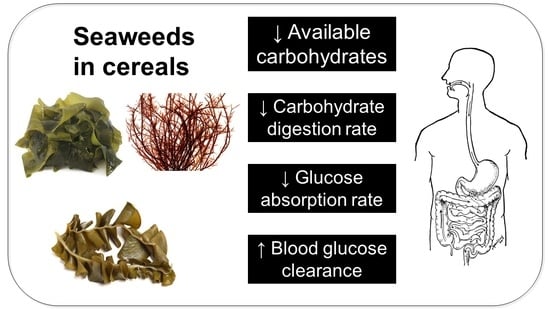
Seaweeds as Ingredients to Lower Glycemic Potency of Cereal Foods Synergistically—A Perspective
Abstract
:1. Introduction
2. Reducing the Glycemic Potency of Cereal Foods: A Diet Challenge
3. Seaweed: The Unconventional Potential of the Traditional Food Ingredients in Lowering the Glycemic Impact
3.1. Seaweed: A Traditional Food Ingredients in Modern Days
3.2. Carbohydrates: Dietary Fiber and Polysaccharides
3.2.1. Alginate
3.2.2. Fucoidan
3.2.3. Other Dietary Fibers and Polysaccharides
3.3. Protein
3.4. Lipids
3.5. Polyphenols
3.6. Carotenoids
4. Proposed Strategies for Seaweeds-Derived Functional Ingredients in Glycemic Control
4.1. Reducing the Available Carbohydrate
4.2. Reducing Post-Consumption Digestion Rate
4.3. Reduce the Rate of Post-Digestion Glucose Absorption
4.4. Increase the Rate of Postprandial Glucose Clearance
4.5. Future Perspective in Clinical Evidence and Application
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Diabetes Federation. IDF Atlas 10th Edition. 2021. Available online: https://idf.org/e-library/epidemiology-research/diabetes-atlas.html?id=171 (accessed on 8 February 2022).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard Overview of Current Cases. Available online: https://covid19.who.int/ (accessed on 23 January 2022).
- Holman, N.; Knighton, P.; Kar, P.; O’Keefe, J.; Curley, M.; Weaver, A.; Barron, E.; Bakhai, C.; Khunti, K.; Wareham, N.J.; et al. Risk Factors for COVID-19-Related Mortality in People with Type 1 and Type 2 Diabetes in England: A Population-Based Cohort Study. Lancet Diabetes Endocrinol. 2020, 8, 823–833. [Google Scholar] [CrossRef]
- Singh, A.K.; Gillies, C.L.; Singh, R.; Singh, A.; Chudasama, Y.; Coles, B.; Seidu, S.; Zaccardi, F.; Davies, M.J.; Khunti, K. Prevalence of Co-Morbidities and Their Association with Mortality in Patients with COVID-19: A Systematic Review and Meta-Analysis. Diabetes Obes. Metab. 2020, 22, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, J.; Sun, X.; Wang, L.; Xu, Y.; Zhang, Y.; Liu, X.; Dong, C. Influence of Diabetes Mellitus on the Severity and Fatality of SARS-CoV-2 (COVID-19) Infection. Diabetes Obes. Metab. 2020, 22, 1907–1914. [Google Scholar] [CrossRef]
- Dennis, J.M.; Mateen, B.A.; Sonabend, R.; Thomas, N.J.; Patel, K.A.; Hattersley, A.T.; Denaxas, S.; McGovern, A.P.; Vollmer, S.J. Type 2 Diabetes and COVID-19-Related Mortality in the Critical Care Setting: A National Cohort Study in England, March-July 2020. Diabetes Care 2021, 44, 50–57. [Google Scholar] [CrossRef]
- Hayek, S.; Ben-Shlomo, Y.; Balicer, R.; Byrne, K.; Katz, M.; Kepten, E.; Raz, I.; Roitman, E.; Zychma, M.; Barda, N. Preinfection Glycaemic Control and Disease Severity among Patients with Type 2 Diabetes and COVID-19: A Retrospective, Cohort Study. Diabetes Obes. Metab. 2021, 23, 1995–2000. [Google Scholar] [CrossRef]
- Tan, W.S.K.; Tan, S.-Y.; Henry, C.J. Ethnic Variability in Glycemic Response to Sucrose and Isomaltulose. Nutrients 2017, 9, 347. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, M.; Venn, B.J.; Williams, S.M.; Te Morenga, L.A.; Heemels, I.M.; Mann, J.I. Glycaemic Responses to Glucose and Rice in People of Chinese and European Ethnicity. Diabet. Med. 2013, 30, e101–e107. [Google Scholar] [CrossRef]
- Dickinson, S.; Colagiuri, S.; Faramus, E.; Petocz, P.; Brand-Miller, J.C. Postprandial Hyperglycemia and Insulin Sensitivity Differ among Lean Young Adults of Different Ethnicities. J. Nutr. 2002, 132, 2574–2579. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic Index of Foods: A Physiological Basis for Carbohydrate Exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic Index, Glycemic Load and Glycemic Response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations FAOSTAT Statistical Database. Available online: https://www.fao.org/faostat/en/#data/FBS (accessed on 30 January 2022).
