Investigation of Chemical Compounds and Evaluation of Toxicity, Antibacterial, and Anti-Inflammatory Activities of Three Selected Essential Oils and Their Mixtures with Moroccan Thyme Honey
Abstract
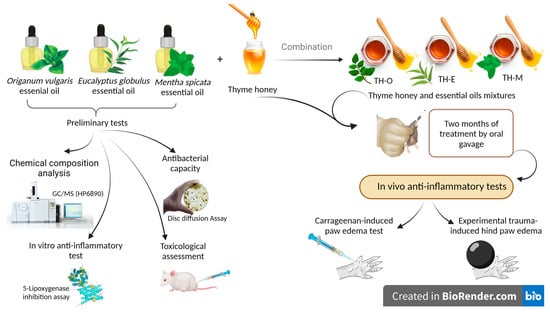
:1. Introduction
2. Materials and Methods
2.1. Honey Sample Collection
2.2. Essential Oils Selection and Their Chemical Composition
2.3. Antibacterial Activity
2.3.1. Preparation of Bacterial Strains
2.3.2. Disc Diffusion Assay
2.3.3. Determination of MIC and MBC
2.4. Acute and Subacute Toxicity Studies of the Essential Oils
2.4.1. Animals
2.4.2. Acute Toxicity
2.4.3. Subacute Oral Toxicity
2.5. Anti-Inflammatory Activity
2.5.1. Animals and Groups
- Groups I was treated daily for two months with the mixture of thyme honey (TH) and essential oil of O. vulgare;
- Group II received, for two months, a daily dose of the mixture of TH and essential oil of M. spicata;
- Group III received, for two months, a daily dose of the mixture of TH and essential oil of E. globulus;
- Group IV was fed daily for two months with TH;
- Group V served as positive control and received indomethacin (10 mg/Kg) one hour before the induction of edema;
- Group VI served as the negative control.
- Groups I was treated daily for two months with the mixture of TH and essential oil of O. vulgare;
- Group II received, for two months, a daily dose of the mixture of TH and essential oil of M. spicata;
- Group III received, for two months, a daily dose of the mixture of TH and essential oil of E. globulus;
- Group IV was fed daily for two months with TH;
- Group V served as positive control and received indomethacin (20 mg/Kg) one hour before the induction of edema;
- Group VI served as the negative control.
2.5.2. Pretreatment Protocol and Dosing Regimen for Thyme Honey and Mixtures between the Honey and Essential Oils of M. spicata, E. globulus, and O. vulgare.
2.5.3. In Vivo Anti-Inflammatory Test Models
2.5.4. In Vitro Anti-Inflammatory Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties of Thymus Vulgaris Honey
3.2. Chemical Composition Property of the Studied Essential Oils
3.3. Antimicrobial Potential of the Studied EOs
3.4. Acute and Subacute Toxicity Studies of the Essential Oils
3.4.1. Acute Toxicity
3.4.2. Subacute Toxicity
3.5. Anti-Inflammatory Activity
3.5.1. In Vivo Anti-Inflammatory Tests
3.5.2. In Vitro Anti-Inflammatory Test (5-Lipoxygenase (5-LOX) Inhibition Ass)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ingawale, D.; Mandlik, S.; Patel, S. An Emphasis on Molecular Mechanisms of Anti-Inflammatory Effects and Glucocorticoid Resistance. J. Complement. Integr Med. 2015, 12, 1–13. [Google Scholar] [CrossRef]
- Roediger, B.; Weninger, W. Resolving a Chronic Inflammation Mystery. Nat. Med. 2017, 23, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A. Honey and Its Nutritional and Anti-Inflammatory Value. BMC Complement. Med. 2021, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Abdallah, E.M.; Elsharkawy, E.R. Physico-Chemical Properties, Antioxidant, and Antimicrobial Activity of Five Varieties of Honey from Saudi Arabia. Asia Pac. J. Mol. Biol. Biotechnol. 2021, 29, 27–34. [Google Scholar] [CrossRef]
- Glevitzky, M.; Mirela, D.; Behl, T.; Laura, M.; Cristina, R.; Carmen, D.; Ursu, F.; Popa, M. The Antimicrobial Activity of Honey and Propolis Extracts from the Central Region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Angioi, R.; Morrin, A. The Rediscovery of Honey for Skin Repair: Recent Advances in Mechanisms for Honey-Mediated Wound Healing and Scaffolded Application Techniques. Appl. Sci. 2021, 11, 5192. [Google Scholar] [CrossRef]
- Astrada, A.; Nakagami, G.; Jais, S.; Sanada, H. Successful Treatment of a Diabetic Foot Ulcer with Exposed Bone Using Trigona Honey: A Case Study. J. Wound Care 2019, 28, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska-Matysek, M.; Stryjecka, M.; Teter, A.; Skałecki, P.; Domaradzki, P.; Florek, M. Relationships between the Content of Phenolic Compounds and the Antioxidant Activity of Polish Honey Varieties as a Tool for Botanical Discrimination. Molecules 2021, 26, 1810. [Google Scholar] [CrossRef]
- Arquam, M. Pure Honey and Black Seed (Nigella Sativa) Is the Treatment and Prevention of Pandemic Disease (Corona Virus). Ann. Rom. Soc. Cell Biol. 2021, 25, 3359–3363. [Google Scholar]
- Hadagali, M.D.; Chua, L.S. The Anti-Inflammatory and Wound Healing Properties of Honey. Eur. Food Res. Technol. 2014, 239, 1003–1014. [Google Scholar] [CrossRef]
- Al-Waili, N.; Salom, K.; Butler, G.; Ghamdi, A. Al Honey and Microbial Infections: A Review Supporting the Use of Honey for Microbial Control. J. Med. Food 2011, 14, 1079–1096. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Zaker, S. Effects of Topical Application of Honey on Cutaneous Wound Healing in Rabbits. Zent. Vet. A 1998, 45, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi, R.; Kazerouni, A.K.O. Evidence for Clinical Use of Honey in Wound Healing as an Anti-Bacterial, Anti-Inflammatory Anti-Oxidant and Anti-Viral Agent: A Review. Jundishapur J. Nat. Pharm Prod. 2013, 8, 100–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Waili, N.; Salom, K.; Al-Ghamdi, A. Honey for Wound Healing, Ulcers, and Burns; Data Supporting Its Use in Clinical Practice. Sci World J. 2011, 11, 766–787. [Google Scholar] [CrossRef]
- Simon, A.; Traynor, K.; Santos, K.; Blaser, G.; Bode, U.M.P. Medical Honey for Wound Care-Still the “Latest Resort”? Evid Based Complement. Altern. Med. 2009, 6, 165–173. [Google Scholar] [CrossRef] [PubMed]
