.8. Polygalacturonase (PG), Pectinesterase (PE), and Cellulase Enzyme Activity Determination
2.9. Determination of Anthocyanins, Flavonoids, Limonin, and Total Phenols
2.10. Dihydroflavonol-4-Reductase (DFR), Anthocyanidin Synthase (ANS), Chalcone Synthase (CHS), and Chalcone Isomerase (CHI) Activity Assay
2.11. Statistical Analysis
3. Results
3.1. Determination of Optimal SNP Concentration
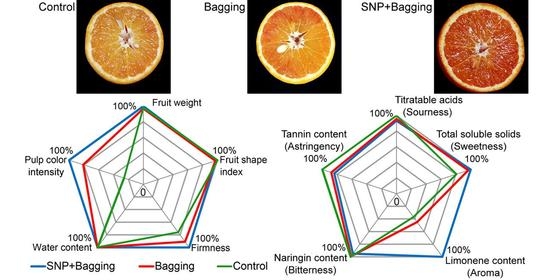
3.2. Effects of Different Treatment Methods on the Physical Properties of Blood Orange
3.3. Effects of Different Treatments on Nutritional Qualities of Blood Orange Fruit
3.4. Effects of Different Treatments on the Texture Qualities of Blood Orange Fruit
3.5. Effects of Different Treatments on Anthocyanins, Flavonoids, and Phenols in Fruits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sicilia, A.; Scialo, E.; Puglisi, I.; Lo Piero, A.R. Anthocyanin biosynthesis and DNA methylation dynamics in sweet orange fruit [Citrus sinensis L. (Osbeck)] under cold stress. J. Agric. Food Chem. 2020, 68, 7024–7031. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Bermejo, A.; Navarro, P.; Forner-Giner, M.N.; Salvador, A. Rootstock effect on fruit quality, anthocyanins, sugars, hydroxycinnamic acids and flavanones content during the harvest of blood oranges ‘moro’ and ‘tarocco rosso’ grown in spain. Food Chem. 2020, 342, 128305. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.J.; Zhang, M.; Fang, Z.X.; Wang, B. Degradation and regulation of edible flower pigments under thermal processing: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Fabroni, S.; Amenta, M.; Timpanaro, N.; Todaro, A.; Rapisarda, P. Change in taste-altering non-volatile components of blood and common orange fruit during cold storage. Food Res. Int. 2019, 131, 108916. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Ramezanian, A.; Guillen, F.; Serrano, M.; Valero, D. Effect of various postharvest treatment on aroma volatile compounds of blood orange fruit exposed to chilling temperature after long-term storage. Food Bioprocess Technol. 2020, 13, 2054–2064. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Fu, Y.F.; Zhou, Y.H.; Wang, C.Q.; Lan, T.; Chen, G.D.; Zeng, J.; Chen, Y.E.; Yuan, M.; Yuan, S.; et al. Nitrogen and nitric oxide regulate Arabidopsis flowering differently. Plant Sci. 2019, 284, 177–184. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Li, M.X.; Huang, B.; Feng, L.Y.; Wu, F.; Fu, Y.F.; Zheng, X.J.; Peng, H.Q.; Chen, Y.E.; Yang, H.N.; et al. Nitric oxide regulates chlorophyllide biosynthesis and singlet oxygen generation differently between Arabidopsis and barley. Nitric Oxide 2018, 76, 6–15. [Google Scholar] [CrossRef]
- Buet, A.; Steelheart, C.; Perini, M.A.; Galatro, A.; Simontacchi, M.; Grozeff, G.E.G. Nitric oxide as a key gasotransmitter in fruit postharvest: An overview. J. Plant Growth Regul. 2021, 40, 2286–2302. [Google Scholar] [CrossRef]
- Palma, J.M.; Freschi, L.; Rodriguez-Ruiz, M.; Gonzalez-Gordo, S.; Corpas, F.J. Nitric oxide in the physiology and quality of fleshy fruits. J. Exp. Bot. 2019, 70, 4405–4417. [Google Scholar] [CrossRef]
- Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Freschi, L.; Corpas, F.J.; Khan, N.A. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environ. Sci. Pollut. Res. 2017, 24, 2273–2285. [Google Scholar] [CrossRef]
- Bruand, C.; Meilhoc, E. Nitric oxide in plants: Pro- or anti-senescence. J. Exp. Bot. 2019, 70, 4419–4427. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Luo, S.; Zhang, G.C.; Feng, L.Y.; Zheng, C.; Zhou, Y.H.; Du, J.B.; Yuan, M.; Chen, Y.E.; Wang, C.Q.; et al. Nitric oxide induces monosaccharide accumulation through enzyme S-nitrosylation. Plant Cell Environ. 2017, 40, 1834–1848. [Google Scholar] [CrossRef]
