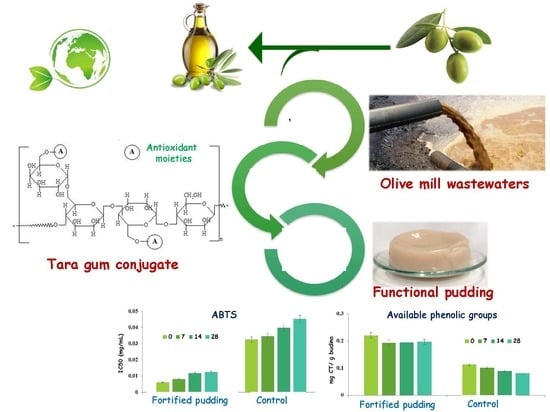
A Tara Gum/Olive Mill Wastewaters Phytochemicals Conjugate as a New Ingredient for the Formulation of an Antioxidant-Enriched Pudding
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Collection and Preparation
2.3. Chemical Characterization of Lyophilized Olive Mill Wastewaters
2.3.1. HPLC-MS Analysis of Lyophilized Olive Mill Wastewaters
2.3.2. H-NMR Analysis of Lyophilized Olive Mill Wastewaters
2.3.3. Polyphenols Total Content
2.3.4. Phenolic Acid Content
2.3.5. Flavonoid Content
2.3.6. Anthocyanin Content
2.3.7. Antioxidant Properties
2.4. Synthesis of the Tara Gum Conjugate by Grafting Procedure
2.5. Characterization of Tara Gum Conjugate
2.5.1. H-NMR Analysis of Tara Gum Conjugate
2.5.2. Differential Scansion Calorimetry (DSC)
- Isotherm at 25 °C for 20 min;
- Heating from 25 to 650 °C at 10 °C min−1.
2.5.3. Antioxidant Properties of the Tara Gum Conjugate
2.5.4. Toxicity of the Tara Gum Conjugate
Cell Culture
Neutral Red Uptake (NRU)
Cell Viability Assay
Con A/o-Pd Assay
2.6. Preparation of Puddings
2.7. Characterization of Pudding
2.7.1. Rheological Characterization
2.7.2. Antioxidant Performances of the Puddings
2.8. Statistical Analysis
3. Result and Discussion
3.1. Oil Mill Wastewater Treatment
3.2. HPLC-MS/MS and 1H-NMR Analyses of Lyophilized Oil Mill Wastewater
3.3. Antioxidant Properties of Lyophilized Oil Mill Wastewater
3.4. Synthesis of the Tara Gum Conjugate
3.5. 1H-NMR Analysis of the Polymers
3.6. Calorimetric Analysis of the Polymers
3.7. Toxicity Evaluation of the Conjugate
3.7.1. NRU Assay
3.7.2. Cell Viability by MTT Assay
3.7.3. Bio-Adhesive Ability Assay
3.8. Antioxidant Performances
3.9. Pudding Preparation and Evaluation of the Antioxidant Properties
3.10. Rheological Analysis of Puddings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spizzirri, U.G.; Aiello, F.; Carullo, G.; Facente, A.; Restuccia, D. Nanotechnologies: An Innovative Tool to Release Natural Extracts with Antimicrobial Properties. Pharmaceutics 2021, 13, 230. [Google Scholar] [CrossRef] [PubMed]
- Roselli, L.; Cicia, G.; Cavallo, C.; Del Giudice, T.; Carlucci, D.; Clodoveo, M.L.; De Gennaro, B.C. Consumers’ willingness to buy innovative traditional food products: The case of extra-virgin olive oil extracted by ultrasound. Food Res. Int. 2018, 108, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Restuccia, D.; Clodoveo, M.L.; Puoci, F.; Ragno, G. Chemometric analysis for discrimination of extra virgin olive oils from whole and stoned olive pastes. Food Chem. 2016, 202, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Amirante, P.; Clodoveo, M.L.; Tamborrino, A.; Leone, A.; Paice, A.G. Influence of the crushing system: Phenol content in virgin olive oil produced from whole and de-stoned pastes. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: London, UK, 2010; pp. 69–76. [Google Scholar]
- Cirillo, G.; Curcio, M.; Spizzirri, U.G.; Vittorio, O.; Valli, E.; Farfalla, A.; Leggio, A.; Nicoletta, F.P.; Iemma, F. Chitosan-Quercetin Bioconjugate as Multi-Functional Component of Antioxidants and Dual-Responsive Hydrogel Networks. Macromol. Mater. Eng. 2019, 304, 1800728. [Google Scholar] [CrossRef]