- El-haskoury, R.; Al-Waili, N.; Kamoun, Z.; Makni, M.; Al-Waili, H.; Lyoussi, B. Antioxidant Activity and Protective Effect of Carob Honey in CCl4-Induced Kidney and Liver Injury. Arch. Med. Res. 2018, 49, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Imtara, H.; Al-Waili, N.; Bakour, M.; Al-Waili, W.; Lyoussi, B. Evaluation of Antioxidant, Diuretic, and Wound Healing Effect of Tulkarm Honey and Its Effect on Kidney Function in Rats. Vet. World 2018, 11, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Imtara, H.; Al-Waili, N.; Aboulghazi, A.; Abdellaoui, A.; Al-Waili, T.; Lyoussi, B. Chemical Composition and Antioxidant Content of Thymus Vulgaris Honey and Origanum Vulgare Essential Oil; Their Effect on Carbon Tetrachloride-Induced Toxicity. Vet. World 2021, 14, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Elamine, Y.; Lyoussi, B.; Miguel, M.G.; Anjos, O.; Estevinho, L.; Alaiz, M.; Girón-Calle, J.; Martín, J.; Vioque, J. Physicochemical Characteristics and Antiproliferative and Antioxidant Activities of Moroccan Zantaz Honey Rich in Methyl Syringate. Food Chem. 2021, 339, 128098. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Al-Waili, N.; Aazza, S.; Elamine, Y.; Zizi, S.; Al-Waili, T.; Al-Waili, A.; Lyoussi, B. Antioxidant and Diuretic Activity of Co-Administration of Capparis spinosa Honey and Propolis in Comparison to Furosemide. Asian Pac. J. Trop. Med. 2017, 10, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Boutoub, O.; El-Guendouz, S.; Manhita, A.; Dias, C.; Estevinho, L.; Paula, V.; Carlier, J.; Costa, M.; Rodrigues, B.; Raposo, S.; et al. Comparative Study of the Antioxidant and Enzyme Inhibitory Activities of Two Types of Moroccan Euphorbia Entire Honey and Their Phenolic Extracts. Foods 2021, 10, 1909. [Google Scholar] [CrossRef] [PubMed]
- El Azzouzi, F.; Zidane, L. La Flore Médicinale Traditionnelle de La Région de Béni- Mellal (Maroc). J. Appl. Biosci. 2015, 91, 8493. [Google Scholar] [CrossRef]
- Hmamouchi, M. Plantes Alimentaires, Aromatiques, Condimentaires, Médicinales et Toxiques Au Maroc. Cah Options Médi-Terranéennes; CIHEAM: Paris, France, 1997. [Google Scholar]
- Tiji, S.; Rokni, Y.; Benayad, O.; Laaraj, N.; Asehraou, A.; Mimouni, M. Chemical Composition Related to Antimicrobial Activity of Moroccan Nigella sativa L. Extracts and Isolated Fractions. Evid.-Based Complement. Altern. Med. 2021, 2021, 8308050. [Google Scholar] [CrossRef] [PubMed]
- Lahlou, Y.; El Amraoui, B.; El-Wahidi, M.; Bamhaoud, T. Chemical Composition, Antioxidants and Antimicrobial Activities of Moroccan Species of Psidium Guajava Extracts. Rocz. Panstw. Zakl. Hig. 2022, 73, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Nafis, A.; Saad, F.E.; Khalloufi, F.E. New Insight into Antimicrobial Activities of Linaria Ventricosa Essential Oil and Its Synergetic Effect with Conventional Antibiotics. Arch. Microbiol. 2021, 203, 4361–4366. [Google Scholar] [CrossRef]
- Merrouni, I.A.; Elachouri, M.; Nf-, B. Anticancer Medicinal Plants Used by Moroccan People: Ethnobotanical, Preclinical, Phytochemical and Clinical Evidence. J. Ethnopharmacol. 2021, 266, 113435. [Google Scholar] [CrossRef]
- Amrati, F.E.Z.; Bourhia, M.; Slighoua, M. Traditional Medicinal Knowledge of Plants Used for Cancer Treatment by Communities of Mountainous Areas of Fez-Meknes-Morocco. Saudi Pharm. J. 2021, 29, 1185–1204. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.E.; Balahbib, A.; Belmehdi, O.; Bakri, Y. Moroccan Antidiabetic Medicinal Plants: Ethnobotanical Studies, Phytochemical Bioactive Compounds, Preclinical Investigations, Toxicological Validations and Clinical Evidences; Challenges, Guidance and Perspectives for Future Management of Diabetes Worldw. Trends Food Sci. Technol. 2021, 115, 147–254. [Google Scholar] [CrossRef]
- Boulfia, M.; Lamchouri, F.; Senhaji, S.; Lachkar, N.; Bouabid, K.; Toufik, H. Mineral Content, Chemical Analysis, in Vitro Antidiabetic and Antioxidant Activities, and Antibacterial Power of Aqueous and Organic Extracts of Moroccan Leopoldia comosa (L.) Parl. Bulbs. Evid.-Based Complement. Altern. Med. 2021, 2021, 9932291. [Google Scholar] [CrossRef]
- Belhaj, S.; Chaachouay, N.; Zidane, L. Ethnobotanical and Toxicology Study of Medicinal Plants Used for the Treatment of Diabetes in the High Atlas Central of Morocco. J. Pharm. Pharmacogn. Res. 2021, 9, 619–662. [Google Scholar] [CrossRef]
- Chlif, N.; Bouymajane, A.; El Majdoub, Y.O.; Diouri, M.; Filali, F.R.; Bentayeb, A.; Cacciola, F. Phenolic Compounds, In Vivo Anti-Inflammatory, Analgesic and Antipyretic Activities of the Aqueous Extracts from Fresh and Dry Aerial Parts of Brocchia Cinerea (Vis.). J. Pharm. Biomed. Anal. 2022, 213, 114695. [Google Scholar] [CrossRef] [PubMed]
- Hamamouchi, J.; El Mahi, M.; Faouzi, M.E.A. Investigation of Origanum Compactum Essential Oil for Analgesic and Anti-Inflammatory Activities. E3S Web Conf. 2021, 319, 01104. [Google Scholar] [CrossRef]
- Bouyahya, A.; Guaouguaou, F.E.; El Omari, N.; El Menyiy, N.; Balahbib, A.; El-Shazly, M.; Bakri, Y. Anti-Inflammatory and Analgesic Properties of Moroccan Medicinal Plants: Phytochemistry, in Vitro and In Vivo Investigations, Mechanism Insights, Clinical Evidences and Perspectives. J. Pharm. Analysis. 2021, 12, 35–57. [Google Scholar] [CrossRef]
- El Ouahdani, K.; Es-Safi, I.; Mechchate, H.; Al-Zahrani, M.; Qurtam, A.A.; Aleissa, M.; Bousta, D. Thymus Algeriensis and Artemisia Herba-Alba Essential Oils: Chemical Analysis, Antioxidant Potential and In Vivo Anti-Inflammatory, Analgesic Activities, and Acute Toxicity. Molecules 2021, 26, 6780. [Google Scholar] [CrossRef] [PubMed]
