Dextran Conjugation Improves the Structural and Functional Properties of Heat-Treated Protein Isolate from Cinnamomum camphora Seed Kernel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
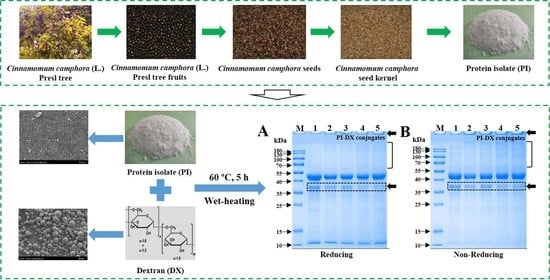
2.2. Preparation of Protein Isolate (PI) from DCCSK
2.2.1. Extraction of Polyphenol
2.2.2. Extraction of PI
2.3. Preparation of PI-DX Conjugates
2.4. Measurement of UV Absorbance
2.5. Determination of Degree of Grafting (DG)
2.6. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.7. Determination of Z-Average Size and Zeta Potential
2.8. Structural Characterization of PI-DX Conjugates
2.8.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.8.2. Intrinsic Fluorescence Spectroscopy
2.8.3. Surface Hydrophobicity (H0)
2.8.4. Scanning Electron Microscopy (SEM)
2.9. Functional Properties of PI-DX Conjugates
2.9.1. Protein Solubility and Emulsifying Properties
2.9.2. Differential Scanning Calorimetry (DSC)
2.9.3. Antioxidant Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Formation of PI-DX Conjugates
3.2. SDS-PAGE
3.3. Z-Average Size and Zeta Potential
3.4. Structural Properties of PI and PI-DX Conjugates
3.4.1. FTIR Spectroscopy
3.4.2. Intrinsic Fluorescence Spectroscopy
3.4.3. Surface Hydrophobicity (H0)
3.4.4. Scanning Electron Microscopy (SEM)
3.5. Functional Properties of PI and PI-DX Conjugates
3.5.1. Solubility
3.5.2. Emulsifying Properties
3.5.3. DSC
3.5.4. Antioxidant Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, X.; Liang, S.; Peng, T.; Zhang, G.; Zeng, Z.; Yu, P.; Gong, D.; Deng, S. Influence of phenolic compounds on physicochemical and functional properties of protein isolate from Cinnamomum camphora seed kernel. Food Hydrocoll. 2020, 102, 105612. [Google Scholar] [CrossRef]
- Bao, X.; Yan, X.; Zhang, G.; Zhao, J.; Zeng, Z.; Yu, P.; Gong, D. Improving effect of phytase treatment on the functional properties and in vitro digestibility of protein isolate from Cinnamomum camphora seed kernel. LWT 2022, 155, 112948. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Food protein amyloid fibrils: Origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019, 269, 334–356. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.N.; Nickerson, M.T. Review on plant protein–polysaccharide complex coacervation, and the functionality and applicability of formed complexes. J. Sci. Food Agric. 2018, 98, 5559–5571. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, J.; Zeng, Z.; Ma, M.; Xia, J.; Tian, W.; Zhang, G.; Gong, X.; Gong, D.; Yu, P. Effects of preheat treatment and polyphenol grafting on the structural, emulsifying and rheological properties of protein isolate from Cinnamomum camphora seed kernel. Food Chem. 2022, 377, 132044. [Google Scholar] [CrossRef]
- Nikbakht Nasrabadi, M.; Sedaghat Doost, A.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Lu, Z.; Wu, F.; Fu, G.; Zheng, B.; Tian, Y. Structural characteristics and emulsifying properties of lotus seed protein isolate-dextran glycoconjugates induced by a dynamic high pressure microfluidization Maillard reaction. LWT 2022, 160, 113309. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, N.; Wu, Y.; Zhao, L.; Ma, G.; Pei, F.; Hu, Q. Characterization and functional evaluation of oat protein isolate-Pleurotus ostreatus β-glucan conjugates formed via Maillard reaction. Food Hydrocoll. 2019, 87, 459–469. [Google Scholar] [CrossRef]
- Saatchi, A.; Kiani, H.; Labbafi, M. A new functional protein-polysaccharide conjugate based on protein concentrate from sesame processing by-products: Functional and physico-chemical properties. Int. J. Biol. Macromol. 2019, 122, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Cui, Q.; Wang, G.; Liu, J.; Chen, S.; Wang, X.; Wang, X.; Jiang, L. Relationship between surface functional properties and flexibility of soy protein isolate-glucose conjugates. Food Hydrocoll. 2019, 95, 349–357. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. Pea protein isolate-gum Arabic Maillard conjugates improves physical and oxidative stability of oil-in-water emulsions. Food Chem. 2019, 285, 130–138. [Google Scholar] [CrossRef]
