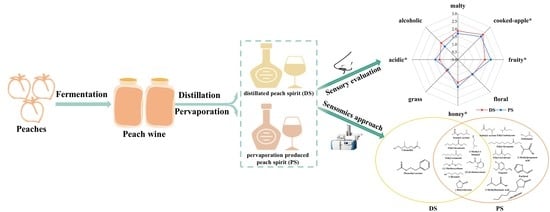
Characterization of Key Aroma-Active Compounds in Two Types of Peach Spirits Produced by Distillation and Pervaporation by Means of the Sensomics Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Samples
2.2. Chemicals
2.3. Aroma Extract Dilution Analysis with GC-O/GC-MS
2.3.1. Liquid-Liquid Extraction (LLE)
2.3.2. Headspace Solid-Phase Microextraction (HS-SPME)
2.3.3. Aroma Extract Dilution Analysis (AEDA)
2.3.4. Conditions of GC-MS/GC-O
2.3.5. Identification of Aroma Compounds
2.4. Quantitation of Aroma Compounds
2.4.1. Direct Injection (DI) Combined with GC-MS
2.4.2. LLE Combined with GC-MS
2.4.3. HS-SPME Combined with GC-MS
2.5. Sensory Analysis
2.6. Aroma Reconstitution Experiments
2.7. Omission Experiments
2.8. Statistical Analysis
3. Results and Discussion
3.1. Aroma Profile Analysis of Peach Spirits
3.2. Identification of Aroma Compounds in Peach Spirits
| No. | Compounds | CAS | Odor Descriptor | Identification a | RI b | FD Factor | |||
|---|---|---|---|---|---|---|---|---|---|
| DS | PS | ||||||||
| LLE c (AF/NBF) | SPME c | LLE c (AF/NBF) | SPME c | ||||||
| Esters | |||||||||
| 1 | Ethyl acetate | 141-78-6 | Fruity | MS, RI, aroma, S | 880 | -/81 | 3 | - | 9 |
| 2 | Ethyl isobutyrate | 97-62-1 | Fruity | MS, RI, aroma, S | 878 | - | - | - | 27 |
| 3 | Isobutyl acetate | 110-19-0 | Fruity | MS, RI, aroma, S | 900 | - | 3 | - | 27 |
| 4 | Ethyl 2-methylbutyrate | 7452-79-1 | Fruity | MS, RI, aroma | 960 | - | - | -/27 | 9 |
| 5 | Ethyl isovalerate | 108-64-5 | Fruity | MS, RI, aroma, S | 1103 | - | - | -/243 | 27 |
| 6 | Ethyl butanoate | 105-54-4 | Pineapple | MS, RI, aroma, S | 1110 | - | 3 | - | 27 |
| 7 | Isoamyl acetate | 123-92-2 | Banana | MS, RI, aroma, S | 1138 | -/729 | 9 | -/81 | 27 |
| 8 | Ethyl hexanoate | 123-66-0 | Sweet, fruity | MS, RI, aroma, S | 1252 | -/243 | 9 | -/27 | 9 |
| 9 | Hexyl acetate | 142-92-7 | Fruity, green | MS, RI, aroma, S | 1277 | -/3 | - | -/9 | 1 |
| 10 | Ethyl 2-hexenoate | 1552-67-6 | Fruity | MS, RI, aroma | 1335 | -/3 | - | -/1 | - |
| 11 | Ethyl lactate | 97-64-3 | Fruity | MS, RI, aroma, S | 1339 | -/9 | - | -/1 | - |
| 12 | Ethyl octanoate | 106-32-1 | Fruity | MS, RI, aroma, S | 1413 | -/9 | 9 | - | 9 |
| 13 | Ethyl 2-hydroxyisovalerate | 2441-06-7 | Fruity | MS, RI, aroma | 1422 | - | - | 1/9 | 3 |
| 14 | Ethyl 3-hydroxybutyrate | 5405-41-4 | Fruity | MS, RI, aroma | 1500 | - | - | 1/- | - |
| 15 | Ethyl 2-hydroxy-4-methyl valerate | 10348-47-7 | Woody, fruity | MS, RI, aroma | 1513 | -/9 | - | -/9 | 1 |
| 16 | Isoamyl lactate | 19329-89-6 | Fruity, nutty | MS, RI, aroma, S | 1543 | -/3 | - | -/3 | - |
| 17 | Ethyl methyl succinate | - | Fruity | MS, RI | 1590 | - | - | -/3 | - |
| 19 | Ethyl decanoate | 110-38-3 | Fruity | MS, RI, aroma, S | 1610 | -/9 | 9 | -/3 | - |
| 20 | Diethyl succinate | 123-25-1 | Fruity | MS, RI, aroma, S | 1645 | -/81 | - | -/3 | - |
| 21 | Trimethylene acetate | 628-66-0 | Green | MS, RI | 1672 | - | - | 1/1 | - |
| 22 | Ethyl dodecanoate | 106-33-2 | Floral, sweet | MS, RI, aroma, S | 1837 | -/9 | 3 | - | - |
| 23 | Isoamyl decanoate | 2306-91-4 | Waxy, fruity | MS, RI, aroma, S | 1858 | -/27 | - | - | - |