- Makbal, R.; Idrissi, F.E.J.; Ouchbani, T.; Tastift, M.A.; Kiai, H.; Hafidi, A.; Gadhi, C. Anti-Inflammatory, Antioxidant, Chemical Characterization, and Safety Assessment of Argania Spinosa Fruit Shell Extract from South-Western Morocco. BioMed. Res. Int. 2021, 2021, 5536030. [Google Scholar] [CrossRef]
- Mekkaoui, M.; Assaggaf, H.; Qasem, A.; El-Shemi, A.; Abdallah, E.M.; Bouidida, E.H.; Mrabti, H.N.; Cherrah, Y.; Alaoui, K. Ethnopharmacological Survey and Comparative Study of the Healing Activity of Moroccan Thyme Honey and Its Mixture with Selected Essential Oils on Two Types of Wounds on Albino Rabbits. Foods 2022, 11, 28. [Google Scholar] [CrossRef]
- Boukraâ, L. Synergistic Effect of Monofloral Honeys and Essential Oils against Pseudomonas aeruginosa. Br. Microbiol. Res. J. 2013, 3, 564–573. [Google Scholar] [CrossRef]
- Imtara, H.; Elamine, Y.; Lyoussi, B. Honey Antibacterial Effect Boosting Using Origanum vulgare L. Essential Oil. Evid. -Based Complement. Altern. Med. 2018, 2018, 2583. [Google Scholar] [CrossRef] [Green Version]
- Khay, E.O.; Bouyahya, A.; El Issaoui, K.; Zinebi, S.; Abrini, J. Study of Synergy between Mentha Pulegium Essential Oil, Honey and Bacteriocin-like Inhibitory Substance E204 against Listeria Monocytogenes CECT 4032 and Escherichia Coli K12. Int. J. Curr. Res. Biosci. Plant Biol. 2016, 3, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Ebadi, P.; Fazeli, M. South African Journal of Botany Evaluation of the Potential in Vitro Effects of Propolis and Honey on Wound Healing in Human Dermal Fi Broblast Cells. South Afr. J. Bot. 2021, 137, 414–422. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Filali, F.R.; Lo Presti, V.; Zekkori, B.; Nalbone, L.; Bouymajane, A.; Trabelsi, N.; Lamberta, F.; Bentayeb, A.; Giuffrida, A.; et al. Chemical Composition, Antioxidant Capacity and Antibacterial Action of Five Moroccan Essential Oils against Listeria Monocytogenes and Different Serotypes of Salmonella Enterica. Microb. Pathog. 2020, 149, 104510. [Google Scholar] [CrossRef] [PubMed]
- Eloff, J.N. A Sensitive and Quick Microplate Method to Determine the Minimal Inhibitory Concentration of Plant Extracts for Bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litchfield, J.J.; Wilcoxon, F. A Simplified Method of Evaluating Dose-Effect Experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- Boniface, M.; Boniface, B.; Cazin, J.C.; Cazin, N.; Luyckx, M. Calcul Sur Ordinateur de La Dose Efficace Par la Méthode Du Probit. Application Au Calcul d’une Dose Léthale 50. Bull. Soc. Pharm. Lille 1972, 4, 187–189. [Google Scholar]
- Alnamer, R.; Alaoui, K.; Bouidida, E.H.; Benjouad, A. Toxicity and Psychotropic Activity of Essential Oils of Rosmarinus officinalis and Lavandula officinalis from Morocco. J. Biol. Act. Prod. Nat. 2011, 1, 37–41. [Google Scholar] [CrossRef]
- Alaoui, K.; Belabbes, M.; Cherrah, Y.; Hassar, M.; Mcharrout, Z.; Amarouch, H.; Roguebert, J. Acute and Chronic Toxicity of Saponine from Argania Spinosa. Ann. Pharm. Françaises 1998, 65, 213–219. [Google Scholar]
- Olfert, E.; Cross, B.; McWilliam, A. Manuel Sur Le Soin et l’utilisation Des Animaux d’expérimentation. Cons. Can. De Prot. Des. Animaux 1993, 1, 1–210. [Google Scholar]
- Laroche, M.; Rousselet, F. Les Animaux Du Laboratoire: Éthique et Bonnes Pratiques; Masson: Paris, France, 1990. [Google Scholar]
- Assaggaf, H.M.; Naceiri Mrabti, H.; Rajab, B.S.; Attar, A.A.; Hamed, M.; Sheikh, R.A.; Bouyahya, A. Singular and Combined Effects of Essential Oil and Honey of Eucalyptus Globulus on Anti-Inflammatory, Antioxidant, Dermatoprotective, and Antimicrobial Properties: In Vitro and In Vivo Findings. Molecules 2022, 27, 5121. [Google Scholar] [CrossRef]
- Alaoui, K.; Lagorce, J.F.; Cherrah, Y.; Hassar, M.; Amarouch, H.; Roquebert, J. Activité Analgésique et Anti-Inflammatoire Des Saponines d’Argania Spinosa [Analgesic and Anti-Inflammatory Activity of Saponins of Argania Spinoza]. Ann. Pharm Fr. 1998, 56, 220–228. [Google Scholar]
- Al-Sobarry, M.; Alwashli, A.; Cherrah, Y.; Cherrah, Y.; Katim, A. Antiinflammatory Activities of Methanolic Extract of Jatropha Unicostata Balf (Sibru) and Ethanolic Extract of Aloe Perryi Baker (Taife), as Endemic Plants in Yemen. Int. J. Pharma Bio Sci. 2011, 2, 375–381. [Google Scholar]
- Winter, C.; Risley, E.; Nuss, G. Carrageenin-Induced Edema in Hind Paw of the Rat as an Assay for Antiiflammatory Drugs. Proc. Soc. Exp. Biol Med. 1962, 111, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Kharbach, M.; Eljemli, M.; Bouklouze, A.; Cherrah, Y.; Alaoui, K. Activité Anti-Inflammatoire In Vivo de l’huile d’argan. Phytothérapie 2019, 18, 255–261. [Google Scholar] [CrossRef]
- El Jemli, M.; Kamal, R.; Marmouzi, I.; Doukkali, Z.; Bouidida, E.H.; Touati, D.; Nejjari, R.; El Guessabi, L.; Cherrah, Y.; Alaoui, K. Chemical Composition, Acute Toxicity, Antioxidant and Anti-Inflammatory Activities of Moroccan Tetraclinis articulata L. J. Tradit. Complement. Med. 2017, 7, 281–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, C.; Ferreres, F.; Gomes, N.G.M.; Duangsrisai, S.; Srisombat, N.; Vajrodaya, S.; Pereira, D.M.; Gil-izquierdo, A.; Andrade, P.B. Phenolic Profiling and Biological Potential of Ficus Curtipes Corner Leaves and Stem Bark: 5-Lipoxygenase Inhibition and Interference with NO Levels in LPS-Stimulated RAW 264. 7 Macrophages. Biomolecules 2019, 9, 400. [Google Scholar] [CrossRef] [Green Version]