- Zhu, S.H.; Sun, L.N.; ZHou, J. Effects of nitric oxide fumigation on phenolic metabolism of postharvest Chinese winter jujube (Zizyphus jujuba Mill. cv. Dongzao) in relation to fruit quality. LWT-Food Sci. Technol. 2009, 42, 1009–1014. [Google Scholar] [CrossRef]
- Li, G.J.; Zhu, S.H.; Wu, W.X.; Zhang, C.; Peng, Y.; Wang, Q.G.; Shi, J.Y. Exogenous nitric oxide induces disease resistance against Monilinia fructicola through activating the phenylpropanoid pathway in peach fruit. J. Sci. Food Agric. 2017, 97, 3030–3038. [Google Scholar] [CrossRef]
- Zaharah, S.S.; Singh, Z. Mode of action of nitric oxide in inhibiting ethylene biosynthesis and fruit softening during ripening and cool storage of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 2011, 62, 258–266. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, Z.; Swinny, E.E. Postharvest nitric oxide fumigation delays fruit ripening and alleviates chilling injury during cold storage of Japanese plums (Prunus salicina Lindell). Postharvest Biol. Technol. 2009, 53, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Xu, J.; Chen, Y.; Wei, J.; Wu, B. Nitric oxide treatment maintains postharvest quality of table grapes by mitigation of oxidative damage. Postharvest Biol. Technol. 2019, 152, 9–18. [Google Scholar] [CrossRef]
- Tan, B.Z.; Close, D.C.; Quin, P.R.; Swarts, N.D. Nitrogen use efficiency, allocation, and remobilization in apple trees: Uptake is optimized with pre-harvest N supply. Front. Plant Sci. 2021, 12, 657070. [Google Scholar] [CrossRef]
- Yin, H.; Li, B.; Wang, X.; Xi, Z. Effect of ammonium and nitrate supplies on nitrogen and sucrose metabolism of Cabernet Sauvignon (Vitis vinifera cv.). J. Sci. Food Agric. 2020, 100, 5239–5250. [Google Scholar] [CrossRef]
- Barboriak, D.P.; Padua, A.O.; York, G.E.; Macfall, J.R. Creation of DICOM—aware applications using ImageJ. J. Digit. Imaging 2005, 18, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.; Vercambre, G.; Kang, S.Z.; Bertin, N.; Gautier, H.; Genard, M. Fruit water content as an indication of sugar metabolism improves simulation of carbohydrate accumulation in tomato fruit. J. Exp. Bot. 2020, 71, 5010–5026. [Google Scholar] [CrossRef]
- Davidović, S.; Miljković, M.; Gordic, M.; Cabrera-Barjas, G.; Nesic, A.; Dimitrijević-Branković, S. Dextran-based edible coatings to prolong the shelf life of blueberries. Polymers 2021, 13, 4252. [Google Scholar] [CrossRef]
- Akkad, R.; Buchko, A.; Johnston, S.P.; Han, J.; House, J.D.; Curtis, J.M. Sprouting improves the flavour quality of faba bean flours. Food Chem. 2021, 364, 130355. [Google Scholar] [CrossRef]
- Bai, H.W.; Hong, S.H.; Park, C.H.; Jang, D.M.; Kim, T.H.; Chung, B.Y. Degradation of Limonene by Gamma Radiation for Improving Bioethanol Production. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 1–4. [Google Scholar] [CrossRef]
- Shamloo, M.M.; Sharifani, M.; Garmakhany, A.D.; Seifi, E. Alternation of secondary metabolites and quality attributes in Valencia Orange fruit (Citrus sinensis) as influenced by storage period and edible covers. J. Food Sci. Technol. 2015, 52, 1936–1947. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.W.; Wu, P.; Zhang, W.B.; Yang, Z.F.; Liu, H.Y.; Ahammed, G.J.; Cui, J.X. Calcium is involved in exogenous NO-induced enhancement of photosynthesis in cucumber (Cucumis sativus L.) seedlings under low temperature. Sci. Hortic. 2020, 261, 108953. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.H.; Ning, H.F.; Zhang, X.X.; Li, S.; Pang, J.; Wang, G.S.; Sun, J.S. Optimizing irrigation frequency and amount to balance yield, fruit quality and water use efficiency of greenhouse tomato. Agric. Water Manag. 2019, 226, 105787. [Google Scholar] [CrossRef]
