Cultivation Conditions of Spinach and Rocket Influence Epiphytic Growth of Listeria monocytogenes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Leafy Vegetables (Experimental Farm Produce)
2.2. Preparation of L. monocytogenes for Inoculation of Leafy Vegetables
2.3. Preparation of the Polypropylene Bags
2.4. Preparation and Subsequent Inoculation and Storage of Leafy Vegetables
2.5. Product pH and Water Activity and Subsequent Batch Determination
2.6. Sampling of the Leafy Vegetable Packs and L. monocytogenes Analysis
2.7. Total Bacteria Counts (TBCs)
2.8. Statistical Analysis
3. Results
3.1. Water Activity and pH
3.2. Influence of Leafy Vegetable Products, Variety, Cultivation Method, and Seasonality of Cultivation) on the Growth of L. monocytogenes on Experimental Farm Produce
3.3. L. monocytogenes Growth Curve Parameters from Growth Potential Experiments (Three-Strain L. monocytogenes Mixed Cultures)
3.4. Parameters from Growth Curves of L. monocytogenes on Experimental Farm Produce Inoculated with Single L. monocytogenes Cultures
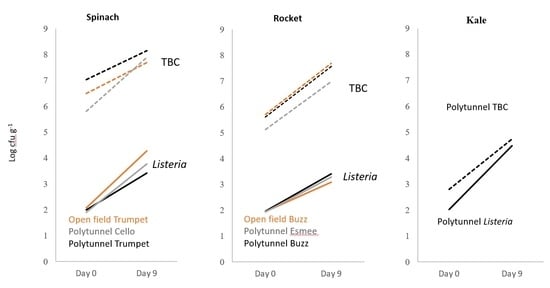
3.5. Comparison of Experimental Farm Leafy Vegetables’ Total Bacteria Counts (TBCs) and Subsequent Influence on Growth of L. monocytogenes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CDC. Listeria Outbreak Linked to Packaged Salads Produced by Dole, 2022. Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/listeria/outbreaks/packaged-salad-mix-12-21/index.html (accessed on 1 August 2022).
- Self, J.L.; Conrad, A.; Stroika, S.; Jackson, A.; Whitlock, L.; Jackson, K.A.; Beal, J.; Wellman, A.; Fatica, M.K.; Bidol, S.; et al. Multistate outbreak of listeriosis associated with packaged leafy green salads, United States and Canada, 2015–2016. Emerg. Infect. Dis. 2019, 25, 1461–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Gooneratne, R.; Hussain, M. Listeria monocytogenes in fresh produce: Outbreaks, prevalence and contamination levels. Foods 2017, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-leaf and multi-leaf of green and red lettuces are suitable raw materials for the fresh-cut industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Prohens, J.; Rodríguez-Burruezo, A.; Fita, A. Potential of wall rocket (Diplotaxis erucoides) as a new crop: Influence of the growing conditions on the visual quality of the final product. Sci. Hortic. 2019, 258, 108778. [Google Scholar] [CrossRef]
- Smith, A.; Moorhouse, E.; Monaghan, J.; Taylor, C.; Singleton, I. Sources and survival of Listeria monocytogenes on fresh, leafy produce. J. Appl. Microbiol. 2018, 125, 930–942. [Google Scholar] [CrossRef] [Green Version]
- Magdovitz, B.F.; Gummalla, S.; Garren, D.; Thippareddi, H.; Berrang, M.E.; Harrison, M.A. Prevalence of Listeria species and Listeria monocytogenes on raw produce arriving at frozen food manufacturing facilities. J. Food Prot. 2021, 84, 1898–1903. [Google Scholar] [CrossRef]
- Townsend, A.; Strawn, L.K.; Chapman, B.J.; Dunn, L.L. A systematic review of Listeria Species and Listeria monocytogenes prevalence, persistence, and diversity throughout the fresh produce supply chain. Foods 2021, 10, 1427. [Google Scholar] [CrossRef]
- Nag, R.; Russell, L.; Nolan, S.; Auer, A.; Markey, B.K.; Whyte, P.; O’Flaherty, V.; Bolton, D.; Fenton, O.; Richards, K.G.; et al. Quantitative microbial risk assessment associated with ready-to-eat salads following the application of farmyard manure and slurry or anaerobic digestate to arable lands. Sci. Total Environ. 2021, 806, 151227. [Google Scholar] [CrossRef]
- Strawn, L.K.; Fortes, E.D.; Bihn, E.A.; Nightingale, K.K.; Gröhn, Y.T.; Worobo, R.W.; Wiedmann, M.; Bergholz, P.W. Landscape and meteorological factors affecting prevalence of three food-borne pathogens in fruit and vegetable farms. Appl. Environ. Microbiol. 2013, 79, 588–600. [Google Scholar] [CrossRef] [Green Version]
- Strawn, L.K.; Gröhn, Y.T.; Warchocki, S.; Worobo, R.W.; Bihn, E.A.; Wiedmann, M. Risk factors associated with Salmonella and Listeria monocytogenes contamination of produce fields. Appl. Environ. Microbiol. 2013, 79, 7618–7627. [Google Scholar] [CrossRef] [Green Version]
- Weller, D.; Wiedmann, M.; Strawn, L.K.; Schaffner, D.W. Spatial and temporal factors associated with an increased prevalence of Listeria monocytogenes in spinach fields in New York State. Appl. Environ. Microbiol. 2015, 81, 6059–6069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denis, N.; Zhang, H.; Leroux, A.; Trudel, R.; Bietlot, H. Prevalence and trends of bacterial contamination in fresh fruits and vegetables sold at retail in Canada. Food Control 2016, 67, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Technical Guidance Document for Conducting Shelf-Life Studies on Listeria Monocytogenes in Ready-to-Eat Foods Version 3-Amended (21 February 2019); EURL Listeria monocytogenes; ANSES: Maisons-Alfort, France, 2019.