- Ould Si Said, Z. Activités Biologiques Des Huiles Essentielles Des Feuilles et Du Fruit d’une Plante Médicinale Eucalyptus Globulus. Master’s Thesis, Université Abderrahmane Mira, Béjaïa, Algeria, 2014. [Google Scholar]
- Harkat-Madouri, L.; Asma, B.; Madani, K.; Said, Z.B.-O.S.; Rigou, P. Chemical Composition, Antibacterial and Antioxidant Activities of Essential Oil of Eucalyptus Globulus from Algeria. Ind. Crops Prod. 2015, 78, 148–153. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Carol, A.; Graziano, E.; Cardile, V. Oregano (Origanum vulgare L.) Essential Oil Provides Anti-Inflammatory Activity and Facilitates Wound Healing in a Human Keratinocytes Cell Model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef]
- Radünz, M.; Mota Camargo, T.; dos Santos Hackbart, H.C.; Inchauspe Correa Alves, P.; Radünz, A.L.; Avila Gandra, E.; da Rosa Zavareze, E. Chemical Composition and in Vitro Antioxidant and Antihyperglycemic Activities of Clove, Thyme, Oregano, and Sweet Orange Essential Oils. LWT 2021, 138, 110632. [Google Scholar] [CrossRef]
- Santos, F.; Silva, R.; Campos, A.; Araújo, R.; Júnior, R.; Rao, V. 1,8-Cineole (Eucalyptol), a Monoterpene Oxide Attenuates the Colonic Damage in Rats on Acute TNBS-Colitis. Food Chem. Toxicol. 2004, 42, 579–584. [Google Scholar] [CrossRef]
- Belkhodja, H.; Meddah, B.; Medjadel, B.; Sidelarbi, K.; Brakna, A.; Bouhadi, D.; Engineering, M.; Safety, H. In Vitro and In Vivo Anti-Inflammatory Potential of Eucalyptus Globulus Essential Oil. J. Appl. Biol. Sci. 2022, 16, 80–88. [Google Scholar] [CrossRef]
- Yoon, W.; Kim, S.; Oh, T.; Lee, N.; Hyun, C. Cryptomeria japonica Essential Oil Inhibits the Growth of Drug-Resistant Skin Pathogens and LPS-Induced NO and pro-Inflammatory Cytokine Production. Pol. J. Microbiol. 2009, 58, 61–68. [Google Scholar]
- Juergens, U.; Dethlefsen, U.; Steinkamp, G.; Gillissen, A.; Repges, R.; Vetter, H. Anti-Inflammatory Activity of 1.8-Cineol (Eucalyptol) in Bronchial Asthma: A Double-Blind Placebo-Controlled Trial. Respir. Med. 2003, 97, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbaghi, G.; Groleau, M.C.; López de los Santos, Y.; Doucet, N.; Déziel, E.; Castonguay, A. Cationic RuII Cyclopentadienyl Complexes with Antifungal Activity against Several Candida Species. ChemBioChem 2020, 21, 3112–3119. [Google Scholar] [CrossRef]
- Zhou, J.W.; Jia, A.Q.; Tan, X.J.; Chen, H.; Sun, B.; Huang, T.Z.; He, Y.; Li, P.L.; Liu, E.Q. 1-(4-Amino-2-Hydroxyphenyl)Ethenone Suppresses Agrobacterium Tumefaciens Virulence and Metabolism. Front. Microbiol. 2020, 11, 584767. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Recalde, M.; Ruiz Arias, I.E.; Hermida, É.B. Could Essential Oils Enhance Biopolymers Performance for Wound Healing? A Systematic Review. Phytomedicine 2018, 38, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Özkalp, B.; Sevgi, F.; Özcan, M.; Özcan, M.M. The Antibacterial Activity of Essential Oil of Oregano (Origanum vulgare L.). J. Food Agric. Environ. 2010, 8, 272–274. [Google Scholar]
- Sales, A.; de Felipe, L.O.; Bicas, J.L. Production, Properties, and Applications of α-Terpineol. Food Bioprocess. Technol. 2020, 13, 1261–1279. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A Review of Applications of Tea Tree Oil in Dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef] [PubMed]
- de Assis, K.M.A.; da Silva Leite, J.M.; de Melo, D.F.; Borges, J.C.; Santana, L.M.B.; dos Reis, M.M.L.; Moreira, V.M.; da Rocha, W.R.V.; Catão, R.M.R.; dos Santos, S.G.; et al. Bicontinuous Microemulsions Containing Melaleuca Alternifolia Essential Oil as a Therapeutic Agent for Cutaneous Wound Healing. Drug Deliv. Transl. Res. 2020, 10, 1748–1763. [Google Scholar] [CrossRef]
- Jiang, Z.; Guo, X.; Zhang, K.; Sekaran, G.; Cao, B.; Zhao, Q.; Zhang, S.; Kirby, G.M.; Zhang, X.Y. The Essential Oils and Eucalyptol from Artemisia vulgaris L. Prevent Acetaminophen-Induced Liver Injury by Activating Nrf2-Keap1 and Enhancing APAP Clearance through Non-Toxic Metabolic Pathway. Front. Pharmacol. 2019, 10, 782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, M.; Du, J. Anti-Inflammatory and Protective Effects of D-Carvone on Lipopolysaccharide (LPS)-Induced Acute Lung Injury in Mice. J. King Saud Univ.-Sci. 2020, 32, 1592–1596. [Google Scholar] [CrossRef]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Kehili, S.; Boukhatem, M.A.; Belkadi, A.; Boulaghmen, F.; Ferhat, M.A.; Setzer, W.N. Spearmint (Mentha spicata L.) Essential Oil from Tipaza (Algeria): In Vivo Anti-Inflammatory and Analgesic Activities in Experimental Animal Models. Acta Pharm. Hung. 2020, 90, 15–26. [Google Scholar] [CrossRef]
- Yahia, I.B.H.; Jaouadi, R.; Trimech, R.; Boussaid, M.; Zaouali, Y. Variation of Chemical Composition and Antioxidant Activity of Essential Oils of Mentha x rotundifolia (L.) Huds.(Lamiaceae) Collected from Different Bioclimatic Areas of Tunisia. Biochem. Syst. Ecol 2019, 84, 8–16. [Google Scholar] [CrossRef]
- Mannu, A.; Melito, S.; Petretto, G.L.; Manconi, P.; Pintore, G.M.; Chessa, M. Geographical Variation of the Chemical Composition in Essential Oils Extracted from Sardinian Salvia Verbenaca. Nat. Prod. Res. 2022, 36, 367–370. [Google Scholar] [CrossRef]