- Wu, Z.F.; Tu, M.M.; Yang, X.P.; Xu, J.H.; Yu, Z.F. Effect of cutting and storage temperature on sucrose and organic acids metabolism in postharvest melon fruit. Postharvest Biol. Technol. 2020, 161, 111081. [Google Scholar] [CrossRef]
- Wistaff, E.A.; Beller, S.; Schmid, A.; Neville, J.J.; Nietner, T. Chemometric analysis of amino acid profiles for detection of fruit juice adulterations—Application to verify authenticity of blood orange juice. Food Chem. 2021, 343, 128452. [Google Scholar] [CrossRef]
- Bao, Y.L.; Boeren, S.; Ertbjerg, P. Myofibrillar protein oxidation affects filament charges, aggregation and water-holding. Meat Sci. 2018, 135, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.Q.; Dang, H.; Zhang, T.; Dong, J.R.; Chen, H.W.; Xiang, W. Nutrient variation induced by rodent disturbance in Haloxylon ammodendron as a target transfer strategy. Ecol. Evol. 2021, 11, 17260–17272. [Google Scholar] [CrossRef] [PubMed]
- Paunovic, S.M.; Maskovic, P.; Milinkovic, M. Determination of primary metabolites, vitamins and minerals in black mulberry (morus nigra) berries depending on altitude. Erwerbs-Obstbau 2020, 62, 355–360. [Google Scholar] [CrossRef]
- Rezaeinejad, R.; Khademi, H.; Ayoubi, S.; Mosaddeghi, M.R. Roots under water stress induce K release from phlogopite, bio-transforming to vermiculite. Rhizosphere 2021, 17, 100310. [Google Scholar] [CrossRef]
- Bagci, Y.; Arslan, D.; Ozcan, M.M.; Dursun, N. Determination of mineral contents of bee honeys produced in Middle Anatolia. Int. J. Food Sci. Nutr. 2007, 58, 668–676. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.S.; Kommula, V.P. Exploration on the characteristics of cellulose microfibers from Palmyra palm fruits. Int. J. Polym. Anal. Charact. 2016, 21, 286–295. [Google Scholar] [CrossRef]
- Lin, X.; Luo, C.L.; Chen, Y.L. Effects of vacuum impregnation with sucrose solution on mango tissue. J. Food Sci. 2016, 81, 1412–1418. [Google Scholar] [CrossRef]
- Cao, J.K.; Jiang, W.B.; Zhao, Y.M. Guidance on Postharvest Physiological and Biochemical Experiments of Fruits and Vegetables; China Light Industry Press: Beijing, China, 2007; Chapter 2; pp. 89–92. [Google Scholar]
- Lohani, S.; Trivedi, P.K.; Nath, P. Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: Effect of 1-MCP, ABA and IAA. Postharvest Biol. Technol. 2004, 31, 119–126. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Lin, H.; Hung, Y.C.; Zhang, S.; Lin, Y.; Lin, T. Paper-based 1-MCP treatment suppresses cell wall metabolism and delays softening of Huanghua pears during storage. J. Sci. Food Agric. 2017, 97, 2547–2552. [Google Scholar] [CrossRef]
- Abu-Goukh, A.B.A.; Bashir, H.A. Changes in pectic enzymes and cellulase activity during guava fruit ripening. Food Chem. 2003, 83, 213–218. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Z.L. Plant Physiology Experiment Guide, 5th ed.; Higher Education Press: Beijing, China, 2016; Chapter 5; pp. 124–125. [Google Scholar]
- Jia, Z.S.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Jiang, Z.M.; Wang, L.J.; Liu, W.J.; Wang, H.Y.; Xiao, P.T.; Zhou, P.; Bi, Z.M.; Liu, E.H. Development and validation of a supercritical fluid chromatography method for fast analysis of six flavonoids in Citri Reticulatae Pericarpium. J. Chromatogr. B 2019, 1133, 121845. [Google Scholar] [CrossRef]
- Gordon, R.M.; Washington, T.L.; Sims, C.A.; Goodrich-Schneider, R.; Baker, S.M.; Yagiz, Y.; Gu, L.W. Performance of macroporous resins for debittering HLB-affected grapefruit juice and its impacts on furanocoumarin and consumer sensory acceptability. Food Chem. 2021, 352, 129367. [Google Scholar] [CrossRef]