| 24 | Ethyl tetradecanoate | 124-06-1 | Sweet | MS, RI, aroma, S | 2037 | -/9 | 9 | - | - |
| 25 | 3-Methylbutyl dodecanoate | 6309-51-9 | Sweet, overripe fruit | MS, RI, aroma, S | 2058 | -/9 | - | - | - |
| 26 | Ethyl pentadecanoate | 41114-00-5 | Sweet, honey | MS, RI, aroma, S | 2138 | -/27 | - | - | - |
| 27 | Ethyl hexadecanoate | 628-97-7 | Waxy, oily | MS, RI, aroma, S | 2250 | 1/9 | - | -/3 | - |
| 28 | Ethyl 9-decenoate | 67233-91-4 | Unpleasant | MS, RI | 2262 | -/9 | 3 | - | - |
| 29 | Ethyl octadecanoate | 111-61-5 | Waxy | MS, RI, aroma, S | 2449 | -/3 | - | -/3 | - |
| 30 | Ethyl oleate | 111-62-6 | Waxy, oily | MS, RI, aroma, S | 2469 | -/9 | - | - | - |
| Alcohols | |||||||||
| 31 | 1-Propanol | 71-23-8 | Alcohol | MS, RI, aroma, S | 1024 | - | 9 | 3/3 | - |
| 32 | Isobutanol | 78-83-1 | Burnt | MS, RI, aroma, S | 1092 | - | - | 3/1 | 81 |
| 33 | 1-Butanol | 71-36-3 | Fruity | MS, RI, aroma, S | 1120 | - | - | 9/1 | 3 |
| 34 | 3-Methyl-1-butanol | 123-51-3 | Malty, nail polish-like | MS, RI, aroma, S | 1199 | 243/6561 | 81 | 243/6561 | 243 |
| 35 | 3-Methyl-3-buten-1-ol | 763-32-6 | Fruity | MS, RI, aroma, S | 1245 | - | - | 1/3 | - |
| 36 | 1-Pentanol | 71-41-0 | Fruity, sour | MS, RI, aroma | 1258 | /3 | /1 | 1 | |
| 37 | 4-Methyl-2-pentanol | 108-11-2 | Almond, toasted | MS, RI, aroma | 1300 | -/3 | - | - | - |
| 38 | 3-Methyl-1-pentanol | 589-35-5 | Fruity | MS, RI, aroma, S | 1318 | - | - | -/1 | - |
| 39 | 1-Hexanol | 111-27-3 | Green | MS, RI, aroma, S | 1346 | 1/81 | 81 | 9/81 | 9 |
| 40 | 3-Hexenol | 544-12-7 | Green | MS, RI, aroma, S | 1350 | -/9 | - | 3/9 | 1 |
| 41 | Leaf alcohol | 928-96-1 | Green | MS, RI, aroma, S | 1365 | -/9 | - | - | - |
| 42 | 1-Heptanol | 111-70-6 | Green | MS, RI, aroma, S | 1428 | -/81 | - | -/3 | - |
| 43 | 2-Ethyl-1-hexanol | 104-76-7 | Fruity | MS, RI, aroma, S | 1495 | - | - | -/1 | - |
| 44 | 1-Octanol | 111-87-5 | Fruity | MS, RI, aroma, S | 1529 | -/3 | - | - | - |
| Acetals | |||||||||
| 45 | 1,1-Diethoxyethane | 105-57-7 | Sweet, fruity | MS, RI, aroma, S | 906 | - | 27 | - | 27 |
| Carbonyl compounds | |||||||||
| 46 | 3-Hydroxy-2-butanone | 513-86-0 | Fruity | MS, RI, aroma, S | 1285 | - | - | 9/3 | - |
| 47 | Furfural | 98-01-1 | Woody, almond | MS, RI, aroma, S | 1419 | 1/81 | - | 3/27 | - |
| 48 | Decanal | 112-31-2 | Green | MS, RI, aroma, S | 1512 | - | - | - | 3 |
| 49 | Ethyl 2-furoate | 614-99-3 | Fruity, burnt | MS, RI, aroma, S | 1586 | -/81 | - | -/1 | 3 |
| Lactones | |||||||||
| 50 | γ-Butyrolactone | 96-48-0 | Fruity | MS, RI, aroma, S | 1590 | -/9 | - | -/3 | - |
| 51 | γ-Hexalactone | 695-06-7 | Tobacco | MS, RI, aroma, S | 1621 | - | - | -/9 | - |
| 52 | γ-Octalactone | 104-50-7 | Apricot and peach | MS, RI, aroma, S | 1810 | - | - | -/9 | - |
| 53 | γ-Nonalactone | 104-61-0 | Milky notes | MS, RI, aroma, S | 1957 | - | - | /243 | - |
| 54 | γ-Decalactone | 706-14-9 | Apricot and peach | MS, RI, aroma, S | 2158 | - | - | /729 | - |
| Aromatic compound | |||||||||
| 55 | Benzaldehyde | 100-52-7 | Almond, woody | MS, RI, aroma, S | 1485 | -/243 | - | -/9 | 9 |
| 56 | Ethyl benzoate | 93-89-0 | Floral, fruity | MS, RI, aroma, S | 1639 | -/81 | 3 | -/1 | 3 |
| 57 | Benzyl acetate | 140-11-4 | Floral, sweet | MS, RI, aroma, S | 1695 | -/243 | - | -/1 | 3 |