- Bakhtiar, A.; Khaghani, S.; Pirbalouti, A.G.; Gomarian, M.; Chavoshi, S. Essential Oil Variation among Different Populations of Ziziphora tenuior L. Cultivated at Semiarid Climate. J. Essent. Oil Res. 2021, 33, 385–393. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.; Latva-Kala, K.; Mattila-Sandholm, T. Characterization of the Action of Selected Essential Oil Components on Gram-Negative Bacteria. J. Agric. Food Chem. 1989, 46, 3590–3595. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Disruption of Escherichia Coli, Listeria Monocytogenes and Lactobacillus Sakei Cellular Membranes by Plant Oil Aromatics. Int. J. Food Microbiol. 2006, 15, 1–9. [Google Scholar] [CrossRef]
- Prabuseenivasan, S.; Jayakumar, M.; Ignacimuthu, S. In Vitro Antibacterial Activity of Some Plant Essential Oils. BMC Complement. Altern. Med. 2006, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Božović, M.; Pirolli, A.; Ragno, R. Mentha Suaveolens Ehrh. (Lamiaceae) Essential Oil and Its Main Constituent Piperitenone Oxide: Biological Activities and Chemistry. Molecules 2015, 20, 8605–8633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carolina, A.; Pires, L.; Campos, D.; Daniela, R.; Nandi, S.; Scandorieiro, S.; Chue, M.; Fonseca, G.; Dibo, M.; Pinto, L.; et al. Antimicrobial Effect of Origanum vulgare L. Essential Oil as an Alternative for Conventional Additives in the Minas Cheese Manufacture. LWT 2022, 157, 113063. [Google Scholar] [CrossRef]
- Miroslava, K.; Vukovič, N.; Hleba, L.; Bobková, A.; Pavelková, A.; Rovná, K.; Arpášová, H. Antimicrobial and Antiradicals Activity of Origanum vulgare L. and Thymus Vulgaris Essential Oils. Food Sci. 2012, 2, 263–271. [Google Scholar]
- Badekova, K.Z.; Atazhanova, G.A.; Kacergius, T. Composition and Screening of Origanum Vulgare Essential Oil for Antimicrobial Activity Composition and Screening of Origanum Vulgare Essential Oil for Antimicrobial Activity. J. Taibah Univ. Med. Sci. 2021, 16, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Bencheikh, D.; Gueddah, A.; Soualat, K.; Ben-aissi, H. Polyphenolic Contents, Antioxidant and Antibacterial Activities of Aqueous Extracts of Eucalyptus globulus L. and Trigonella Foenum-Greacum L. J. Appl. Biol. Sci. 2021, 15, 53–63. [Google Scholar]
- Sharma, A.D.; Kaur, I.; Singh, N. Synthesis, Characterization, and In Vitro Drug Release and In Vitro Antibacterial Activity of O/W Nanoemulsions Loaded with Natural Eucalyptus Globulus Essential Oil. Int. J. Nanosci. Nanotechnol. 2021, 17, 191–207. [Google Scholar]
- Sharma, A.D.; Farmaha, M.; Kaur, I.; Singh, N. Phytochemical Analysis Using GC-FID, FPLC Fingerprinting, Antioxidant, Antimicrobial, Anti- Inflammatory Activities Analysis of Traditionally Used Eucalyptus Globulus Essential Oil. Drug Anal. Res. 2021, 5, 26–38. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Bakchiche, B.; Alsalamat, H.A.; Rezzoug, M.; Gherib, A.; Flamini, G. Chemical Composition, Antioxidant, Antimicrobial and Antiproliferative Activities of Essential Oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan Atlas. BMC Complement. Altern. Med. 2018, 18, 201. [Google Scholar] [CrossRef] [Green Version]
- Karaca, N.; Demirci, B.; Demirci, F. Evaluation of Lavandula stoechas L. Subsp. Stoechas L., Mentha spicata L. Subsp. Spicata L. Essential Oils and Their Main Components against Sinusitis Pathogens. Z. Nat. 2018, 73, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.; Dahiya, P. In Vitro Antimicrobial Activity, Phytochemical Analysis and Total Phenolic Content of Essential Oil from Mentha Spicata and Mentha Piperita. Int. Food Res. J. 2015, 22, 2440–2445. [Google Scholar]
- Mahboubi, M. Mentha spicata L. Essential Oil, Phytochemistry and Its Effectiveness in Fl Atulence. J. Tradit. Chin. Med. Sci. 2021, 11, 75–81. [Google Scholar] [CrossRef]
- Othman, S.I.; Kamel, F.H. In Vitro Antibacterial Activity of Mentha Spicata Essential Oil against Some Pathogenic Bacteria. Polytech. J. 2021, 11, 13–15. [Google Scholar] [CrossRef]
- Adli, D.E.; Brahmi, M.; Ziani, K.; Brahmi, K.; Kahloula, K.; Slimani, M. Chemical Composition, in Vitro Antioxidant, Antimicrobial and Cytotoxic Activities of Mentha Spicata Essential Oil: A Review. Phytothérapie 2022. review. [Google Scholar] [CrossRef]
- Omer, A.M.; Tamer, T.M.; Khalifa, R.E.; Eltaweil, A.S.; Agwa, M.M.; Sabra, S.; Abd-Elmonem, M.S.; Mohy-Eldin, M.S.; Ziora, Z.M. Formulation and Antibacterial Activity Evaluation of Quaternized Aminochitosan Membrane for Wound Dressing Applications. Polymers 2021, 13, 2428. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M. Antibacterial Activity of Hibiscus sabdariffa L. Calyces against Hospital Isolates of Multidrug Resistant Acinetobacter Baumannii. J. Acute Dis. 2016, 5, 512–516. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, C.; Thomas, M.; Athis, J.; Ballantyne, B.; Marrs, T.; Turner, P. Principles of Testing for Acute Toxic Effects. In General and Applied Toxicology. Stockt. Press: New York 1993, 1, 49–87. [Google Scholar]
- Naidu, J.R.; Ismail, R.; Sasidharan, S. Acute Oral Toxicity and Brine Shrimp Lethality of Methanol Extract of Mentha Spicata L (Lamiaceae). Trop. J. Pharm. Res. 2014, 13, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Hassen, A.; Debella, A.; Gebru, G.; Eshetu, M.; Asefa, M.; Admas, A.; Abebe, A. Subchronic Toxicity Study of Herbal Tea of Moringa Stenopetala (Baker f.) Cudof. and Mentha spicata L. Leaves Formulation in Wistar Albino Rats. Toxicol. Rep. 2022, 9, 797–805. [Google Scholar] [CrossRef]
- Bayani, M.; Ahmadi-Hamedani, M.; Jebelli Javan, A. Study of Hypoglycemic, Hypocholesterolemic and Antioxidant Activities of Iranian Mentha Spicata Leaves Aqueous Extract in Diabetic Rats. Iran. J. Pharm. Res. 2017, 16, 75–82. [Google Scholar]