- Beuchat, L.R.; Brackett, R.E. Survival and growth of Listeria monocytogenes on lettuce as influenced by shredding, chlorine treatment, modified atmosphere packaging and temperature. J. Food Sci. 1990, 55, 755–758. [Google Scholar] [CrossRef]
- Boyacioglu, O.; Sulakvelidze, A.; Sharma, M.; Goktepe, I. Effect of a bacteriophage cocktail in combination with modified atmosphere packaging in controlling Listeria monocytogenes on fresh-cut spinach. Ir. J. Agr. Food Res. 2016, 55, 74–79. [Google Scholar] [CrossRef] [Green Version]
- McManamon, O.; Scollard, J.; Schmalenberger, A. Inoculation density is affecting growth conditions of Listeria monocytogenes on fresh cut lettuce. World J. Microbiol. Biotechnol. 2017, 33, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sant’Ana, A.S.; Barbosa, M.S.; Destro, M.T.; Landgraf, M.; Franco, B.D.G.M. Growth potential of Salmonella spp. and Listeria monocytogenes in nine types of ready-to-eat vegetables stored at variable temperature conditions during shelf-life. Int. J. Food Microbiol. 2012, 157, 52–58. [Google Scholar] [CrossRef]
- Ziegler, M.; Kent, D.; Stephan, R.; Guldimann, C. Growth potential of Listeria monocytogenes in twelve different types of RTE salads: Impact of food matrix, storage temperature and storage time. Int. J. Food Microbiol. 2019, 296, 83–92. [Google Scholar] [CrossRef]
- Cai, S.; Worobo, R.W.; Snyder, A.B. Combined effect of storage condition, surface integrity, and length of shelf life on the growth of Listeria monocytogenes and spoilage microbiota on refrigerated ready-to-eat products. J. Food Prot. 2019, 82, 1423–1432. [Google Scholar] [CrossRef]
- Culliney, P.; Schmalenberger, A. Growth potential of Listeria monocytogenes on refrigerated spinach and rocket leaves in modified atmosphere packaging. Foods 2020, 9, 1211. [Google Scholar] [CrossRef]
- Lokerse, R.F.A.; Maslowska-Corker, K.A.; van de Wardt, L.C.; Wijtzes, T. Growth capacity of Listeria monocytogenes in ingredients of ready-to-eat salads. Food Control 2016, 60, 338–345. [Google Scholar] [CrossRef]
- Söderqvist, K.; Lambertz, S.T.; Vågsholm, I.; Fernström, L.-L.; Alsanius, B.; Mogren, L.; Boqvist, S. Fate of Listeria monocytogenes, pathogenic Yersinia enterocolitica, and Escherichia coli O157:H7 gfp+ in ready-to-eat salad during cold storage: What is the risk to consumers? J. Food Prot. 2017, 80, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Moreira Calix, J.F. Effect of Storage Temperage on the Survival or Growth of Listeria monocytogenes on Whole and Fresh-Cut Produce; Louisiana State University: Baton Rouge, LA, USA, 2019. [Google Scholar]
- Ross, T.; McMeekin, T.A. Modeling Microbial Growth within Food Safety Risk Assessments. Risk Anal. 2003, 23, 179–197. [Google Scholar] [CrossRef] [PubMed]
- McManamon, O.; Kaupper, T.; Scollard, J.; Schmalenberger, A. Nisin application delays growth of Listeria monocytogenes on fresh-cut iceberg lettuce in modified atmosphere packaging, while the bacterial community structure changes within one week of storage. Postharvest Biol. Technol. 2019, 147, 185–195. [Google Scholar] [CrossRef]
- ISO. ISO 1842:1991; Fruit and Vegetable Products-Determination of pH. International Organization for Standardization: Geneva, Switzerland, 1991.