| 58 | Phenethyl acetate | 103-45-7 | Honey | MS, RI, aroma, S | 1787 | 1/2187 | 9 | -/2187 | 3 |
| 59 | Benzyl alcohol | 100-51-6 | Fruit | MS, RI, aroma, S | 1843 | 3/- | - | 81/81 | 27 |
| 60 | Phenylethyl alcohol | 60-12-8 | Dried rose | MS, RI, aroma, S | 1879 | 1/6561 | 3 | 81/6561 | 81 |
| Terpene | |||||||||
| 61 | Linalool | 78-70-6 | Floral | MS, RI, aroma, S | 1521 | -/9 | - | -/9 | 1 |
| 62 | Citronellol | 106-22-9 | Rose-like | MS, RI, aroma, S | 1740 | -/81 | - | 9/- | - |
| 63 | (E)-β-Damascenone | 23696-85-7 | Cooked apple | MS, RI, aroma, S | 1820 | -/2187 | 3 | -/81 | 81 |
| 64 | Dihydro-β-ionol | 3293-47-8 | Woody, floral | MS, RI, aroma | 1950 | -/243 | 3 | -/3 | 27 |
| 65 | trans-Nerolidol | 40716-66-3 | Floral, tea | MS, RI, aroma, S | 2017 | -/729 | - | - | - |
| 66 | trans-β-ionone | 79-77-6 | Sweet, floral | MS, RI, aroma, S | 2065 | -/27 | - | - | - |
| 67 | Eugenol | 97-53-0 | Sweet | MS, RI, aroma, S | 2130 | - | - | -/9 | - |
| 68 | (E,E)-Farnesol | 106-28-5 | Sweet, floral | MS, RI, aroma, S | 2326 | -/27 | - | - | - |
| Acids | |||||||||
| 69 | Acetic acid | 64-19-7 | Acidic | MS, RI, aroma, S | 1405 | 3/- | - | 1/- | 3 |
| 70 | 2-Methylpropanoic acid | 79-31-2 | Acidic | MS, RI, aroma, S | 1526 | 3/- | - | 9/- | 1 |
| 71 | Butyric acid | 107-92-6 | Acidic | MS, RI, aroma, S | 1598 | - | - | 9/- | - |
| 72 | 3-Methylbutanoic acid | 503-74-2 | Sweat | MS, RI, aroma, S | 1629 | 81/- | - | 243/- | 27 |
| 73 | Hexanoic acid | 142-62-1 | Acidic, cheese | MS, RI, aroma, S | 1804 | 3/- | - | 27/- | - |
| 74 | Heptanoic acid | 111-14-8 | Acidic | MS, RI, aroma, S | 1913 | 1/- | - | - | - |
| 75 | Octanoic acid | 124-07-2 | Vegetable, fatty | MS, RI, aroma, S | 2021 | 9/- | 1 | - | 9 |
| 76 | Nonanoic acid | 112-05-0 | Coffee | MS, RI, aroma, S | 2130 | 81/- | - | 9/- | 27 |
| 77 | Decanoic acid | 334-48-5 | Fatty | MS, RI, aroma, S | 2235 | 3/- | - | - | - |
| Sulfides | |||||||||
| 78 | Blackberry thiophenone | 13679-85-1 | Fruity berry, toasted | MS, RI, aroma | 1492 | -/2187 | - | - | - |
| 79 | 3-Methylthiopropanol | 505-10-2 | Cooked potato, roasted | MS, RI, aroma, S | 1681 | 81/729 | - | 9/9 | - |
| 80 | p-toluenesulfonic acid n-butyl ester | 778-28-9 | Unpleasant | MS, RI, aroma, S | 1757 | - | 9 | - | - |
| Unknown | |||||||||
| 81 | Unknown1 | Woody | - | 1572 | -/27 | - | - | 1 | |
| 82 | Unknown2 | Roasted | - | 1652 | -/3 | - | - | ||
| 83 | Unknown3 | Coffee, roasted | - | 1960 | 3/- | - | - | ||
| 84 | Unknown4 | Animal | 2063 | - | - | -/27 | |||
| 85 | Unknown5 | Cheese | 2154 | - | - | 27/- | |||
3.3. Quantification and OAV Analysis of Aroma Compounds
| No. | Compounds | Concentrations (μg/L) | Thresholds (μg/L) | OAVs | ||
|---|---|---|---|---|---|---|
| DS | PS | DS | PS | |||
| Esters | ||||||
| 1 | Ethyl acetate b | 42,106.30 ± 3614.85 | 45,362.25 ± 375.58 | 32,551.6 d | 1 | 1 |
| 2 | Ethyl isobutyrate b | - | 1254.23 ± 8.24 | 57.47 d | - | 21 |
| 3 | Isobutyl acetate b | Trace | 981.85 ± 8.14 | 922 d | - | 1 |
| 5 | Ethyl isovalerate a | - | 32.79 ± 0.04 | 6.89 d | - | 5 |
| 6 | Ethyl butanoate b | 306.77 ± 10.42 | 165.08 ± 6.85 | 81.5 d | 4 | 2 |
| 7 | Isoamyl acetate b | 3180.61 ± 113.57 | 6281.25 ± 60.21 | 93.93 d | 35 | 67 |