- Zhou, D.D.; Li, R.; Zhang, H.; Chen, S.X.; Tu, K. Hot air and UV-C treatments promote anthocyanin accumulation in peach fruit through their regulations of sugars and organic acids. Food Chem. 2020, 309, 125726. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, M.; Mateos, R.M.; Codesido, V.; Corpas, F.J.; Palma, J.M. Characterization of the galactono-1,4-lactone dehydrogenase from pepper fruits and its modulation in the ascorbate biosynthesis. Role of nitric oxide. Redox Biol. 2017, 12, 171–181. [Google Scholar] [CrossRef]
- Shi, K.K.; Liu, Z.C.; Wang, J.W.; Zhu, S.H.; Huang, D.D. Nitric oxide modulates sugar metabolism and maintains the quality of red raspberry during storage. Sci. Hortic. 2019, 256, 108611. [Google Scholar] [CrossRef]
- Khan, G.A.; Persson, S. Cell Wall Biology: Dual Control of Cellulose Synthase Guidance. Curr. Biol. 2020, 30, R232–R234. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, S.; Wang, Y. Compositional changes in cell wall polyuronides and enzyme activities associated with melting/mealy textural property during ripening following long-term storage of ‘Comice’ and ‘d’Anjou’ pears. Postharvest Biol. Technol. 2018, 135, 131–140. [Google Scholar] [CrossRef]
- Qi, X.H.; Ji, Z.J.; Lin, C.; Li, S.F.; Liu, J.; Kan, J.; Zhang, M.; Jin, C.H.; Qian, C.L. Nitric oxide alleviates lignification and softening of water bamboo (Zizania latifolia) shoots during postharvest storage. Food Chem. 2020, 332, 127416. [Google Scholar] [CrossRef]
- Dong, T.; Xia, R.X.; Xiao, Z.Y.; Wang, P.; Song, W.H. Effect of pre-harvest application of calcium and boron on dietary fibre, hydrolases and ultrastructure in ‘Cara Cara’ navel orange (Citrus sinensis L. Osbeck) fruit. Sci. Hortic. 2009, 121, 272–277. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Li, J.; Dawuda, M.M.; Ali, B.; Wu, Y.; Yu, J.; Tang, Z.; Lyu, J.; Xiao, X.; et al. Exogenous application of 5-aminolevulinic acid promotes coloration and improves the quality of tomato fruit by regulating carotenoid metabolism. Front. Plant Sci. 2021, 12, 683868. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.C.; Qu, H.X.; Li, L.; Mao, B.Z.; Xu, Y.Q.; Lin, X.Y.; Luo, Z.S. Delaying the biosynthesis of aromatic secondary metabolites in postharvest strawberry fruit exposed to elevated CO2 atmosphere. Food Chem. 2020, 306, 125611. [Google Scholar] [CrossRef]
- Takahashi, M.; Hirose, N.; Ohno, S.; Arakaki, M.; Wada, K. Flavor characteristics and antioxidant capacities of hihatsumodoki (Piper retrofractum Vahl) fresh fruit at three edible maturity stages. J. Food Sci. Technol. 2018, 55, 1295–1305. [Google Scholar] [CrossRef]
- Lopez-Carrion, A.I.; Castellano, R.; Rosales, M.A.; Ruiz, J.M.; Romero, L. Role of nitric oxide under saline stress: Implications on proline metabolism. Biol. Plant. 2008, 52, 587–591. [Google Scholar] [CrossRef]
- Yang, L.T.; Chen, L.S.; Peng, H.Y.; Guo, P.; Wang, P.; Ma, C.L. Organic acid metabolism in Citrus grandis leaves and roots is differently affected by nitric oxide and aluminum interactions. Sci. Hortic. 2012, 133, 40–46. [Google Scholar] [CrossRef]
- Sdiri, S.; Bermejo, A.; Aleza, P.; Navarro, P.; Salvador, A. Phenolic composition, organic acids, sugars, vitamin C and antioxidant activity in the juice of two new triploid late-season mandarins. Front. Plant Sci. 2012, 49, 462–468. [Google Scholar] [CrossRef]
