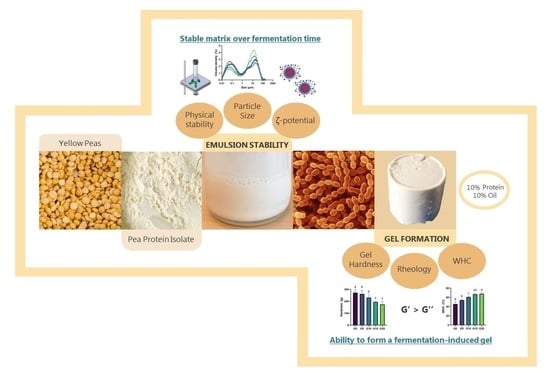
Design of a Functional Pea Protein Matrix for Fermented Plant-Based Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Pea Matrices
2.2.2. Physical Stability
2.2.3. Particle Size and -Potential Measurements
2.2.4. Fermentation of Pea Matrices
2.2.5. Textural Characterization
Texture Analysis
Rheological Measurements
2.2.6. Gel Structure Visualization
2.2.7. Water-Holding Capacity
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. Matrix Formulation
3.2. Physical Stability of PPI Emulsions
3.3. Oil Droplet Aggregation
3.4. Electrostatic Interactions between Proteins
3.5. Gel Formation during Fermentation
3.6. Oscillation Measurements of Fermented PPI Gels
3.7. Effect of Oil on Water-Holding Capacity of Fermented PPI Gels
3.8. Protein Network Visualization in Fermented PPI Gels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPI | Pea Protein Isolate |
| WHC | Water-Holding Capacity |
| CLSM | Confocal Laser Scan Microscopy |
References
- Burger, T.G.; Zhang, Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- McClements, D.J. Development of Next-Generation Nutritionally fortified Plant-Based Milk Substitutes: Structural Design Principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef] [Green Version]
- Floury, J.; Desrumaux, A.; Lardières, J. Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions. Innov. Food Sci. Emerg. Technol. 2000, 1, 127–134. [Google Scholar] [CrossRef]
- Ben-Harb, S.; Panouillé, M.; Huc-Mathis, D.; Moulin, G.; Saint-Eve, A.; Irlinger, F.; Bonnarme, P.; Michon, C.; Souchon, I. The rheological and microstructural properties of pea, milk, mixed pea/milk gels and gelled emulsions designed by thermal, acid, and enzyme treatments. Food Hydrocoll. 2018, 77, 75–84. [Google Scholar] [CrossRef]
- Pelgrom, P.J.; Vissers, A.M.; Boom, R.M.; Schutyser, M.A. Dry fractionation for production of functional pea protein concentrates. Food Res. Int. 2013, 53, 232–239. [Google Scholar] [CrossRef]
- Moreno, H.M.; Domínguez-Timón, F.; Díaz, M.T.; Pedrosa, M.M.; Borderías, A.J.; Tovar, C.A. Evaluation of gels made with different commercial pea protein isolate: Rheological, structural and functional properties. Food Hydrocoll. 2020, 99, 105375. [Google Scholar] [CrossRef]
- Kornet, C.; Venema, P.; Nijsse, J.; van der Linden, E.; van der Goot, A.J.; Meinders, M. Yellow pea aqueous fractionation increases the specific volume fraction and viscosity of its dispersions. Food Hydrocoll. 2020, 99, 105332. [Google Scholar] [CrossRef]
- Kornet, R.; Veenemans, J.; Venema, P.; van der Goot, A.J.; Meinders, M.; Sagis, L.; van der Linden, E. Less is more: Limited fractionation yields stronger gels for pea proteins. Food Hydrocoll. 2021, 112, 106285. [Google Scholar] [CrossRef]
- Lam, A.C.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Klost, M.S. Fermentation-Induced Gelation of Pea Protein: Molecular Interactions and Rheological Properties. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2021. [Google Scholar]
- O’Kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; Van Boekel, M.A. Characterization of Pea Vicilin. 2. Consequences of Compositional Heterogeneity on Heat-Induced Gelation Behavior. J. Agric. Food Chem. 2004, 52, 3149–3154. [Google Scholar] [CrossRef]
- O’Kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; Van Boekel, M.A. Heat-induced gelation of pea legumin: Comparison with soybean glycinin. J. Agric. Food Chem. 2004, 52, 5071–5078. [Google Scholar] [CrossRef]
- Sun, X.D.; Arntfield, S.D. Molecular forces involved in heat-induced pea protein gelation: Effects of various reagents on the rheological properties of salt-extracted pea protein gels. Food Hydrocoll. 2012, 28, 325–332. [Google Scholar] [CrossRef]