| 8 | Ethyl hexanoate b | 416.76 ± 18.24 | 6522.65 ± 50.00 | 55 d | 8 | 127 |
| 9 | Hexyl acetate a | 161.24 ± 3.34 | 355.67 ± 0.18 | 1500 e | <1 | <1 |
| 11 | Ethyl lactate a | 1222.83 ± 12.33 | Trace | 128,000 d | <1 | - |
| 12 | Ethyl octanoate a | 533.49 ± 10.49 | 341.48 ± 1.56 | 12.87 d | 42 | 27 |
| 16 | Isoamyl lactate a | 13.05 ± 0.58 | 207.76 ± 0.39 | 131,703.4 d | <1 | <1 |
| 19 | Ethyl decanoate a | 73.12 ± 0.26 | 5060.85 ± 0.97 | 1120 d | <1 | 5 |
| 20 | Diethyl succinate a | 8.63 ± 0.61 | 1445.60 ± 50 | 353,193.25 d | <1 | <1 |
| 22 | Ethyl dodecanoate a | 150.45 ± 1.36 | 129.25 ± 4.37 | 500 e | <1 | <1 |
| 24 | Ethyl tetradecanoate a | 96.61 ± 2.70 | 129.24 ± 4.37 | 447,068.16 f | <1 | - |
| Alcohols | ||||||
| 32 | Isobutanol c | - | 848,679.75 ± 40,462.05 | 40,000 e | - | 22 |
| 33 | 1-Butanol a | - | 983.92 ± 0.39 | 2733 d | - | <1 |
| 34 | 3-Methyl-1-butanol c | 1,133,697.91 ± 30335.64 | 1,149,273.6 ± 21,513.27 | 179,000 d | 7 | 7 |
| 36 | 1-Pentanol a | Trace | 107.46 ± 0.08 | 4000 e | - | <1 |
| 38 | 3-Methyl-1-pentanol a | - | 120.89 ± 1.25 | 1000 i | - | <1 |
| 39 | 1-Hexanol c | 15,903.45 ± 438.73 | 32,594.58 ± 1737.89 | 5370 e | 3 | 6 |
| 40 | 3-Hexenol a | 90.68 ± 5.19 | Trace | 1257 h | <1 | - |
| 41 | Leaf alcohol a | 143.46 ± 12.6 | - | 1000 i | <1 | - |
| 42 | 1-Heptanol a | 149.03 ± 7.54 | Trace | 26,600 e | <1 | - |
| 43 | 2-Ethyl-1-hexanol a | - | 15.44 ± 0.04 | 1280 g | - | <1 |
| Acetals | ||||||
| 45 | 1,1-Diethoxyethane b | 4775.19 ± 85.41 | 4489.08 ± 62.68 | 69 e | 70 | 65 |
| Carbonyl compounds | ||||||
| 47 | Furfural a | 166.71 ± 23.15 | 335.47 ± 0.39 | 122 e | 2 | 3 |
| 49 | Ethyl 2-furoate a | 30.97 ± 0.37 | Trace | 16,000 g | <1 | - |
| Lactones | ||||||
| 50 | γ-Butyrolactone a | 84.54 ± 0.30 | 428.94 ± 0.08 | 20 m | 4 | 21 |
| 51 | γ-Hexalactone a | - | 116.97 ± 5.00 | 359,000 e | - | <1 |
| 53 | γ-Nonanolactone a | - | 3.36 ± 0.039 | 90.66 d | - | <1 |
| 54 | γ-Decalactone a | - | 50.96 ± 0.18 | 10.87 d | - | 5 |
| Aromatic compounds | ||||||
| 55 | Benzaldehyde a | 74.56 ± 1.46 | 1995.96 ± 1.95 | 4203.1 d | <1 | <1 |
| 56 | Ethyl benzoate a | 109.04 ± 0.64 | 246.5 ± 0.39 | 4203 d | <1 | <1 |
| 57 | Benzyl acetate a | 39.06 ± 0.27 | 38.71 ± 1.25 | 270 g | <1 | <1 |
| 58 | Phenethyl acetate a | 3738.40 ± 144.96 | 359.93 ± 0.39 | 908.83 d | 4 | <1 |
| 59 | Benzyl alcohol a | 27.02 ± 3.16 | 446.02 ± 0.70 | 40,900 e | <1 | <1 |
| 60 | Phenylethyl alcohol a | 1085.98 ± 120.13 | 10,530.78 ± 622.24 | 8500 d | <1 | 1 |
| Terpenes | ||||||
| 61 | Linalool a | 19.67 ± 0.19 | Trace | 23 l | 1 | - |
| 62 | Citronellol a | 372.06 ± 9.17 | 12.32 ± 0.04 | 18 j | 21 | <1 |
| 63 | (E)-β-damascenone a | 626.89 ± 22.22 | 35.18 ± 0.01 | 0.4 e | 1622 | 88 |
| 65 | trans-Nerolidol a | 63.36 ± 1.16 | - | 400 j | <1 | - |
| 66 | trans-β-ionone a | 2.34 ± 0.07 | - | 4.5 j | <1 | - |
| 67 | Eugenol a | - | 77.72 ± 0.19 | 21 e | - | 4 |
| 68 | (E,E)-Farnesol a | 11.09 ± 1.70 | - | 1000 k | <1 | - |
| Acids | ||||||
| 69 | Acetic acid a | 179.9 ± 0.52 | 6827.92 ± 1.95 | 160,000 e | <1 | <1 |
| 70 | 2-Methylpropanoic acid a | 292.58 ± 23.08 | 12,938.45 ± 481.50 | 1580 e | <1 | 8 |
| 71 | Butyric acid a | - | 386.28 ± 0.39 | 964 d | - | <1 |