- Tepe, H.D.; Aydemir, T. Investigation of the changes in low molecular weight organic acids and other physiological parameters by nitric oxide application in two wheat cultivars exposed to boron stress. J. Plant Nutr. 2017, 40, 1287–1299. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium a central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Conn, S.J.; Gilliham, M.; Athman, A.; Schreiber, A.W.; Baumann, U.; Moller, I.; Cheng, N.H.; Stancombe, M.A.; Hirschi, K.D.; Webb, A.A.; et al. Cell-specific vacuolar calcium storage mediated by CAX1 regulates apoplastic calcium concentration, gas exchange, and plant productivity in Arabidopsis. Plant Cell 2011, 23, 240–257. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.Y.; Dong, Y.J.; Wang, Q.H.; Xu, L.L.; Kong, J.; Liu, S. Effects of lead and nitric oxide on photosynthesis, antioxidative ability, and mineral element content of perennial ryegrass. Biol. Plant. 2015, 59, 163–170. [Google Scholar] [CrossRef]
- Shen, J.C.; Shao, W.L.; Du, Z.K.; Lu, H.F.; Li, J.M. Integrated metabolomic and transcriptomic analyses reveal differences in the biosynthetic pathway of anthocyanins in Fragaria nilgerrensis and Fragaria pentaphylla. Sci. Hortic. 2020, 271, 109476. [Google Scholar] [CrossRef]
- Shi, S.G.; Li, S.J.; Kang, Y.X.; Liu, J.J. Molecular characterization and expression analyses of an anthocyanin synthase gene from Magnolia sprengeri Pamp. Appl. Biochem. Biotechnol. 2015, 175, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Recalde, L.; Vazquez, A.; Groppa, M.D.; Benavides, M.P. Reactive oxygen species and nitric oxide are involved in polyamine-induced growth inhibition in wheat plants. Protoplasma 2018, 255, 1295–1307. [Google Scholar] [CrossRef]
- Ruenroengklin, N.; Yang, B.; Lin, H.T.; Chen, F.; Jiang, Y.M. Degradation of anthocyanin from litchi fruit pericarp by H2O2 and hydroxyl radical. Food Chem. 2009, 116, 995–998. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Akagi, T.; Kanzaki, S.; Gao, M.; Tao, R.; Parfitt, D.E.; Yonemori, K. Quantitative real-time PCR to determine allele number for the astringency locus by analysis of a linked marker in Diospyros kaki Thunb. Tree Genet. Genomes 2009, 5, 483–492. [Google Scholar] [CrossRef]
- Shi, J.Y.; Li, J.X.; Zhu, S.H.; Zhou, J. Browning inhibition on fresh-cut chestnut kernel by exogenous nitric oxide. Int. J. Food Sci. Technol. 2011, 46, 944–950. [Google Scholar] [CrossRef]
- Wodala, B.; Ordög, A.; Horváth, F. The cost and risk of using sodium nitroprusside as a NO donor in chlorophyll fluorescence experiments. J. Plant Physiol. 2010, 167, 1109–1111. [Google Scholar] [CrossRef]
- Ratnasooriya, W.D.; Jayakody, J.R.; Dharmasiri, M.G. Sodium nitroprusside impairs sexual competence of male rats. Hum. Exp. Toxicol. 2004, 23, 187–192. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-W.; Liu, H.; Li, H.; Yang, X.-Y.; Fu, Y.-F.; Kang, Q.; Wang, C.-Q.; Yuan, M.; Chen, Y.-E.; Yuan, S. Beneficial Effects of Sodium Nitroprusside on the Aroma, Flavors, and Anthocyanin Accumulation in Blood Orange Fruits. Foods 2022, 11, 2218. https://doi.org/10.3390/foods11152218
Zhang Z-W, Liu H, Li H, Yang X-Y, Fu Y-F, Kang Q, Wang C-Q, Yuan M, Chen Y-E, Yuan S. Beneficial Effects of Sodium Nitroprusside on the Aroma, Flavors, and Anthocyanin Accumulation in Blood Orange Fruits. Foods. 2022; 11(15):2218. https://doi.org/10.3390/foods11152218
Chicago/Turabian StyleZhang, Zhong-Wei, Han Liu, Hao Li, Xin-Yue Yang, Yu-Fan Fu, Qi Kang, Chang-Quan Wang, Ming Yuan, Yang-Er Chen, and Shu Yuan. 2022. "Beneficial Effects of Sodium Nitroprusside on the Aroma, Flavors, and Anthocyanin Accumulation in Blood Orange Fruits" Foods 11, no. 15: 2218. https://doi.org/10.3390/foods11152218
APA StyleZhang, Z. -W., Liu, H., Li, H., Yang, X. -Y., Fu, Y. -F., Kang, Q., Wang, C. -Q., Yuan, M., Chen, Y. -E., & Yuan, S. (2022). Beneficial Effects of Sodium Nitroprusside on the Aroma, Flavors, and Anthocyanin Accumulation in Blood Orange Fruits. Foods, 11(15), 2218. https://doi.org/10.3390/foods11152218