- Sim, S.Y.; Karwe, M.V.; Moraru, C.I. High pressure structuring of pea protein concentrates. J. Food Process. Eng. 2019, 42, e13261. [Google Scholar] [CrossRef]
- Mession, J.L.; Chihi, M.L.; Sok, N.; Saurel, R. Effect of globular pea proteins fractionation on their heat-induced aggregation and acid cold-set gelation. Food Hydrocoll. 2014, 46, 233–243. [Google Scholar] [CrossRef]
- Yousseef, M.; Lafarge, C.; Valentin, D.; Lubbers, S.; Husson, F. Fermentation of cow milk and/or pea milk mixtures by different starter cultures: Physico-chemical and sensorial properties. LWT-Food Sci. Technol. 2016, 69, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Agrawal, U.S.; Sharma, A.K.; Sarkar, B.C.; Sharma, H.K. Optimization of process parameters for the development of a cheese analogue from pigeon pea (Cajanus cajan) and soy milk using response surface methodology. Int. J. Dairy Technol. 2005, 58, 51–58. [Google Scholar] [CrossRef]
- Ben-Harb, S.; Saint-Eve, A.; Panouillé, M.; Souchon, I.; Bonnarme, P.; Dugat-Bony, E.; Irlinger, F. Design of microbial consortia for the fermentation of pea-protein-enriched emulsions. Int. J. Food Microbiol. 2019, 293, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Klost, M.; Giménez-Ribes, G.; Drusch, S. Enzymatic hydrolysis of pea protein: Interactions and protein fractions involved in fermentation induced gels and their influence on rheological properties. Food Hydrocoll. 2020, 105, 105793. [Google Scholar] [CrossRef]
- Klost, M.; Drusch, S. Structure formation and rheological properties of pea protein-based gels. Food Hydrocoll. 2019, 94, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Youssef, C.E.; Bonnarme, P.; Fraud, S.; Péron, A.C.; Helinck, S.; Landaud, S. Sensory improvement of a pea protein-based product using microbial co-cultures of lactic acid bacteria and yeasts. Foods 2020, 9, 349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Q.; Biviano, M.; Mettu, S.; Zhou, M.; Dagastine, R.; Ashokkumar, M. Modification of pea protein isolate for ultrasonic encapsulation of functional liquids. RSC Adv. 2016, 6, 106130–106140. [Google Scholar] [CrossRef]
- Paseiro-Losada, P.; López-Fabal, M.F.; Pérez-Lamela, C.; Sanmartín-Fenollera, P.; Paz-Abuín, S. Two Rp-Hplc Methods To Quantify and Identify Bisphenol a Diglycidyl Ether (Badge): European Union Fatty Food Simulant (Olive Oil). Cienc. Tecnol. Aliment. 1999, 2, 202–209. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.B.; Panigrahi, S.; Sarmah, A.K.; Dubey, B.K. Mixture design applied for formulation and characterization of vegetal-based fermented products. Sci. Total Environ. 2021, 146, 135907. [Google Scholar] [CrossRef]
- Munialo, C.D.; van der Linden, E.; Ako, K.; de Jongh, H.H. Quantitative analysis of the network structure that underlines the transitioning in mechanical responses of pea protein gels. Food Hydrocoll. 2015, 49, 104–117. [Google Scholar] [CrossRef]
- Hong, X.; Zhao, Q.; Liu, Y.; Li, J. Recent advances on food-grade water-in-oil emulsions: Instability mechanism, fabrication, characterization, application, and research trends. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. The Rheology of Emulsion-Based Food Products; Woodhead Publishing Limited: Sawston, UK, 2003; Volume 1, pp. 3–35. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Kennedy, D.; Hogan, S.A.; Kelly, P.M.; Thapa, K.; Murphy, K.M.; Fenelon, M.A. Emulsification properties of pea protein isolate using homogenization, microfluidization and ultrasonication. Food Res. Int. 2016, 89, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Franks, G.V.; Hosken, R.W. Particle sizes and stability of UHT bovine, cereal and grain milks. Food Hydrocoll. 2003, 17, 671–678. [Google Scholar] [CrossRef]