| 72 | 3-Methylbutanoic acid a | 387.43 ± 7.79 | 4033.45 ± 309.44 | 1050 e | <1 | 4 |
| 73 | Hexanoic acid a | 610.95 ± 24.63 | 1307.33 ± 1.17 | 2520 d | <1 | <1 |
| 75 | Octanoic acid a | 272.16 ± 23.36 | 1040.49 ± 0.78 | 2700 d | <1 | <1 |
| 77 | Decanoic acid a | 219.86 ± 17.54 | - | 13,737 d | <1 | - |
| Sulfides | ||||||
| 79 | 3-Methylthiopropanol a | 29.51 ± 3.23 | 406.02 ± 0.39 | 2110 d | <1 | <1 |
3.4. Aroma Reconstitution Test
3.5. Omission Test
3.6. PCA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davidovic, S.M.; Veljovic, M.S.; Pantelic, M.M.; Baosic, R.M.; Natic, M.M.; Dabic, D.C.; Pecic, S.P.; Vukosavljevic, P.V. Physicochemical, Antioxidant and Sensory Properties of Peach Wine Made from Redhaven Cultivar. J. Agric. Food Chem. 2013, 61, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, K.; Qu, X.; Jia, J.; Shi, J.; Jin, D.; Wang, B. Construction and characterization of a bacterial artificial chromosome library of peach. Theor. Appl. Genet. 2001, 103, 1174–1179. [Google Scholar] [CrossRef]
- Liu, Q.; Weng, P.; Wu, Z. Quality and aroma characteristics of honey peach wines as influenced by different maturity. Int. J. Food Prop. 2020, 23, 445–458. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Kader, A.A. Antioxidant Capacities, Phenolic Compounds, Carotenoids, and Vitamin C Contents of Nectarine, Peach, and Plum Cultivars from California. J. Agric. Food Chem. 2002, 50, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.; Gonçalves, A.C.; Silva, B.; Silva, L.R. Peach (Prunus Persica): Phytochemicals and Health Benefits. Food Res. Int. 2020, 1–32. [Google Scholar] [CrossRef]
- Wang, L. Current situation and development suggestions of peach industry in China. China Fruits 2021, 10, 1–5. [Google Scholar] [CrossRef]
- The Crops and Livestock Products of Peaches and Nectarines. Available online: https://www.fao.org/faostat/zh/#data/QI (accessed on 29 June 2022).
- Gao, H.; Zhang, Z.K.; Chai, H.K.; Cheng, N.; Yang, Y.; Wang, D.N.; Yang, T.; Cao, W. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biol. Technol. 2016, 118, 103–110. [Google Scholar] [CrossRef]
- Li, A.; Zhao, J.; Xi, J.; Yang, X.; Jin, X.; Chen, Q.; Pan, L. Geographical authentication of peach in China based on stable isotope combined with multielement analysis of peach juice. Food Control 2021, 127, 108126. [Google Scholar] [CrossRef]
- Drogoudi, P.; Gerasopoulos, D.; Kafkaletou, M.; Tsantili, E. Phenotypic characterization of qualitative parameters and antioxidant contents in peach and nectarine fruit and changes after jam preparation. J. Sci. Food Agric. 2017, 97, 3374–3383. [Google Scholar] [CrossRef]
- Kingsly, R.P.; Goyal, R.K.; Manikantan, M.R.; Ilyas, S.M. Effects of pretreatments and drying air temperature on drying behaviour of peach slice. J. Food Sci. Technol. 2007, 42, 65–69. [Google Scholar] [CrossRef]
- Dhiman, A.; Attri, S. Production of Brandy. In Handbook of Enology: Principles, Practices and Recent Innovations, 1st ed.; Asiatech Publisher, Inc.: New Delhi, India, 2011; Volume 3, pp. 3–60. [Google Scholar]
- Regulation (EU) No 787/2019 of the European Parliament and of the Council of 17 April 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02019R0787-20210525 (accessed on 27 June 2022).