- Protte, K.; Weiss, J.; Hinrichs, J.; Knaapila, A. Thermally stabilised whey protein-pectin complexes modulate thethermodynamic incompatibility in hydrocolloid matrixes: A feasibility-study on sensory and rheological characteristics in dairy desserts. LWT-Food Sci. Technol. 2019, 105, 336–343. [Google Scholar] [CrossRef]
- Lim, H.S.; Narsimhan, G. Pasting and rheological behavior of soy protein-based pudding. LWT-Food Sci. Technol. 2006, 39, 344–350. [Google Scholar] [CrossRef]
- Alamprese, C.; Mariotti, M. Effects of different milk substitutes on pasting, rheological and textural properties of puddings. LWT-Food Sci. Technol. 2011, 44, 2019–2025. [Google Scholar] [CrossRef]
- Ares, G.; Baixauli, R.; Sanz, T.; Varela, P. New functional fibre in milk puddings: Effect on sensory properties and consumers’ acceptability. LWT-Food Sci. Technol. 2009, 42, 710–716. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, W.; He, Q. The gelation properties of tara gum blended with κ-carrageenan or xanthan. Food Hydrocoll. 2018, 77, 764–771. [Google Scholar] [CrossRef]
- Huamaní-Meléndez, V.J.; Mauro, M.A.; Darros-Barbosa, R. Physicochemical and rheological properties of aqueous Tara gum solutions. Food Hydrocoll. 2021, 111, 106195. [Google Scholar] [CrossRef]
- Menegon Rosário, F.; Biduski, B.; Fernando dos Santos, D.; Hadlish, E.V.; Tormen, L.; Fidelis dos Santos, G.H.; Zanella Pinto, V. Red araçá pulp microencapsulation by hydrolyzed pinhão starch, and tara and arabic gums. J. Sci. Food Agric. 2021, 101, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Ren, Y.; Wang, L. Investigation of antioxidant activity and release kinetics of curcumin from tara gum/polyvinyl alcohol active film. Food Hydrocoll. 2017, 70, 286–292. [Google Scholar] [CrossRef]
- D’Antuono, I.; Kontogianni, V.G.; Kotsiou, K.; Linsalata, V.; Logrieco, A.F.; Tasioula-Margari, M.; Cardinali, A. Polyphenolic characterization of olive mill wastewaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 2014, 65, 301–310. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive mill waste extracts: Polyphenols content, antioxidant, and antimicrobial activities. Adv. Pharmacol. Sci. 2015, 2015, 714138. [Google Scholar] [CrossRef]
- De Santis, S.; Liso, M.; Verna, G.; Curci, F.; Milani, G.; Faienza, M.F.; Franchini, C.; Moschetta, A.; Chieppa, M.; Clodoveo, M.L.; et al. Extra Virgin Olive Oil Extracts Modulate the Inflammatory Ability of Murine Dendritic Cells Based on Their Polyphenols Pattern: Correlation between Chemical Composition and Biological Function. Antioxidants 2021, 10, 1016. [Google Scholar] [CrossRef] [PubMed]