- Cui, Z.; Dibley, M.J. Trends in Dietary Energy, Fat, Carbohydrate and Protein Intake in Chinese Children and Adolescents from 1991 to 2009. Br. J. Nutr. 2012, 108, 1292–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seah, J.Y.H.; Koh, W.-P.; Yuan, J.-M.; Van Dam, R.M. Rice Intake and Risk of Type 2 Diabetes: The Singapore Chinese Health Study. Eur. J. Nutr. 2019, 58, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Zuñiga, Y.L.M.; Rebello, S.A.; Oi, P.L.; Zheng, H.; Lee, J.; Tai, E.S.; Van Dam, R.M. Rice and Noodle Consumption Is Associated with Insulin Resistance and Hyperglycaemia in an Asian Population. Br. J. Nutr. 2014, 111, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Spiegelman, D.; Van Dam, R.M.; Holmes, M.D.; Malik, V.S.; Willett, W.C.; Hu, F.B. White Rice, Brown Rice, and Risk of Type 2 Diabetes in US Men and Women. Arch. Intern. Med. 2010, 170, 961–969. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Romundstad, P.; Vatten, L.J. Whole Grain and Refined Grain Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-Analysis of Cohort Studies. Eur. J. Epidemiol. 2013, 28, 845–858. [Google Scholar] [CrossRef]
- Lu, L.W.; Monro, J.; Lu, J.; Rush, E. The Effect of Cold Treatment of Parboiled Rice with Lowered Glycaemic Potency on Consumer Liking and Acceptability. Foods 2018, 7, 207. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Ursin, G.; Veierød, M.B. Meat Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Diabetologia 2009, 52, 2277–2287. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Z.; Du, W.; Huang, F.; Jiang, H.; Bai, J.; Zhang, X.; Zhang, B.; Wang, H. Twenty-Five-Year Trends in Dietary Patterns among Chinese Adults from 1991 to 2015. Nutrients 2021, 13, 1327. [Google Scholar] [CrossRef]
- Batis, C.; Sotres-Alvarez, D.; Gordon-Larsen, P.; Mendez, M.A.; Adair, L.; Popkin, B. Longitudinal Analysis of Dietary Patterns in Chinese Adults from 1991 to 2009. Br. J. Nutr. 2014, 111, 1441–1451. [Google Scholar] [CrossRef] [Green Version]
- Wee, M.S.M.; Henry, C.J. Reducing the Glycemic Impact of Carbohydrates on Foods and Meals: Strategies for the Food Industry and Consumers with Special Focus on Asia. Compr. Rev. Food Sci. Food Saf. 2020, 19, 670–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajapakse, N.; Kim, S.-K. Nutritional and Digestive Health Benefits of Seaweed. Adv. Food Nutr. Res. 2011, 64, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive Compounds in Seaweed: Functional Food Applications and Legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef] [PubMed]
- Afonso, N.C.; Catarino, M.D.; Silva, A.; Cardoso, S.M. Brown Macroalgae as Valuable Food Ingredients. Antioxidants 2019, 8, 365. [Google Scholar] [CrossRef] [Green Version]
- Shannon, E.; Abu-Ghannam, N. Seaweeds as Nutraceuticals for Health and Nutrition. Phycologia 2019, 58, 563–577. [Google Scholar] [CrossRef] [Green Version]