- ISO. EN ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 1: Detection Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- Baranyi, J.; Tamplin, M.L. ComBase: A common database on microbial responses to food environments. J. Food Prot. 2004, 67, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Omac, B.; Moreira, R.G.; Castell-Perez, E. Quantifying growth of cold-adapted Listeria monocytogenes and Listeria innocua on fresh spinach leaves at refrigeration temperatures. J. Food Eng. 2018, 224, 17–26. [Google Scholar] [CrossRef]
- Pla, M.-L.; Oltra, S.; Esteban, M.-D.; Andreu, S.; Palop, A. Comparison of primary models to predict microbial growth by the plate count and absorbance methods. BioMed Res. Int. 2015, 2015, 365025. [Google Scholar] [CrossRef] [Green Version]
- Sant’Ana, A.S.; Franco, B.D.G.M.; Schaffner, D.W. Modeling the growth rate and lag time of different strains of Salmonella enterica and Listeria monocytogenes in ready-to-eat lettuce. Food Microbiol. 2012, 30, 267–273. [Google Scholar] [CrossRef]
- Schmalenberger, A.; Schwieger, F.; Tebbe, C.C. Effect of primers hybridizing to different evolutionarily conserved regions of the small-subunit rRNA gene in PCR-based microbial community analyses and genetic profiling. Appl. Environ. Microbiol. 2001, 67, 3557–3563. [Google Scholar] [CrossRef] [Green Version]
- Doumith, M.; Buchrieser, C.; Glaser, P.; Jacquet, C.; Martin, P. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J. Clin. Microbiol. 2004, 42, 3819–3822. [Google Scholar] [CrossRef] [Green Version]
- McManamon, O. Control and Monitoring of Listeria monocytogenes with the Use of Natural Antimicrobial Techniques Including the Bacteriocin Nisin. Master’s Thesis, University of Limerick, Limerick, Ireland, 2016. [Google Scholar]
- Johnston, L.M.; Jaykus, L.-A.; Moll, D.; Martinez, M.C.; Anciso, J.; Mora, B.; Moe, C.L. A Field Study of the Microbiological Quality of Fresh Produce. J. Food Prot. 2005, 68, 1840–1847. [Google Scholar] [CrossRef]
- Mansur, A.R.; Oh, D.-H. Combined effects of thermosonication and slightly acidic electrolyzed water on the microbial quality and shelf life extension of fresh-cut kale during refrigeration storage. Food Microbiol. 2015, 51, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Marik, C.M.; Zuchel, J.; Schaffner, D.W.; Strawn, L.K. Growth and survival of Listeria monocytogenes on intact fruit and vegetable surfaces during postharvest handling: A systematic literature review. J. Food Prot. 2019, 83, 108–128. [Google Scholar] [CrossRef] [PubMed]
- Darlison, J.; Mieli, M.; Bengtsson, T.; Hartmann, R.; Mogren, L.; Vågsholm, I.; Karlsson, M.; Alsanius, B.W. Plant species affects establishment of Escherichia coli O157:H7 gfp+ on leafy vegetables. J. Appl. Microbiol. 2019, 127, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Macarisin, D.; Patel, J.; Bauchan, G.; Giron, J.A.; Ravishankar, S. Effect of spinach cultivar and bacterial adherence factors on survival of Escherichia coli O157:H7 on spinach leaves. J. Food Prot. 2013, 76, 1829–1837. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Velasco, G.; Welbaum, G.E.; Falkinham Iii, J.O.; Ponder, M.A. Phyllopshere bacterial community structure of spinach (Spinacia oleracea) as affected by cultivar and environmental conditions at time of harvest. Diversity 2011, 3, 721–738. [Google Scholar] [CrossRef]
- Kang, J.-H.; Song, K.B. Antibacterial activity of the noni fruit extract against Listeria monocytogenes and its applicability as a natural sanitizer for the washing of fresh-cut produce. Food Microbiol. 2019, 84, 103260. [Google Scholar] [CrossRef]
- Bonsaglia, E.C.R.; Silva, N.C.C.; Fernades Júnior, A.; Araújo Júnior, J.P.; Tsunemi, M.H.; Rall, V.L.M. Production of biofilm by Listeria monocytogenes in different materials and temperatures. Food Control 2014, 35, 386–391. [Google Scholar] [CrossRef]