- Zha, F.; Dong, S.; Rao, J.; Chen, B. The structural modification of pea protein concentrate with gum Arabic by controlled Maillard reaction enhances its functional properties and flavor attributes. Food Hydrocoll. 2019, 92, 30–40. [Google Scholar] [CrossRef]
- Naik, R.R.; Wang, Y.; Selomulya, C. Improvements of plant protein functionalities by Maillard conjugation and Maillard reaction products. Crit. Rev. Food Sci. Nutr. 2021, 62, 7036–7061. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Formation of whey protein isolate (WPI)-dextran conjugates in aqueous solutions. J. Agric. Food Chem. 2008, 56, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qi, J.-R.; Li, K.-K.; Yin, S.-W.; Wang, J.-M.; Zhu, J.-H.; Yang, X.-Q. Characterization of soy β-conglycinin–dextran conjugate prepared by Maillard reaction in crowded liquid system. Food Res. Int. 2012, 49, 648–654. [Google Scholar] [CrossRef]
- Guimarães Drummond e Silva, F.; Miralles, B.; Hernández-Ledesma, B.; Amigo, L.; Iglesias, A.H.; Reyes Reyes, F.G.; Netto, F.M. Influence of protein–phenolic complex on the antioxidant capacity of flaxseed (Linum usitatissimum L.) products. J. Agric. Food Chem. 2017, 65, 800–809. [Google Scholar] [CrossRef] [Green Version]
- Pirestani, S.; Nasirpour, A.; Keramat, J.; Desobry, S. Preparation of chemically modified canola protein isolate with gum Arabic by means of Maillard reaction under wet-heating conditions. Carbohydr. Polym. 2017, 155, 201–207. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, M.; Zhang, G.; Hu, X.; Pan, J. Novel insights into the interaction mechanism of 5-hydroxymethyl-2-furaldehyde with β-casein and its effects on the structure and function of β-casein. LWT 2021, 152, 112360. [Google Scholar] [CrossRef]
- Wang, P.; Wang, W.; Chen, C.; Fu, X.; Liu, R. Effect of Fructus Mori. bioactive polysaccharide conjugation on improving functional and antioxidant activity of whey protein. Int. J. Biol. Macromol. 2020, 148, 761–767. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Qin, W.; Gu, J.; Zhang, H.; Duan, Y.; Ma, H. Structure and functional properties of soy protein isolate-lentinan conjugates obtained in Maillard reaction by slit divergent ultrasonic assisted wet heating and the stability of oil-in-water emulsions. Food Chem. 2020, 331, 127374. [Google Scholar] [CrossRef]
- Yan, X.; Gao, Y.; Liu, S.; Zhang, G.; Zhao, J.; Cheng, D.; Zeng, Z.; Gong, X.; Yu, P.; Gong, D. Covalent modification by phenolic extract improves the structural properties and antioxidant activities of the protein isolate from Cinnamomum camphora seed kernel. Food Chem. 2021, 352, 129377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chang, Y.; Luo, B.; Teng, H.; Chen, L. Molecular structure modification of ovalbumin through controlled glycosylation with dextran for its emulsibility improvement. Int. J. Biol. Macromol. 2022, 194, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Xia, J.; Gong, Y.; Deng, H.; Wu, Z.; Li, X.; Tong, P.; Chen, H. Changes in the structure, digestibility and immunoreactivities of glycinin induced by the cross-linking of microbial transglutaminase following heat denaturation. Int. J. Food Sci. 2017, 52, 2265–2273. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, D.; Xu, P.; Geng, Z.; Xiong, G.; Zou, Y.; Wang, D.; Xu, W. Structural and antimicrobial properties of Maillard reaction products in chicken liver protein hydrolysate after sonication. Food Chem. 2021, 343, 128417. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Wei, Z.; Zhang, H.; Dong, M.; Huang, M.; Han, M.; Xu, X.; Zhou, G. Modification of myofibrillar protein via glycation: Physicochemical characterization, rheological behavior and solubility property. Food Hydrocoll. 2020, 105, 105852. [Google Scholar] [CrossRef]