- Yousuf, T.; Akter, R.; Ahmed, J.; Mazumdar, S.; Talukder, D.; Nandi, N.C.; Nurulamin, M. Evaluation of Acute Oral Toxicity, Cytotoxicity, Antidepressant and Antioxidant Activities of Japanese Mint (Mentha arvensis L.) Oil. Phytomedicine Plus 2021, 1, 100140. [Google Scholar] [CrossRef]
- Gardner, Z.; McGuffin, M. Botanical Safety Handbook, 2nd ed.; Zoe, E., Ed.; Taylor & Francis Group: Abingdon, UK, 2013. [Google Scholar]
- Berhan, M.; Tizazu, Z.; Kassahun, D.; Ermias, L.; Awol, M.; Zegeye, N.; Shiferaw, Y. Safety Evaluation of Eucalyptus Globulus Essential Oils through Acute and Sub-Acute Toxicity and Skin Irritation in Mice and Rats. Curr. Chem. Biol. 2020, 14, 187–195. [Google Scholar] [CrossRef]
- Gebremickael, A. Acute and Sub-Chronic Oral Toxicity Evaluation of Eucalyptus Globulus Essential Oil-Water Emulsion in Mice. J. Cytol. Histol. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Ajilore, B.S.; Oluwadairo, T.O.; Olorunnisola, O.S.; Fadahunsi, O.S.; Adegbola, P.I. GC–MS Analysis, Toxicological and Oral Glucose Tolerance Assessments of Methanolic Leaf Extract of Eucalyptus Globulus. Future J. Pharm. Sci. 2021, 7, 162. [Google Scholar] [CrossRef]
- Selim, S.A.; Aziz, M.H.A.; Mashait, M.S.; Warrad, M.F. Antibacterial Activities, Chemical Constitutes and Acute Toxicity of Egyptian Origanum majorana L., Peganum harmala L. and Salvia officinalis L. Essential Oils. Afr. J. Pharm. Pharmacol. 2014, 7, 725–735. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Pichardo, S.; Jos, A.; Moyano, R.; Cameán, A. A Subchronic 90-Day Oral Toxicity Study of Origanum vulgare Essential Oil in Rats; University of Seville: Seville, Spain, 2017. [Google Scholar]
- Nasiri, A.A.; Shahdad, M.P.H.; Balouchi, A.; Sepehri, Z.; Ghalenov, A.R. A Comparative Study of Dimethicone and Supermint Anti-Flatulence Effects on Reducing Flatulence in Patients with Irritable Bowel Syndrome. Der Pharm Lett 2015, 7, 432–436. [Google Scholar]
- RoknAbadi, M.; Sarafraz, N. Effect of Supermint Oral Drop (Mentha Spicata Essential Oil) on Primary Dysmenorrhea Compared with Ibuprofen: A Randomized Clinical Trial. J. Qom. Univ. Med. Sci. 2011, 5, 37–41. [Google Scholar]
- Kamal, R.; Kharbach, M.; Vander Heyden, Y.; Doukkali, Z.; Ghchime, R.; Bouklouze, A.; Cherrah, Y.; Alaoui, K. In Vivo Anti-Inflammatory Response and Bioactive Compounds’ Profile of Polyphenolic Extracts from Edible Argan Oil (Argania spinosa L.), Obtained by Two Extraction Methods. J. Food Biochem. 2019, 43, 13066. [Google Scholar] [CrossRef] [PubMed]
- Vinegar, R.; Schreiber, W.; Hugo, R. Biphasic Development of Carrageenan Edema in Rats. J. Pharmacol. Exp. Ther. 1969, 166, 96–103. [Google Scholar] [PubMed]
- Mequanint, W.; Makonnen, E.; Urga, K. In Vivo Anti-inflamma-Tory Activities of Leaf Extracts of Ocimum Lamiifolium in Mice Model. J. Ethnopharmacol. 2011, 134, 32–36. [Google Scholar] [CrossRef]
- Panthong, A.; Supraditaporn, W.; Kanjanapothi, D.; Taesotikul, T.; Reutrakul, V. Analgesic, Anti-inflammatory and Venotonic Effects of Cissus Quadrangularis Linn. J. Ethnopharmacol. 2007, 110, 264–270. [Google Scholar] [CrossRef]
- Yonathan, M.; Asres, K.; Assefa, A.; Bucar, F. In Vivo Anti-inflammatory and Anti-nociceptive Activities of Cheilanthes Farinosa. J. Ethnopharmacol. 2006, 108, 462–470. [Google Scholar] [CrossRef]
- Gomes, A.; Fernandes, E.; Lima, J.L.; Mira, L.; Corvo, M.L. Molecular Mechanisms of Anti-inflammatory Activity Mediated by Flavonoids. Curr. Med. Chem. 2008, 15, 1586–1605. [Google Scholar] [CrossRef]
- Bounihi, A.; Hajjaj, G.; Alnamer, R.; Cherrah, Y.; Zellou, A. In Vivo Potential Anti-inflammatory Activity of Melissa officinalis L. Essential Oil. Adv. Pharmacol. Sci. 2013, 2013, 101759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sipahi, H.; Aydogan, G.; Helvacioglu, S.; Charehsaz, M.; Guzelmeric, E.; Aydin, A. Antioxidant, Antiinflammatory and Antimutagenic Activities of Various Kinds of Turkish Honey. FABAD J. Pharm. Sci. 2017, 42, 7–13. [Google Scholar]
- Joo, T.; Sowndhararajan, K.; Hong, S.; Lee, J.; Park, S.; Kim, S.; Jhoo, J. Inhibition of Nitric Oxide Production in LPS-Stimulated RAW 264.7 Cells by Stem Bark of Ulmus pumila L. Saudi J. Biol Sci 2014, 21, 427–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiemer, A.; Müller, C.; Vollmar, A. Inhibition of LPSinduced Nitric Oxide and TNF-Alpha Production by Alpha-Lipoic Acid in Rat Kupffer Cells and in RAW 264.7 Murine Macrophages. Immunol Cell Biol 2002, 80, 550–557. [Google Scholar] [CrossRef]
- Hashemian, F.; Baghbanian, N.; Majd, Z.; Rouini, M.-R.; Hashemian, J.J.F. The Effect of Thyme Honey Nasal Spray on Chronic Rhinosinusitis: A Double-Blind Randomized Controlled Clinical Trial. Eur Arch. Otorhinolaryngol 2015, 272, 1429–1435. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.; Harizanis, P.; Polissiou, M. Comparison of the Volatile Composition in Thyme Honeys from Several Origins in Greece. J. Agric. Food Chem 2007, 55, 8152. [Google Scholar] [CrossRef]
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a Component of Thyme Oil, Activates PPAR a and c and Suppresses COX-2 Expression. J. Lipid Res. 2010, 51, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Seibert, K.; Masferrer, J. Role of Inducible Cyclooxygenase (COX-2) in Inflammation. Receptor 1994, 4, 17–23. [Google Scholar]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- Chepulis, L.M. The Effect of Honey Compared to Sucrose, Mixed Sugars, and a Sugar-Free Diet on Weight Gain in Young Rats. J. Food Sci. 2007, 72, 224–229. [Google Scholar] [CrossRef]