- Xiang, X.F.; Lan, Y.B.; Gao, X.T.; Xie, H.; An, Z.Y.; Lv, Z.H.; Yin, S.; Duan, C.Q.; Wu, G.F. Characterization of odor-active compounds in the head, heart, and tail fractions of freshly distilled spirit from Spine grape (Vitis davidii Foex) wine by gas chromatography-olfactometry and gas chromatography-mass spectrometry. Food Res. Int. 2020, 137, 11. [Google Scholar] [CrossRef] [PubMed]
- Tgarguifa, A.; Abderafi, S.; Bounahmidi, T. Energetic optimization of Moroccan distillery using simulation and response surface methodology. Renew. Sustain. Energy Rev. 2017, 75, 415–425. [Google Scholar] [CrossRef]
- Castro-Munoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fila, V. Membrane technologies assisting plant-based and agro-food by-products processing: A comprehensive review. Trends Food Sci. Technol. 2020, 95, 219–232. [Google Scholar] [CrossRef]
- Karp, J.R.; Hamerski, F.; da Silva, V.R.; Medeiros, A.B.P. Membrane processing of the Brazilian spirit Cachaca. J. Inst. Brew. 2019, 125, 383–388. [Google Scholar] [CrossRef]
- Sun, X.F.; Dang, G.F.; Ding, X.B.; Shen, C.X.; Liu, G.P.; Zuo, C.Y.; Chen, X.J.; Xing, W.H.; Jin, W.Q. Production of alcohol-free wine and grape spirit by pervaporation membrane technology. Food Bioprod. Proc. 2020, 123, 262–273. [Google Scholar] [CrossRef]
- Tsakiris, A.; Kallithraka, S.; Kourkoutas, Y. Grape brandy production, composition and sensory evaluation. J. Sci. Food Agric. 2014, 94, 404–414. [Google Scholar] [CrossRef]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s Chemical Signatures in Human Olfaction: A Foodborne Perspective for Future Biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef]
- Grosch, W. Evaluation of the key odorants of foods by dilution experiments, aroma models and omission. Chem. Senses 2001, 26, 533–545. [Google Scholar] [CrossRef]
- Jiang, X.; Li, Y. GC-MS analysis of the characteristic aroma components of peach brandy. Sino-Overseas Grapevine Wine 2014, 5, 32–35. [Google Scholar] [CrossRef]
- Li, H.H.; Qin, D.; Wu, Z.Y.; Sun, B.G.; Sun, X.T.; Huang, M.Q.; Sun, J.Y.; Zheng, F.P. Characterization of key aroma compounds in Chinese Guojing sesame-flavor Baijiu by means of molecular sensory science. Food Chem. 2019, 284, 100–107. [Google Scholar] [CrossRef]
- Fan, W.L.; Qian, M.C. Headspace solid phase microextraction and gas chromatography-olfactometry dilution analysis of young and aged Chinese “Yanghe Daqu” liquors. J. Agric. Food Chem. 2005, 53, 7931–7938. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shi, D.; Sun, J.; Li, A.; Sun, B.; Zhao, M.; Chen, F.; Sun, X.; Li, H.; Huang, M.Q. Characterization of key aroma compounds in Gujinggong Chinese Baijiu by gas chromatography–olfactometry, quantitative measurements, and sensory evaluation. Food Res. Int. 2018, 105, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Al-Dalali, S.; Zheng, F.P.; Sun, B.G.; Zhou, C.X.; Li, M.; Chen, F. Effects of different brewing processes on the volatile flavor profiles of Chinese vinegar determined by HS-SPME-AEDA with GC-MS and GC-O. LWT-Food Sci. Technol. 2020, 133, 109969. [Google Scholar] [CrossRef]
- Wang, J.; Ming, Y.Z.; Li, Y.M.; Huang, M.Q.; Luo, S.Q.; Li, H.F.; Li, H.H.; Wu, J.H.; Sun, X.T.; Luo, X.L. Characterization and comparative study of the key odorants in Caoyuanwang mild-flavor style Baijiu using gas chromatography-olfactometry and sensory approaches. Food Chem. 2021, 347, 129028. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, X.L.; Song, X.B.; Zheng, F.P.; Li, H.H.; Chen, F.; Zhang, Y.H.; Zhang, F.Y. Evolution of the key odorants and aroma profiles in traditional Laowuzeng baijiu during its one-year ageing. Food Chem. 2020, 310, 125898. [Google Scholar] [CrossRef]
- Marcq, P.; Schieberle, P. Characterization of the Key Aroma Compounds in a Commercial Amontillado Sherry Wine by Means of the Sensomics Approach. J. Agric. Food Chem. 2015, 63, 4761–4770. [Google Scholar] [CrossRef]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices, 2nd ed.; Springer Science+Business Media: New York, NY, USA, 2010; pp. 79–99. [Google Scholar]