- Carullo, G.; Scarpelli, F.; Belsito, E.L.; Caputo, P.; Rossi Oliverio, C.; Mincione, A.; Leggio, A.; Crispini, A.; Restuccia, D.; Spizzirri, U.G.; et al. Formulation of New Baking (+)-Catechin Based Leavening Agents: Effects on Rheology, Sensory and Antioxidant Features during Muffin Preparation. Foods 2020, 9, 1569. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Lin, R. The Effect of Simulated Digestion In Vitro on Bioactivity of Wheat Bread with Tartary Buckwheat Flavones Addition. LWT-Food Sci. Technol. 2009, 42, 137–143. [Google Scholar] [CrossRef]
- Ardestani, A.; Yazdanparast, R. Antioxidant and free radical scavenging potential of Achillea santolina extracts. Food Chem. 2007, 104, 21–29. [Google Scholar] [CrossRef]
- Rababah, T.M.; Al-Omoush, M.; Brewer, S.; Alhamad, M.; Yang, W.; Alrababah, M.; Al-Ghzawi, A.A.-M.; Al-U’datt, M.; Ereifej, K.; Alsheyab, F.; et al. Total phenol, antioxidant activity, flavonoids, anthocyanins and color of honey as affected by floral origin found in the arid and semiarid mediterranean areas. J. Food Process. Preserv. 2014, 38, 1119–1128. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Anthocyanins: Characterization and measurement with W-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Spizzirri, U.G.; Carullo, G.; Aiello, F.; Paolino, D.; Restuccia, D. Valorisation of olive oil pomace extracts for a functional pear beverage formulation. Int. J. Food Sci. Technol. 2021, 56, 5497–5505. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Carullo, G.; De Cicco, L.; Crispini, A.; Scarpelli, F.; Restuccia, D.; Aiello, F. Synthesis and characterization of a (+)-catechin and L-(+)-ascorbic acid cocrystal as a new functional ingredient for tea drinks. Heliyon 2019, 5, e02291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellett, M.E.; Greenspan, P.; Pegg, R.B. Modification of the cellular antioxidant activity (CAA) assay to study phenolic antioxidants in a Caco-2 cell line. Food Chem. 2018, 244, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Giorgi, G.; Spizzirri, U.G.; Sciubba, F.; Capuani, G.; Rago, V.; Carullo, G.; Aiello, F. Autochthonous white grape pomaces as bioactive source for functional jams. Int. J. Food Sci. Technol. 2019, 54, 1313–1320. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Altimari, I.; Puoci, F.; Parisi, O.I.; Iemma, F.; Picci, N. Innovative antioxidant thermo-responsive hydrogels by radical grafting of catechin on inulin chain. Carbohydr. Polym. 2011, 84, 517–523. [Google Scholar] [CrossRef]
- Stokes, W.S.; Casati, S.; Strickland, J.; Paris, M. Neutral Red Uptake Cytotoxicity Tests for Estimating Starting Doses for Acute Oral Toxicity Tests. Curr. Protoc. Toxicol. 2008, 36, 20.4.1–20.4.20. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Rizza, L.; Frasca, G.; Nicholls, M.; Puglia, C.; Cardile, V. Caco-2 cell line as a model to evaluate mucoprotective proprieties. Int. J. Pharm. 2012, 422, 318–322. [Google Scholar] [CrossRef]
- Sun, Y.; Hayakawa, S.; Ogawa, M.; Izumori, K. Antioxidant properties of custard pudding dessert containing rare hexose, D-psicose. Food Control 2007, 18, 220–227. [Google Scholar] [CrossRef]
- Paraskeva, P.; Diamadopoulos, E. Technologies for olive mill wastewater (OMW) treatment: A review. J. Chem. Technol. Biotechnol. 2006, 81, 1475–1485. [Google Scholar] [CrossRef]