- Chepulis, L.; Starkey, N. The Long-Term Effects of Feeding Honey Compared with Sucrose and a Sugar-Free Diet on Weight Gain, Lipid Profiles, and DEXA Measurements in Rats. Food Sci. 2008, 73, H1–H7. [Google Scholar] [CrossRef] [PubMed]
- Nemoseck, T.M.; Carmody, E.G.; Gleason, A.F.-E.; Li, A.; Potter, H. Honey Promotes Lower Weight Gain, Adiposity, and Triglycerides than Sucrose in Rats. Nutr. Res. 2011, 31, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Niijima, A.; Nagai, K. Effect of Olfactory Stimulation with Flavor of Grapefruit Oil and Lemon Oil on the Activity of Sympathetic Branch in the White Adipose Tissue of the Epididymis. Exp. Biol. Med. 2003, 228, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Dahu, L.; Houjiu, W.; Huating, D. Weight Loss Effect of Sweet Orange Essential Oil Microcapsules on Obese SD Rats Induced by High-Fat Diet. Biosci. Biotechnol. Biochem. 2019, 83, 923–932. [Google Scholar] [CrossRef]
- Asnaashari, S.; Delazar, A.; Habibi, B.; Vasfi, R.; Nahar, L.; Hamedeyazdan, S. Essential Oil from Citrus Aurantifolia Prevents Ketotifen-Induced Weight-Gain in Mice. Phytother. Res. 2010, 24, 1893–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, H.-S.; Lee, H.-J.; Lee, H.-J.; Sohn, E.J.; Yun, M.; Lee, M.-H.; Kim, S.-H. Essential Oil of Pinus Koraiensis Exerts Antiobesic and Hypolipidemic Activity via Inhibition of Peroxisome Proliferator-Activated Receptors Gamma Signaling. Evid. -Based Complement. Altern. Med. 2013, 2013, 947037. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Xiang, Q.; Wang, J.; Wei, H.; Peng, J. Effects of Oregano Essential Oil or Quercetin Supplementation on Body Weight Loss, Carcass Characteristics, Meat Quality and Antioxidant Status in Finishing Pigs under Transport Stress. Livest. Sci. 2016, 192, 33–38. [Google Scholar] [CrossRef]
- Ataabadi, M.S.; Alaee, S.; Mohammad Jafar Bagheri, S.B. Role of Essential Oil of Mentha Spicata (Spearmint) in Addressing Reverse Hormonal and Folliculogenesis Disturbances in a Polycystic Ovarian Syndrome in a Rat Model. Adv. Pharm Bull. 2017, 7, 651–654. [Google Scholar] [CrossRef]
- Farhadi, D.; Karimi, A.; Sadeghi, G.; Sheikhahmadi, A.; Habibian, M.; Raei, A.; Sobhani, K. Effects of Using Eucalyptus (Eucalyptus globulus L.) Leaf Powder and Its Essential Oil on Growth Performance and Immune Response of Broiler Chickens. Iran. J. Vet. Res. 2017, 18, 60–62. [Google Scholar]
- Unusan, N. Essential Oils and Microbiota: Implications for Diet and Weight Control. Trends Food Sci. Technol. 2020, 104, 60–71. [Google Scholar] [CrossRef]
- Njengaa, E.W.; Viljoen, A.M. In Vitro 5-Lipoxygenase Inhibition and Anti-Oxidant Activity of Eriocephalus L. (Asteraceae) Species. South Afr. J. Bot. 2006, 72, 637–641. [Google Scholar] [CrossRef]



| Sample | Moisture (%) | pH | Free Acidity (meq kg−1) | Lactonic Acidity (meq kg−1) | Total Acid-ity (meq kg−1) | Electrical Conductivity (ms cm−1) | Sugar Conten (°Brix) | Ash (%) | HMF (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|
| THYME HONEY | 17.15 ± 0.30 | 4.37 ± 0.26 | 35.88 ± 3.32 | 3.33 ± 0.72 | 39.21 ± 2.72 | 0.82 ± 0.083 | 79.83 ± 2.46 | 0.27 ± 0.12 | 8.44 ± 3.43 |
| CODEX | ≤21% | 3.4–6.1 | ≤50 meq/kg | _ | 8.68–59.49 meq/kg | ≥0.700 (ms cm−1) | ≥60 °Brix | ≤0.6% | ≤40 mg/kg |
| NO | O. vulgare EO a | M. spicata EO b | E. globulus EO c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Compound | RT * | % | Compound | RT | % | Compound | RT | % | |
| 1 | α-Pinene | 1.390 | 0.4 | α-Pinene | 1.356 | 0.84 | α-Pinene | 2.093 | 3.85 |
| 2 | Camphene | 1.502 | 0.09 | Camphene | 1.457 | 0.24 | Camphene | 2.251 | 0.48 |
| 3 | β-Phellandrene | 2.257 | 0.78 | β-Pinene | 1.762 | 1.84 | 2,4-Thujadiene | 2.363 | 0.09 |
| 4 | 2-Carene | 2.483 | 1.04 | Limonene | 2.697 | 21.56 | β-Pinene | 2.713 | 0.62 |
| 5 | Benzene, 1,2,3,4-tetramethyl | 2.764 | 8.15 | 3-Octanol, acetate | 5.154 | 0.43 | β-Myrcene | 3.186 | 0.58 |
| 6 | p-Cymene | 3.114 | 0.68 | Borneol | 5.638 | 1.00 | α-Phellandrene | 3.299 | 0.96 |
| 7 | γ-Terpinene | 3.441 | 8.00 | Terpinen-4-ol | 5.931 | 0.4 | Eucalyptol (1,8-cineol) | 4.268 | 90.14 |
| 8 | Benzene, 2-butenyl | 4.579 | 0.12 | Carvone | 8.050 | 60.37 | β-Ocimene | 4.380 | 0.28 |
| 9 | Linalool | 5.029 | 1.01 | β-Bourbonene | 10.596 | 2.30 | γ-Terpinene | 4.651 | 2.39 |
| 10 | α-Terpineol | 6.280 | 0.24 | Caryophyllene | 11.385 | 1.51 | p-Cymenene | 5.237 | 0.08 |
| 11 | Thymol | 10.247 | 54.21 | __ | __ | __ | 2,4,6-Octatriene, 3,4-dimethyl | 6.330 | 0.07 |
| 12 | Carvacrol | 10.867 | 19.08 | __ | __ | __ | trans-Sabinol | 7.952 | 0.08 |
| 13 | Caryophyllene | 11.757 | 2.26 | __ | __ | __ | 1_7_7-Trimethylbicyclo_2.2.1_hept-5-en-2-ol | 10.330 | 0.09 |
| 14 | Humulene | 12.703 | 0.11 | __ | __ | __ | __ | __ | __ |
| 15 | σ-Cadinene | 15.149 | 0.20 | __ | __ | __ | __ | __ | __ |
| Total identified compounds% | 96.57 | 88.98 | 99.71 | ||||||
| Monoterpene hydrocarbons% | 19.26 | 24.48 | 9.4 | ||||||
| Oxygenated monoterpenes % | 74.54 | 62.2 | 90.31 | ||||||
| Sesquiterpenes hydrocarbons% | 2.57 | 2.3 | __ | ||||||
| Oxygenated sesquiterpenes% | __ | __ | __ | ||||||
| Microorganisms | Mean Zone of Inhibition in Millimeters (Mean ± Standard Deviation) * | |||
|---|---|---|---|---|
| O. vulgare EO | E. globulus EO | M. spicata EO | Chloramphenicol (30 μg) | |
| E. coli ATCC 25922 | 25.1 ± 0.4 | 28.3 ± 1.1 | 21.5 ± 0.5 | 21.0 ± 0.4 |
| S. typhimurium ATCC 700408 | 23.1 ± 0.6 | 21.8 ± 0.9 | 17.5 ± 0.6 | 12.6 ± 0.4 |
| S. aureus ATCC 29213 | 30.4 ± 0.9 | 32 ± 1.43 | 26.8 ± 1.1 | 23.0 ± 0.6 |
| L. monocytogenes ATCC 13932 | 34.4 ± 1.2 | 36 ± 0.5 | 22.6 ± 0.9 | 26.7 ± 0.9 |