- Dong, X.; Zhang, J. Analysis of volatile aroma components of different peach original brandy products. Food Ferment. Ind. 2015, 41, 174–178. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Zhang, B.; Shen, C.; Xu, Y.; Tang, K. Identification, quantitation and sensorial contribution of lactones in brandies between China and France. Food Chem. 2021, 357, 129761. [Google Scholar] [CrossRef]
- Langen, J.; Wang, C.Y.; Slabizki, P.; Wall, K.; Schmarr, H.-G. Quantitative analysis of γ- and δ-lactones in wines using gas chromatography with selective tandem mass spectrometric detection. Rapid Commun. Mass Spectrom. 2013, 27, 2751–2759. [Google Scholar] [CrossRef]
- Awad, P.; Athes, V.; Decloux, M.E.; Ferrari, G.; Snakkers, G.; Raguenaud, P.; Giarnpaoli, P. Evolution of Volatile Compounds during the Distillation of Cognac Spirit. J. Agric. Food Chem. 2017, 65, 7736–7748. [Google Scholar] [CrossRef]
- Diéguez, S.C.; de la Peña, M.L.G.; Gómez, E.F. Approaches to Spirit Aroma: Contribution of Some Aromatic Compounds to the Primary Aroma in Samples of Orujo Spirits. J. Agric. Food Chem. 2003, 51, 7385–7390. [Google Scholar] [CrossRef] [PubMed]
- Uselmann, V.; Schieberle, P. Decoding the Combinatorial Aroma Code of a Commercial Cognac by Application of the Sensomics Concept and First Insights into Differences from a German Brandy. J. Agric. Food Chem. 2015, 63, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R. Pervaporation: The emerging technique for extracting aroma compounds from food systems. J. Food Eng. 2019, 253, 27–39. [Google Scholar] [CrossRef]
- Niu, Y.; Zhang, J.; Xiao, Z.; Zhu, J. Evaluation of the Perceptual Interactions Between Higher Alcohols and Off-Odor Acids in Laimao Baijiu by σ–τ Plot and Partition Coefficient. J. Agric. Food Chem. 2020, 68, 14938–14949. [Google Scholar] [CrossRef]
- Rao, Y.S. Recent advances in the chemistry of unsaturated lactones. Chem. Rev. 1976, 76, 625–694. [Google Scholar] [CrossRef]
- Baudot, A.; Marin, M. Pervaporation of Aroma Compounds: Comparison of Membrane Performances with Vapour-Liquid Equilibria and Engineering Aspects of Process Improvement. Food Bioprod. Process. 1997, 75, 117–142. [Google Scholar] [CrossRef]
- Gunata, Y.Z.; Bayonove, C.L.; Baumes, R.L.; Cordonnier, R.E. The aroma of grapes I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J. Chromatogr. A 1985, 331, 83–90. [Google Scholar] [CrossRef]
- Sefton, M.A.; Francis, I.L.; Williams, P.J. Free and Bound Volatile Secondary Metabolites of Vitis Vhifera Grape cv. Sauvignon Blanc. J. Food Sci. 1994, 59, 142–147. [Google Scholar] [CrossRef]
- Lukić, I.; Tomas, S.; Miličević, B.; Radeka, S.; Peršurić, Đ. Behaviour of Volatile Compounds During Traditional Alembic Distillation of Fermented Muscat Blanc and Muškat Ruža Porečki Grape Marcs. J. Inst. Brew. 2011, 117, 440–450. [Google Scholar] [CrossRef]
- Kanasawud, P.; Crouzet, J.C. Mechanism of formation of volatile compounds by thermal degradation of carotenoids in aqueous medium. 1. beta.-Carotene degradation. J. Agric. Food Chem. 1990, 38, 237–243. [Google Scholar] [CrossRef]
- Yoshizaki, Y.; Takamine, K.; Shimada, S.; Uchihori, K.; Okutsu, K.; Tamaki, H.; Ito, K.; Sameshima, Y. The Formation of β-Damascenone in Sweet Potato Shochu. J. Inst. Brew. 2011, 117, 217–223. [Google Scholar] [CrossRef]
- Franitza, L.; Granvogl, M.; Schieberle, P. Influence of the Production Process on the Key Aroma Compounds of Rum: From Molasses to the Spirit. J. Agric. Food Chem. 2016, 64, 9041–9053. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Xu, Y. Determination of odor thresholds of volatile aroma compounds in Baijiu by A forced-choice ascending concentration series method of limits. Liquor Mak. 2011, 38, 80–84. [Google Scholar]
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef]
- Du, J.Y.; Li, Y.; Xu, J.; Huang, M.Q.; Wang, J.; Chao, J.; Wu, J.; Sun, H.; Ding, H.; Ye, H. Characterization of key odorants in Langyatai Baijiu with Jian flavour by sensory-directed analysis. Food Chem. 2021, 352, 129363. [Google Scholar] [CrossRef]
- Van Gemert, L. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media, 2nd ed.; Cardon & Company B.V.: Utrecht, The Netherlands, 2003; pp. 1–25. [Google Scholar]