- He, J.; Alister-Briggs, M.; Lyster, T.D.; Jones, G.P. Stability and antioxidant potential of purified olive mill wastewater extracts. Food Chem. 2012, 131, 1312–1321. [Google Scholar] [CrossRef]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and Removal of Phenolic Compounds from Olive Mill Wastewater. J. Am. Oil Chem. Soc. 2013, 91, 1–18. [Google Scholar] [CrossRef]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanzio, V. Verbascoside, isoverbascoside, and their derivatives recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Obied, H.K.; Bedgood, D.R., Jr.; Prenzler, P.D.; Robards, K. Chemical screening of olive biophenol extracts by hyphenated liquid chromatography. Anal. Chim. Acta 2007, 603, 176–189. [Google Scholar] [CrossRef]
- Kiritsakis, K.; Rodríguez-Pérez, C.; Gerasopoulos, D.; Segura- Carretero, A. Olive oil enrichment in phenolic compounds during malaxation in the presence of olive leaves or olive mill wastewater extracts. Eur. J. Lip. Sci. Technol. 2017, 119, 1600425. [Google Scholar] [CrossRef]
- Mattonai, M.; Vinci, A.; Degano, I.; Ribechini, E.; Franceschi, M.; Modugno, F. Olive mill wastewaters: Quantitation of the phenolic content and profiling of elenolic acid derivatives using HPLC-DAD and HPLC/MS2 with an embedded polar group stationary phase. Nat. Prod. Res. 2019, 33, 3171–3175. [Google Scholar] [CrossRef]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.). Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Lacueva, C.; Medina-Remon, A.; Llorach, H.; Urpi-Sarda, R.; Khan, M.; Chiva-Blanch, N.; Zamora-Ros, G.; Rotches-Ribalta, R.; Lamuela-Raventòs, R.M. Phenolic Compounds: Chemistry and Occurrence in Fruits and Vegetables. In Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability; De la Rosa, L., Alvarez-Parrilla, E., Gonzalez-Aguilar, G.A., Eds.; John Wiley and Sons: London, UK, 2010; pp. 53–88. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Pascual-Teresa, S.; Moreno, D.A.; Garcìa-Viguera, C. Flavanols and Anthocyanins in Cardiovascular Health: A Review of Current Evidence. Int. J. Mol. Sci. 2010, 11, 1679–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Giovanelli, G.; Brenna, O.V. Evolution of some phenolic components, carotenoids and chlorophylls during ripening of three Italian grape varieties. Eur. Food Res. Technol. 2007, 225, 145–150. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compost. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Rutz, J.K.; Zambiazi, R.C.; Borges, C.D.; Krumreich, F.D.; Da Luz, S.R.; Hartwig, N.; Da Rosa, C.G. Microencapsulation of purple Brazilian cherry juice in xanthan, tara gums and xanthan-tara hydrogel matrixes. Carbohydr. Polym. 2013, 98, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Kurisawa, M.; Chung, J.E.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and antioxidant properties of poly(rutin). Biomacromolecules 2003, 4, 1394–1399. [Google Scholar] [CrossRef]