| Microorganisms | O. vulgare EO | E. globulus EO | M. spicata EO | Chloramphenicol | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (mg/mL) | MBC (mg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| E. coli ATCC 25922 | 1.56 ± 0.00 | 1.56 ± 0.11 | 1.56 ± 0.26 | 1.56 ± 0.03 | 1.68 ± 0.024 | 3.12 ± 0.093 | 4 | 4 |
| S. typhimurium ATCC 700408 | 3.12 ± 0.221 | 3.12 ± 0.72 | 3.12 ± 0.83 | 4.5 ± 1.44 | 12.5 ± 0.09 | 25 ± 0.219 | 64 | 64 |
| S. aureus ATCC 29213 | 0.78 ± 0.11 | 0.78 ± 0.20 | 0.78 ± 0.09 | 0.78 ± 0.00 | 1.56 ± 0.36 | 2.56 ± 0.008 | 4 | 4 |
| L. monocytogenes ATCC 13932 | 0.78 ± 0.018 | 0.78 ± 0.00 | 0.68 ± 0.36 | 1.56 ± 0.00 | 1.56 ± 0.018 | 3.12 ± 0.048 | 2 | 2 |
| Groups | Treatment | Daily Dose (mg/Kg) | Weight & Weight Gain Percentage | Day 1 | Day 6 | Day 10 | Day 14 | Day 18 | Day 22 | Day 28 |
|---|---|---|---|---|---|---|---|---|---|---|
| Gr I | M. spicata EO | 5000 | Weight (g) SD Weight gain (%) | 245.66 ± 3.61 — | 242.36 ±3.76 −1.34 | 244.94 ±4.11 −0.29 | 238.3 ±4.84 −3.00 | 237.98 ±4.17 −3.12 | 240.5 ±3.97 −2.10 | 244.26 ±3.71. −0.57 |
| Gr II | E. globulus EO | 1759.7 | Weight (g) SD Weight gain (%) | 256.72 ±2.80 — | 245.3 ±1.87 −4.45 | 242.08 ±3.14 −5.70 | 243.97 ±2.77 −4.97 | 242.6 ±2.41 −5.50 | 238.93 ±3.62 −6.93 | 249.32 ±3.44 −2.88 |
| Gr III | O. vulgare EO | 2000 | Weight (g) SD Weight gain (%) | 232.96 ±1.46 — | 227.68 ±2.21 −2.66 | 224.56 ±2.72 −3.60 | 223.32 ±3.94 −4.14 | 221.48 ±3.72 −4.92 | 228.15 ±4.68 −1.80 | 223.02 ±5.01 −4.27 |
| Control | — | — | Weight (g) SD Weight gain (%) | 248.76 ±4.73 — | 246.68 ±3.19 −0.84 | 259.68 ±3.62 4.39 | 264.54 ±3.73 6.34 | 256.71 ±4.11 3.20 | 253.9 ±2.64 2.07 | 247.43 ±3.42 −0.53 |
| Treatment Group | Mean Edema Volume (Left-Right Paw) mL | ||
|---|---|---|---|
| 1 h 30 min | 3 h | 6 h | |
| Control | 0.431 ± 0.011 | 0.546 ± 0.014 | 0.421 ± 0.02 |
| Indomethacin (10 mg/Kg) | 0.135 ± 0.014 * | 0.168 ± 0.016 * | 0.188 ± 0.017 * |
| GrI (mixture of TH and O. vulgare EO) | 0.269 ± 0.015 * | 0.264 ± 0.014 * | 0.240 ± 0.011 * |
| Gr II (mixture of TH and M. spicata EO) | 0.311 ± 0.017 * | 0.381 ± 0.018 * | 0.341 ± 0.013 * |
| Gr III (mixture of TH and E. globulus EO) | 0.288 ± 0.014 * | 0.320 ± 0.013 * | 0.280 ± 0.014 * |
| GrIV (TH) | 0.340 ± 0.016 * | 0.388 ± 0.014 * | 0.358 ± 0.019 * |
| Treatment Group | Mean Edema Volume (Left-Right Paw) mL | ||
|---|---|---|---|
| 1 h 30 min | 3 h | 6 h | |
| Control | 0.43 ± 0.014 | 0.693 ± 0.016 | 0.539 ± 0.014 |
| Indomethacin (20 mg/Kg) | 0.078 ± 0.013 * | 0.117 ± 0.019 * | 0.147 ± 0.014 * |
| GrI (mixture of TH and O. vulgare EO) | 0.278 ± 0.014 * | 0.327 ± 0.017 * | 0.271 ± 0.016 * |
| Gr II (mixture of TH and M. spicata EO) | 0.340 ± 0.013 * | 0.382 ± 0.014 * | 0.351 ± 0.017 * |
| Gr III (mixture of TH and E. globulus EO) | 0.331± 0.014 * | 0.368 ± 0.016 * | 0.347 ± 0.019 * |
| GrIV (TH) | 0.338 ± 0.016 * | 0.414 ± 0.013 * | 0.371 ± 0.014 * |
| Treatment Group | Percentage Inhibition of Edema (%) | ||
|---|---|---|---|
| 1 h 30 min | 3 h | 6 h | |
| Indomethacin (10 mg/Kg) | 68.67 | 69.23 | 55.34 |
| GrI (mixture of TH and O. vulgare EO) | 37.58 * | 51.65 * | 42.99 * |
| Gr II (mixture of TH and M. spicata EO) | 27.84 * | 30.41 * | 19.24 * |
| Gr III (mixture of TH and E. globulus EO) | 33.18 * | 41.39 * | 28.01 * |
| GrIV(TH) | 21.11 * | 28.94 * | 14.96 * |
| Treatment Group | Percentage Inhibition of Edema (%) | ||
|---|---|---|---|
| 1 h 30 min | 3 h | 6 h | |
| Indomethacin (20 mg/Kg) | 81.86 | 83.12 | 72.71 |
| GrI (mixture of TH and O. vulgare EO) | 35.45 * | 52.81 * | 49.72 * |
| Gr II (mixture of TH and M. spicata EO) | 20.93 * | 45.02 * | 35.06 * |
| Gr III (mixture of TH and E. globulus EO) | 23.25 * | 46.90 * | 35.62 * |
| GrIV (TH) | 21.39 * | 40.26 * | 31.17 * |
| TH | M. spicata EO | O. vulgare EO | E. globulus EO | Quercetin | |
|---|---|---|---|---|---|
| IC50 (µg/mL) | 29.53 ± 0.17 d | 37.14 ± 0.07 e | 13.23 ± 0.02 b | 15.53 ± 0.17 c | 1.09 ± 0.05 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mekkaoui, M.; Bouidida, E.H.; Naceiri Mrabti, H.; Ouaamr, A.; Lee, L.-H.; Bouyahya, A.; Cherrah, Y.; Alaoui, K. Investigation of Chemical Compounds and Evaluation of Toxicity, Antibacterial, and Anti-Inflammatory Activities of Three Selected Essential Oils and Their Mixtures with Moroccan Thyme Honey. Foods 2022, 11, 3141. https://doi.org/10.3390/foods11193141
Mekkaoui M, Bouidida EH, Naceiri Mrabti H, Ouaamr A, Lee L-H, Bouyahya A, Cherrah Y, Alaoui K. Investigation of Chemical Compounds and Evaluation of Toxicity, Antibacterial, and Anti-Inflammatory Activities of Three Selected Essential Oils and Their Mixtures with Moroccan Thyme Honey. Foods. 2022; 11(19):3141. https://doi.org/10.3390/foods11193141
Chicago/Turabian StyleMekkaoui, Mouna, El Houcine Bouidida, Hanae Naceiri Mrabti, Ahmed Ouaamr, Learn-Han Lee, Abdelhakim Bouyahya, Yahya Cherrah, and Katim Alaoui. 2022. "Investigation of Chemical Compounds and Evaluation of Toxicity, Antibacterial, and Anti-Inflammatory Activities of Three Selected Essential Oils and Their Mixtures with Moroccan Thyme Honey" Foods 11, no. 19: 3141. https://doi.org/10.3390/foods11193141
APA StyleMekkaoui, M., Bouidida, E. H., Naceiri Mrabti, H., Ouaamr, A., Lee, L. -H., Bouyahya, A., Cherrah, Y., & Alaoui, K. (2022). Investigation of Chemical Compounds and Evaluation of Toxicity, Antibacterial, and Anti-Inflammatory Activities of Three Selected Essential Oils and Their Mixtures with Moroccan Thyme Honey. Foods, 11(19), 3141. https://doi.org/10.3390/foods11193141