- Gorski, L.; Palumbo, J.D.; Mandrell, R.E. Attachment of Listeria monocytogenes to Radish Tissue Is Dependent upon Temperature and Flagellar Motility. Appl. Environ. Microbiol. 2003, 69, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Warning, A.; Datta, A.K. Interdisciplinary engineering approaches to study how pathogenic bacteria interact with fresh produce. J. Food Eng. 2013, 114, 426–448. [Google Scholar] [CrossRef]
- Madloo, P.; Lema, M.; Francisco, M.; Soengas, P. Role of major glucosinolates in the defense of kale against Sclerotinia sclerotiorum and Xanthomonas campestris pv. campestris. Phytopathology 2019, 109, 1246–1256. [Google Scholar] [CrossRef]
- Aires, A.; Marques, E.; Carvalho, R.; Rosa, E.; Saavedra, M. Evaluation of biological value and appraisal of polyphenols and glucosinolates from organic baby-leaf salads as antioxidants and antimicrobials against important human pathogenic bacteria. Molecules 2013, 18, 4651–4668. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Holley, R.A. Effects of changes in pH and temperature on the inhibition of Salmonella and Listeria monocytogenes by Allyl isothiocyanate. Food Control 2013, 34, 414–419. [Google Scholar] [CrossRef]
- Gutiérrez-Rodríguez, E.; Gundersen, A.; Sbodio, A.O.; Suslow, T.V. Variable agronomic practices, cultivar, strain source and initial contamination dose differentially affect survival of Escherichia coli on spinach. J. Appl. Microbiol. 2012, 112, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Koseki, S.; Isobe, S. Growth of Listeria monocytogenes on iceberg lettuce and solid media. Int. J. Food Microbiol. 2005, 101, 217–225. [Google Scholar] [CrossRef]
- Vacher, C.; Hampe, A.; Porté, A.J.; Sauer, U.; Compant, S.; Morris, C.E. The phyllosphere: Microbial jungle at the plant-climate interface. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Comte, I.; Colin, F.; Whalen, J.K.; Grünberger, O.; Caliman, J.-P. Agricultural Practices in Oil Palm Plantations and Their Impact on Hydrological Changes, Nutrient Fluxes and Water Quality in Indonesia. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 116, pp. 71–124. [Google Scholar]
- Tukey, H.B. The leaching of substances from plants. Annu. Rev. Plant Physiol. 1970, 21, 305–324. [Google Scholar] [CrossRef]
- Kyere, E.O.; Palmer, J.; Wargent, J.J.; Fletcher, G.C.; Flint, S. Colonisation of lettuce by Listeria Monocytogenes. Int. J. Food Sci. Technol. 2019, 54, 14–24. [Google Scholar] [CrossRef] [Green Version]
- McCance, R.A.; Widdowson, E.M. McCance and Widdowson’s the Composition of Foods; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Maylani, E.D.; Yuniati, R.; Wardhana, W. The Effect of leaf surface character on the ability of water hyacinth, Eichhornia crassipes (Mart.) Solms. to transpire water. IOP Conf. Ser. Mater. Sci. Eng. 2020, 902, 33–52. [Google Scholar] [CrossRef]
- Acharjee, S.; Ibekwe, A.M.; Ors, S.; Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Influence of seasonal changes and salinity on spinach phyllosphere bacterial functional assemblage. PLoS ONE 2021, 16, e0252242. [Google Scholar] [CrossRef]
- Truchado, P.; Gil, M.I.; Reboleiro, P.; Rodelas, B.; Allende, A. Impact of solar radiation exposure on phyllosphere bacterial community of red-pigmented baby leaf lettuce. Food Microbiol. 2017, 66, 77–85. [Google Scholar] [CrossRef]
- Truchado, P.; Gil, M.I.; Moreno-Candel, M.; Allende, A. Impact of weather conditions, leaf age and irrigation water disinfection on the major epiphytic bacterial genera of baby spinach grown in an open field. Food Microbiol. 2019, 78, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Viñas, I.; Anguera, M.; Abadias, M. Fate of Listeria monocytogenes and Escherichia coli O157:H7 in the presence of natural background microbiota on conventional and organic lettuce. Food Control 2012, 25, 678–683. [Google Scholar] [CrossRef]