- Shi, Y.; Liang, R.; Chen, L.; Liu, H.; Goff, H.D.; Ma, J.; Zhong, F. The antioxidant mechanism of Maillard reaction products in oil-in-water emulsion system. Food Hydrocoll. 2019, 87, 582–592. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, Y.; McClements, D.J.; Liu, X.; Wang, P.; Liu, F. Improving pea protein functionality by combining high-pressure homogenization with an ultrasound-assisted Maillard reaction. Food Hydrocoll. 2022, 126, 107441. [Google Scholar] [CrossRef]
- Xie, H.; Huang, J.; Woo, M.W.; Hu, J.; Xiong, H.; Zhao, Q. Effect of cold and hot enzyme deactivation on the structural and functional properties of rice dreg protein hydrolysates. Food Chem. 2021, 345, 128784. [Google Scholar] [CrossRef]
- Lesmes, U.; McClements, D.J. Controlling lipid digestibility: Response of lipid droplets coated by β-lactoglobulin-dextran Maillard conjugates to simulated gastrointestinal conditions. Food Hydrocoll. 2012, 26, 221–230. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Wang, W.-q.; Bao, Y.-h.; Chen, Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013, 139, 355–361. [Google Scholar] [CrossRef]
- Li, C.; Zhu, W.; Xue, H.; Chen, Z.; Chen, Y.; Wang, X. Physical and structural properties of peanut protein isolate-gum Arabic films prepared by various glycation time. Food Hydrocoll. 2015, 43, 322–328. [Google Scholar] [CrossRef]
- Li, W.; Zhao, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Modification of soy protein hydrolysates by Maillard reaction: Effects of carbohydrate chain length on structural and interfacial properties. Colloids Surf. B Biointerfaces 2016, 138, 70–77. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, X.; Chen, W.; Wang, Z.; He, R.; Ma, H. Effects of ultrasonic and graft treatments on grafting degree, structure, functionality, and digestibility of rapeseed protein isolate-dextran conjugates. Ultrason. Sonochem. 2018, 42, 250–259. [Google Scholar] [CrossRef]
- Yu, J.-j.; Ji, H.; Chen, Y.; Zhang, Y.-f.; Zheng, X.-c.; Li, S.-h.; Chen, Y. Analysis of the glycosylation products of peanut protein and lactose by cold plasma treatment: Solubility and structural characteristics. Food Chem. 2020, 158, 1194–1203. [Google Scholar] [CrossRef]
- Ma, W.; Wang, J.; Wu, D.; Xu, X.; Du, M.; Wu, C. Effects of preheat treatment on the physicochemical and interfacial properties of cod proteins and its relation to the stability of subsequent emulsions. Food Hydrocoll. 2021, 112, 106338. [Google Scholar] [CrossRef]
- Lertittikul, W.; Benjakul, S.; Tanaka, M. Characteristics and antioxidative activity of Maillard reaction products from a porcine plasma protein–glucose model system as influenced by pH. Food Chem. 2007, 100, 669–677. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Salt, L.J.; Ridout, M.J.; Ding, Y.; Wilde, P.J. Fish oil emulsions stabilized with caseinate glycated by dextran: Physicochemical stability and gastrointestinal fate. J. Agric. Food Chem. 2019, 67, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Comparative studies on the physicochemical properties of peanut protein isolate–polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res. Int. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- He, M.; Li, L.; Wu, C.; Zheng, L.; Jiang, L.; Huang, Y.; Teng, F.; Li, Y. Effects of glycation and acylation on the structural characteristics and physicochemical properties of soy protein isolate. J. Food Sci. 2021, 86, 1737–1750. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, C.; Zhang, T.; Ju, X.; He, R. Effects of acylation and glycation treatments on physicochemical and gelation properties of rapeseed protein isolate. RSC Adv. 2018, 8, 40395–40406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaushik, P.; Dowling, K.; McKnight, S.; Barrow, C.J.; Wang, B.; Adhikari, B. Preparation, characterization and functional properties of flax seed protein isolate. Food Chem. 2016, 197, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castaño, L.; Villamiel, M.; López-Fandiño, R. Glycosylation of individual whey proteins by Maillard reaction using dextran of different molecular mass. Food Hydrocoll. 2007, 21, 433–443. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, A.; Li, R.; Wang, X.; Sun, L.; Jiang, L. Ultrasonic treatment affects emulsifying properties and molecular flexibility of soybean protein isolate-glucose conjugates. Food Biosci. 2020, 38, 100747. [Google Scholar] [CrossRef]