- Toti, U.S.; Aminabhavi, T.M. Synthesis and characterization of polyacrylamidegrafted sodium alginate membranes for pervaporation separation of water + isopropanol mixtures. J. Appl. Polym. Sci. 2004, 92, 2030–2037. [Google Scholar] [CrossRef]
- Chouana, T.; Pierre, G.; Vial, C.; Gardarin, C.; Wadouachi, A.; Cailleu, D.; Le Cerf, D.; Boual, Z.; Ould El Hadj, M.D.; Michaud, P.; et al. Structural characterization and rheological properties of a galactomannan from Astragalus gombo Bunge seeds harvested in Algerian Sahara. Carbohydr. Polym. 2017, 175, 387–394. [Google Scholar] [CrossRef]
- Liu, Y.; Nair, M.G. An efficient and economical MTT assay for determining the antioxidant activity of plant natural product extracts and pure compounds. J. Nat. Prod. 2010, 73, 1193–1195. [Google Scholar] [CrossRef]
- Salta, J.; Martins, A.; Santos, R.G.; Neng, N.R.; Nogueira, J.M.F.; Justino, J.; Rauter, A.P. Phenolic composition and antioxidant activity of Rocha pear and other pear cultivars—A comparative study. J. Funct. Foods 2010, 2, 153–157. [Google Scholar] [CrossRef]
- Reiland, H.; Slavin, J. Systematic Review of Pears and Health. Nutr. Today 2015, 50, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Barnes, H.A.; Hutton, J.F.; Walters, K. An Introduction to Rheology; Elsevier Science Publishers: Amsterdam, The Netherlands, 1989; p. 199. [Google Scholar]








| Compound | RT (min) | LOMW (μg mL−1) |
|---|---|---|
| Verbascoside residue | 1.475 | 0.71 ± 0.06 |
| 3,4-dihydroxyphenylglycol | 1.515 | 0.185 ± 0.017 |
| Quinic acid | 1.773 | 4.1±0.4 |
| 3-hydroxytyrosol glucoside isomers 1 | 2.245 | 4.3 ± 0.4 |
| 3-hydroxytyrosol glucoside isomers 2 | 2.470 | 2.9 ± 0.3 |
| Dimer 407 | 2.610 | 1.56 ± 0.14 |
| 3-hydroxytyrosol | 2.957 | 0.092 ± 0.008 |
| Decarboxymethyl-elenolic acid derivative | 3.542 | 1.55 ± 0.14 |
| Hydroxylated product of dialdhydic form of decarboxymethyl elenolic acid | 4.428 | 3.3 ± 0.3 |
| Caffeic acid | 7.996 | 1.91 ± 0.17 |
| Decarboxymethyl-elenolic acid (HyEDA) | 8.075 | 0.27 ± 0.02 |
| Oleuropein aglycone derivative | 8.124 | 5.8 ± 0.5 |
| p-coumaric acid | 10.798 | 2.13 ± 0.19 |
| Compound | Assignment | Chemical Shift (ppm) |
|---|---|---|
| Verbascoside | OH-18; OH-17 | s 9.21; 4.61 |
| Verbascoside | O-CH-O | d 5.23 |
| Sugar residue of glycosides | CH-OH | m 4.17–3.12 |
| Hydroxythyrosol | Ph-CH2- | 2.73 |
| Hydroxythyrosol | CH2-O | 3.76 |
| Oleuropein | CH3 | s 3.86 |
| Oleuropein | Enantiotopic CH2-OH | m 3.42 |
| Caffeic acid | CH2=CH2 | dd 6.05–7.15 |
| Quinic acid | Enantiotopic CH2-CH | 2.27–1.86 |
| Sample | APG (mg CT/g) | PAC (mg CT/g) | FC (mg CT/g) | AC (mg CT/g) | TAC (mg CT/g) | IC50 (mg mL−1) | |
|---|---|---|---|---|---|---|---|
| DPPH Radical | ABTS Radical | ||||||
| LOMW | 75.0 ± 0.7 | 50.8 ± 0.4 | 34.0 ± 0.4 | 0.15 ± 0.01 | 1.10 ± 0.05 | 0.095 ± 0.003 | 0.019 ± 0.001 |
| Sample | T Center Peak 1 (°C) | T Center Peak 2 (°C) | Enthalpy Peak 1 (J g−1) | Enthalpy Peak 2 (J g−1) |
|---|---|---|---|---|
| PLOMW | 141.0 | 311.8 | 125.4 | −154.3 |
| BTG | 137.7 | 291.4 | 76.0 | −11.6 |
| CTG | 100.4 | 302.6 | 261.4 | −83.4 |
| Sample | APG (mg CT/g) | PAC (mg CT/g) | TAC (mg CT/g) | IC50 (mg mL−1) | |