- Gong, T.; Xin, X.F. Phyllosphere microbiota: Community dynamics and its interaction with plant hosts. J. Integr. Plant Biol. 2021, 63, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.A.; O’Beirne, D. Effects of the indigenous microflora of minimally processed lettuce on the survival and growth of Listeria innocua. Int. J. Food Sci. Technol. 2002, 33, 477–488. [Google Scholar] [CrossRef]
- Kruger, M.F.; Barbosa, M.d.S.; Miranda, A.; Landgraf, M.; Destro, M.T.; Todorov, S.D.; Gombossy de Melo Franco, B.D. Isolation of bacteriocinogenic strain of Lactococcus lactis subsp. lactis from rocket salad (Eruca sativa Mill.) and evidences of production of a variant of nisin with modification in the leader-peptide. Food Control 2013, 33, 467–476. [Google Scholar] [CrossRef]
- Barbosa, J.; Albano, H.; Silva, B.; Almeida, M.H.; Nogueira, T.; Teixeira, P. Characterization of a Lactiplantibacillus plantarum R23 isolated from arugula by whole-genome sequencing and its bacteriocin production ability. Int. J. Environ. Res. Public Health 2021, 18, 5515. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Pediocin Applications in Antimicrobial Food Packaging Systems. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 36, pp. 445–454. [Google Scholar] [CrossRef]
- Le Marrec, C.; Hyronimus, B.; Bressollier, P.; Verneuil, B.; Urdaci, M.C. Biochemical and genetic characterization of coagulin, a new antilisterial bacteriocin in the pediocin family of bacteriocins, produced by Bacillus coagulans I4. Appl. Environ. Microbiol. 2000, 66, 5213–5220. [Google Scholar] [CrossRef] [Green Version]
- Caponigro, V.; Ventura, M.; Chiancone, I.; Amato, L.; Parente, E.; Piro, F. Variation of microbial load and visual quality of ready-to-eat salads by vegetable type, season, processor and retailer. Food Microbiol. 2010, 27, 1071–1077. [Google Scholar] [CrossRef]
- Wilson, A.R.; Sigee, D.; Epton, H.A.S. Anti-bacterial activity of Lactobacillus plantarum strain SK1 against Listeria monocytogenes is due to lactic acid production. J. Appl. Microbiol. 2005, 99, 1516–1522. [Google Scholar] [CrossRef]
- Di Cagno, R.; Coda, R.; De Angelis, M.; Gobbetti, M. Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiol. 2013, 33, 1–10. [Google Scholar] [CrossRef]





| Product | Strain | Model | R2 | RMSE | Maximum Growth Rate (μmax) Ln cfu g−1 h−1 |
|---|---|---|---|---|---|
| Spinach | 959 | Linear | 0.836 | 0.193 | 0.0127 |
| Spinach | 959 | Baranyi and Roberts (no lag) | 0.942 | 0.115 | 0.0211 |
| Spinach | 959 | Biphasic model (no lag) | 0.950 | 0.106 | 0.0197 |
| Spinach | 1382 | Linear | 0.916 | 0.142 | 0.0136 |
| Spinach | 1382 | Baranyi and Roberts (no lag) | 0.934 | 0.125 | 0.0158 |
| Spinach | 1382 | Biphasic (no lag) | 0.946 | 0.114 | 0.0157 |
| Rocket | 959 | Linear | 0.972 | 0.0935 | 0.0160 |
| Rocket | 1382 | Linear | 0.950 | 0.117 | 0.0147 |
| Rocket | 1382 | Baranyi and Roberts (no lag) | 0.949 | 0.118 | 0.0162 |
| Rocket | 1382 | Biphasic (no lag) | 0.943 | 0.124 | 0.0148 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Culliney, P.; Schmalenberger, A. Cultivation Conditions of Spinach and Rocket Influence Epiphytic Growth of Listeria monocytogenes. Foods 2022, 11, 3056. https://doi.org/10.3390/foods11193056
Culliney P, Schmalenberger A. Cultivation Conditions of Spinach and Rocket Influence Epiphytic Growth of Listeria monocytogenes. Foods. 2022; 11(19):3056. https://doi.org/10.3390/foods11193056
Chicago/Turabian StyleCulliney, Paul, and Achim Schmalenberger. 2022. "Cultivation Conditions of Spinach and Rocket Influence Epiphytic Growth of Listeria monocytogenes" Foods 11, no. 19: 3056. https://doi.org/10.3390/foods11193056
APA StyleCulliney, P., & Schmalenberger, A. (2022). Cultivation Conditions of Spinach and Rocket Influence Epiphytic Growth of Listeria monocytogenes. Foods, 11(19), 3056. https://doi.org/10.3390/foods11193056