- Klost, M.; Brzeski, C.; Drusch, S. Effect of protein aggregation on rheological properties of pea protein gels. Food Hydrocoll. 2020, 108, 106036. [Google Scholar] [CrossRef]
- Piorkowski, D.T.; McClements, D.J. Beverage emulsions: Recent developments in formulation, production, and applications. Food Hydrocoll. 2014, 42, 5–41. [Google Scholar] [CrossRef]
- Danielsson, C.E. An Electrophoretic Investigation of Vicilin and Legumin from Seeds of Peas. Acta Chem. Scand. 1950, 4, 762–771. [Google Scholar] [CrossRef]
- Nicolai, T.; Durand, D. Controlled food protein aggregation for new functionality. Curr. Opin. Colloid Interface Sci. 2013, 18, 249–256. [Google Scholar] [CrossRef]
- Sim, S.Y.J.; Hua, X.Y.; Henry, C.J. A Novel Approach to Structure Plant-Based Yogurts Using High Pressure Processing. Foods 2020, 9, 1126. [Google Scholar] [CrossRef] [PubMed]
- Farjami, T.; Madadlou, A. An overview on preparation of emulsion-filled gels and emulsion particulate gels. Trends Food Sci. Technol. 2019, 86, 85–94. [Google Scholar] [CrossRef]
- Ramel, P.R.; Marangoni, A.G. Engineering the rheological and thermomechanical properties of model imitation cheese using particle fillers. J. Food Eng. 2018, 235, 9–15. [Google Scholar] [CrossRef]
- Sala, G. Food Gels Filled with Emulsion Droplets Linking Large Deformation Properties to Sensory Perception. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2007; p. 235. [Google Scholar]
- Buldo, P.; Benfeldt, C.; Marie, D.; Bak, H.; Manuel, J.; Sieuwerts, S.; Thygesen, K.; Berg, F.V.D.; Ipsen, R. The role of exopolysaccharide-producing cultures and whey protein ingredients in yoghurt. LWT-Food Sci. Technol. 2016, 72, 189–198. [Google Scholar] [CrossRef]
- Urbonaite, V. Water Holding of Protein Gels. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2015. [Google Scholar]
- Wi, G.; Bae, J.; Kim, H.; Cho, Y.; Choi, M.J. Evaluation of the physicochemical and structural properties and the sensory characteristics of meat analogues prepared with various non-animal based liquid additives. Foods 2020, 9, 461. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Fei, L.; Zhuang, T.; Lei, S.; Ge, Q.; Yu, H.; Wang, J.; Wang, Y. Rheology and microstructure of myofibrillar protein–olive oil composite gels: Effect of different non-meat protein as emulsifier. J. Sci. Food Agric. 2018, 98, 799–806. [Google Scholar] [CrossRef]









| Time (h) | E0 | E5 | E10 | E15 | E20 |
|---|---|---|---|---|---|
| 0 | −33.4 ± 1.3 | −32.4 ± 1.3 | −32.7 ± 2.2 | −33.3 ± 0.7 | −33.1 ± 1.2 |
| 7 | −36.4 ± 0.8 * | −32.6 ± 0.9 | −33.8 ± 1.5 | −33.1 ± 1.2 | −32.6 ± 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masiá, C.; Jensen, P.E.; Petersen, I.L.; Buldo, P. Design of a Functional Pea Protein Matrix for Fermented Plant-Based Cheese. Foods 2022, 11, 178. https://doi.org/10.3390/foods11020178
Masiá C, Jensen PE, Petersen IL, Buldo P. Design of a Functional Pea Protein Matrix for Fermented Plant-Based Cheese. Foods. 2022; 11(2):178. https://doi.org/10.3390/foods11020178
Chicago/Turabian StyleMasiá, Carmen, Poul Erik Jensen, Iben Lykke Petersen, and Patrizia Buldo. 2022. "Design of a Functional Pea Protein Matrix for Fermented Plant-Based Cheese" Foods 11, no. 2: 178. https://doi.org/10.3390/foods11020178
APA StyleMasiá, C., Jensen, P. E., Petersen, I. L., & Buldo, P. (2022). Design of a Functional Pea Protein Matrix for Fermented Plant-Based Cheese. Foods, 11(2), 178. https://doi.org/10.3390/foods11020178