- Zea, L.; Moyano, L.; Moreno, J.; Cortes, B.; Medina, M. Discrimination of the aroma fraction of Sherry wines obtained by oxidative and biological ageing. Food Chem. 2001, 75, 79–84. [Google Scholar] [CrossRef]
- Li, M.X. Study on the Selection of Yellow Peach Wine Saccharomyces Cerevisiae and Fermentation. Master’s Thesis, Jiangnan University, Jiangnan, China, 2021. [Google Scholar]
- Willner, B.; Granvogl, M.; Schieberle, P. Characterization of the Key Aroma Compounds in Bartlett Pear Brandies by Means of the Sensomics Concept. J. Agric. Food Chem. 2013, 61, 9583–9593. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Ferreira, V.; Ortín, N.; Escudero, A.; López, R.; Cacho, J. Chemical Characterization of the Aroma of Grenache Rosé Wines: Aroma Extract Dilution Analysis, Quantitative Determination, and Sensory Reconstitution Studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef]
- Poisson, L.; Schieberle, P. Characterization of the Key Aroma Compounds in an American Bourbon Whisky by Quantitative Measurements, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2008, 56, 5820–5826. [Google Scholar] [CrossRef]
- Lan, Y.; Guo, J.; Qian, X.; Zhu, B.; Shi, Y.; Wu, G.; Duan, C. Characterization of key odor-active compounds in sweet Petit Manseng (Vitis vinifera L.) wine by gas chromatography–olfactometry, aroma reconstitution, and omission tests. J. Food Sci. 2021, 86, 1258–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiao, Z. Characterization of the key aroma compounds in peach by gas chromatography–olfactometry, quantitative measurements and sensory analysis. Eur. Food Res. Technol. 2019, 245, 129–141. [Google Scholar] [CrossRef]




| NO. | Absence of Compounds | DS | PS | ||
|---|---|---|---|---|---|
| N a | Significance b | n | Significance | ||
| 1 | All esters | 9 | * | 14 | *** |
| 1-1 | Ethyl acetate | 7 | - | 8 | - |
| 1-2 | Ethyl isobutyrate | - | - | 13 | *** |
| 1-3 | Isobutyl acetate | - | - | 10 | * |
| 1-4 | Ethyl isovalerate | - | - | 12 | *** |
| 1-5 | Ethyl butyrate | 6 | - | 8 | - |
| 1-6 | Isoamyl acetate | 11 | ** | 12 | *** |
| 1-7 | Ethyl hexanoate | 15 | *** | 13 | *** |
| 1-8 | Ethyl octanoate | 9 | * | 14 | *** |
| 1-9 | Ethyl decanoate | - | - | 9 | * |
| 2 | 1,1-Diethoxyethane | 13 | *** | 15 | *** |
| 3 | All alcohols | 14 | *** | 13 | *** |
| 3-1 | Isobutanol | - | - | 10 | * |
| 3-2 | 3-Methyl-1-butanol | 10 | * | 10 | * |
| 3-3 | 1-Hexanol | 11 | ** | 12 | *** |
| 4 | All terpenes | 8 | - | 12 | *** |
| 4-1 | Linalool | 8 | - | - | - |
| 4-2 | Citronellol | 9 | * | - | - |
| 4-3 | (E)-β-damascenone | 9 | * | 15 | *** |
| 4-4 | Eugenol | - | - | 13 | *** |
| 5 | All acids | - | - | 14 | *** |
| 5-1 | 2-Methylpropanoic acid | - | - | 13 | *** |
| 5-2 | 3-Methylbutanoic acid | - | - | 14 | *** |
| 6 | Furfural | 6 | - | 14 | ** |
| 7 | γ-Butyrolactone | 13 | *** | 13 | *** |
| 8 | γ-Decalactone | - | - | 12 | *** |
| 9 | Phenethyl acetate | 12 | *** | - | - |
| 10 | Phenethyl alcohol | - | - | 7 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Guo, W.; Sun, B.; Li, H.; Zheng, F.; Li, J.; Meng, N. Characterization of Key Aroma-Active Compounds in Two Types of Peach Spirits Produced by Distillation and Pervaporation by Means of the Sensomics Approach. Foods 2022, 11, 2598. https://doi.org/10.3390/foods11172598
Wang X, Guo W, Sun B, Li H, Zheng F, Li J, Meng N. Characterization of Key Aroma-Active Compounds in Two Types of Peach Spirits Produced by Distillation and Pervaporation by Means of the Sensomics Approach. Foods. 2022; 11(17):2598. https://doi.org/10.3390/foods11172598
Chicago/Turabian StyleWang, Xiaoqin, Wentao Guo, Baoguo Sun, Hehe Li, Fuping Zheng, Jinchen Li, and Nan Meng. 2022. "Characterization of Key Aroma-Active Compounds in Two Types of Peach Spirits Produced by Distillation and Pervaporation by Means of the Sensomics Approach" Foods 11, no. 17: 2598. https://doi.org/10.3390/foods11172598
APA StyleWang, X., Guo, W., Sun, B., Li, H., Zheng, F., Li, J., & Meng, N. (2022). Characterization of Key Aroma-Active Compounds in Two Types of Peach Spirits Produced by Distillation and Pervaporation by Means of the Sensomics Approach. Foods, 11(17), 2598. https://doi.org/10.3390/foods11172598