|---|---|---|---|---|---|
| DPPH Radical | ABTS Radical | ||||
| PLOMW | 16.3 ± 0.5 | 15.7 ± 0.4 | 2.26 ± 0.11 | 0.322 ± 0.005 | 0.106 ± 0.005 |
| BTG | - | - | - | - | - |
| Time (Days) | Pudding | APG (mg CT/g Pudding) | PAC (mg CT/g Budino) | IC50 (mg mL−1) |
|---|---|---|---|---|
| ABTS Radical | ||||
| 0 | PPLOMW | 0.220 ± 0.009 a | 0.261 ± 0.011 a | 0.0060 ± 0.0003 a |
| PCTG | 0.113 ± 0.005 b | 0.244 ± 0.009 b | 0.0324 ± 0.0011 b | |
| 7 | PPLOMW | 0.193 ± 0.007 c | 0.136 ± 0.005 c | 0.0082 ± 0.0003 c |
| PCTG | 0.101 ± 0.004 d | 0.070 ± 0.002 d | 0.0345 ± 0.0015 b | |
| 14 | PPLOMW | 0.194 ± 0.006 c | 0.075 ± 0.002 e | 0.0118 ± 0.0005 d |
| PCTG | 0.089 ± 0.003 d | 0.056 ± 0.002 f | 0.0397 ± 0.0018 e | |
| 28 | PPLOMW | 0.196 ± 0.006 c | 0.062 ± 0.002 g | 0.0124 ± 0.0006 d |
| PCTG | 0.080 ± 0.002 e | 0.042 ± 0.001 h | 0.0453 ± 0.0021 f |
| Sample | A ± 100 | z ± 1 |
|---|---|---|
| 5 °C PCGT (t = 28 days) | 8800 | 18 |
| 5 °C PCGT (t = 14 days) | 8200 | 12 |
| 5 °C PCGT (t = 7 days) | 5600 | 11 |
| 5 °C PCGT (t = 0 days) | 2800 | 11 |
| 25 °C PCGT (t = 28 days) | 7200 | 45 |
| 25 °C PCGT (t = 14 days) | 7300 | 12 |
| 25 °C PCGT (t = 7 days) | 4800 | 11 |
| 25 °C PCGT (t = 0 days) | 2000 | 11 |
| 5 °C PPLOMW (t = 28 days) | 9600 | 18 |
| 5 °C PPLOMW (t = 14 days) | 8900 | 10 |
| 5 °C PPLOMW (t = 7 days) | 5100 | 11 |
| 5 °C PPLOMW (t = 0 days) | 3400 | 14 |
| 25 °C PPLOMW (t = 28 days) | 8200 | 32 |
| 25 °C PPLOMW (t = 14 days) | 7200 | 12 |
| 25 °C PPLOMW (t = 7 days) | 4400 | 12 |
| 25 °C PPLOMW (t = 0 days) | 1100 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spizzirri, U.G.; Caputo, P.; Oliviero Rossi, C.; Crupi, P.; Muraglia, M.; Rago, V.; Malivindi, R.; Clodoveo, M.L.; Restuccia, D.; Aiello, F. A Tara Gum/Olive Mill Wastewaters Phytochemicals Conjugate as a New Ingredient for the Formulation of an Antioxidant-Enriched Pudding. Foods 2022, 11, 158. https://doi.org/10.3390/foods11020158
Spizzirri UG, Caputo P, Oliviero Rossi C, Crupi P, Muraglia M, Rago V, Malivindi R, Clodoveo ML, Restuccia D, Aiello F. A Tara Gum/Olive Mill Wastewaters Phytochemicals Conjugate as a New Ingredient for the Formulation of an Antioxidant-Enriched Pudding. Foods. 2022; 11(2):158. https://doi.org/10.3390/foods11020158
Chicago/Turabian StyleSpizzirri, Umile Gianfranco, Paolino Caputo, Cesare Oliviero Rossi, Pasquale Crupi, Marilena Muraglia, Vittoria Rago, Rocco Malivindi, Maria Lisa Clodoveo, Donatella Restuccia, and Francesca Aiello. 2022. "A Tara Gum/Olive Mill Wastewaters Phytochemicals Conjugate as a New Ingredient for the Formulation of an Antioxidant-Enriched Pudding" Foods 11, no. 2: 158. https://doi.org/10.3390/foods11020158
APA StyleSpizzirri, U. G., Caputo, P., Oliviero Rossi, C., Crupi, P., Muraglia, M., Rago, V., Malivindi, R., Clodoveo, M. L., Restuccia, D., & Aiello, F. (2022). A Tara Gum/Olive Mill Wastewaters Phytochemicals Conjugate as a New Ingredient for the Formulation of an Antioxidant-Enriched Pudding. Foods, 11(2), 158. https://doi.org/10.3390/foods11020158