- McClements, D.J.; Jafari, S.M. Improving emulsion formation, stability and performance using mixed emulsifiers: A review. Adv. Colloid Interface Sci. 2018, 251, 55–79. [Google Scholar] [CrossRef]
- Shanmugam, A.; Ashokkumar, M. Ultrasonic preparation of stable flax seed oil emulsions in dairy systems—Physicochemical characterization. Food Hydrocoll. 2014, 39, 151–162. [Google Scholar] [CrossRef]
- Nazari, B.; Mohammadifar, M.A.; Shojaee-Aliabadi, S.; Feizollahi, E.; Mirmoghtadaie, L. Effect of ultrasound treatments on functional properties and structure of millet protein concentrate. Ultrason. Sonochem. 2018, 41, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Robitaille, G.; Ayers, C. Effects of κ-casein glycosylation on heat stability of milk. Food Res. Int. 1995, 28, 17–21. [Google Scholar] [CrossRef]
- Tang, X.; Wu, Q.; Le, G.; Shi, Y. Effects of heat treatment on structural modification and in vivo antioxidant capacity of soy protein. Nutrition 2012, 28, 1180–1185. [Google Scholar] [CrossRef]
- Dong, S.; Panya, A.; Zeng, M.; Chen, B.; McClements, D.J.; Decker, E.A. Characteristics and antioxidant activity of hydrolyzed β-lactoglobulin–glucose Maillard reaction products. Food Res. Int. 2012, 46, 55–61. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, L.; Lan, Q.; Li, M.; Wu, D.; Chen, H.; Liu, Y.; Lin, D.; Qin, W.; Zhang, Z.; et al. Protein glycosylation: A promising way to modify the functional properties and extend the application in food system. Crit. Rev. Food Sci. Nutr. 2019, 59, 2506–2533. [Google Scholar] [CrossRef] [PubMed]




| Samples | Z-Average Size (nm) | Zeta Potential (mV) |
|---|---|---|
| PI | 66.33 ± 1.07 d | −21.40 ± 0.70 a |
| Heated PI | 142.90 ± 1.65 b | −23.13 ± 0.61 b |
| PI-DX conjugate (2:1) | 152.90 ± 2.61 a | −23.47 ± 0.42 bc |
| PI-DX conjugate (1:1) | 145.53 ± 0.55 b | −24.63 ± 0.46 c |
| PI-DX conjugate (1:2) | 124.07 ± 1.50 c | −20.63 ± 0.40 a |
| Samples | α-Helix | β-Sheet | β-Turn | Random Coil |
|---|---|---|---|---|
| PI | 26.51 | 34.89 | 17.93 | 20.66 |
| Heated PI | 24.14 | 30.97 | 21.96 | 22.93 |
| PI-DX conjugate (2:1) | 24.41 | 31.59 | 21.80 | 22.20 |
| PI-DX conjugate (1:1) | 27.72 | 32.65 | 18.89 | 20.74 |
| PI-DX conjugate (1:2) | 31.36 | 34.94 | 17.08 | 16.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Gong, X.; Zeng, Z.; Ma, M.; Zhao, J.; Xia, J.; Li, M.; Yang, Y.; Yu, P.; Gong, D.; et al. Dextran Conjugation Improves the Structural and Functional Properties of Heat-Treated Protein Isolate from Cinnamomum camphora Seed Kernel. Foods 2022, 11, 3066. https://doi.org/10.3390/foods11193066
Yan X, Gong X, Zeng Z, Ma M, Zhao J, Xia J, Li M, Yang Y, Yu P, Gong D, et al. Dextran Conjugation Improves the Structural and Functional Properties of Heat-Treated Protein Isolate from Cinnamomum camphora Seed Kernel. Foods. 2022; 11(19):3066. https://doi.org/10.3390/foods11193066
Chicago/Turabian StyleYan, Xianghui, Xiaofeng Gong, Zheling Zeng, Maomao Ma, Junxin Zhao, Jiaheng Xia, Meina Li, Yujing Yang, Ping Yu, Deming Gong, and et al. 2022. "Dextran Conjugation Improves the Structural and Functional Properties of Heat-Treated Protein Isolate from Cinnamomum camphora Seed Kernel" Foods 11, no. 19: 3066. https://doi.org/10.3390/foods11193066
APA StyleYan, X., Gong, X., Zeng, Z., Ma, M., Zhao, J., Xia, J., Li, M., Yang, Y., Yu, P., Gong, D., & Wan, D. (2022). Dextran Conjugation Improves the Structural and Functional Properties of Heat-Treated Protein Isolate from Cinnamomum camphora Seed Kernel. Foods, 11(19), 3066. https://doi.org/10.3390/foods11193066