- Tak, M.; Shankar, B.; Kadiyala, S. Dietary Transition in India: Temporal and Regional Trends, 1993 to 2012. Food Nutr. Bull. 2019, 40, 254–270. [Google Scholar] [CrossRef]
- Poutanen, K. Past and Future of Cereal Grains as Food for Health. Trends Food Sci. Technol. 2012, 25, 58–62. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.; Liu, S. Is There a Dose-Response Relation of Dietary Glycemic Load to Risk of Type 2 Diabetes? Meta-Analysis of Prospective Cohort Studies. Am. J. Clin. Nutr. 2013, 97, 584–596. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Ranawana, D.V.; Leow, M.K.-S.; Henry, C.J. Effect of Chicken, Fat and Vegetable on Glycaemia and Insulinaemia to a White Rice-Based Meal in Healthy Adults. Eur. J. Nutr. 2014, 53, 1719–1726. [Google Scholar] [CrossRef]
- Kongkachuichai, R.; Charoensiri, R.; Meekhruerod, A.; Kettawan, A. Effect of Processing Conditions on Bioactive Compounds and Glycemic Index of the Selected Landrace Rice Variety in Pre-Diabetes. J. Cereal Sci. 2020, 94, 102994. [Google Scholar] [CrossRef]
- Se, C.-H.; Chuah, K.-A.; Mishra, A.; Wickneswari, R.; Karupaiah, T. Evaluating Crossbred Red Rice Variants for Postprandial Glucometabolic Responses: A Comparison with Commercial Varieties. Nutrients 2016, 8, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shobana, S.; Kokila, A.; Lakshmipriya, N.; Subhashini, S.; Ramya Bai, M.; Mohan, V.; Malleshi, N.G.; Anjana, R.M.; Henry, C.J.K.; Sudha, V. Glycaemic Index of Three Indian Rice Varieties. Int. J. Food Sci. Nutr. 2012, 63, 178–183. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Sun, F.-H.; Wong, S.H.-S.; Huang, Y.-J. Glycemic Index and Glycemic Load of Selected Chinese Traditional Foods. World J. Gastroenterol. 2010, 16, 1512–1517. [Google Scholar] [CrossRef]
- Nicholas, D.; Hazila, K.K.; Chua, H.P.; Rosniyana, A. Nutritional Value and Glycemic Index of Bario Rice Varieties. J. Trop. Agric. Food Sci. 2014, 42, 1–8. [Google Scholar]
- Yang, C.-H.; Chang, C.-W.; Lin, J. White Rice Glycemic Index Measured in Venous and Capillary Blood Samples. Food Sci. Technol. Res. 2017, 23, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Prasad, M.P.R.; Rao, B.D.; Kalpana, K.; Rao, M.V.; Patil, J.V. Glycaemic Index and Glycaemic Load of Sorghum Products. J. Sci. Food Agric. 2015, 95, 1626–1630. [Google Scholar] [CrossRef]
- RamyaBai, M.; Wedick, N.M.; Shanmugam, S.; Arumugam, K.; Nagarajan, L.; Vasudevan, K.; Gunasekaran, G.; Rajagopal, G.; Spiegelman, D.; Malik, V.; et al. Glycemic Index and Microstructure Evaluation of Four Cereal Grain Foods. J. Food Sci. 2019, 84, 3373–3382. [Google Scholar] [CrossRef]
- Ren, X.; Chen, J.; Molla, M.M.; Wang, C.; Diao, X.; Shen, Q. In Vitro Starch Digestibility and in Vivo Glycemic Response of Foxtail Millet and Its Products. Food Funct. 2016, 7, 372–379. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Kim, Y.; Lim, H. Glycaemic Indices and Glycaemic Loads of Common Korean Carbohydrate-Rich Foods. Br. J. Nutr. 2019, 121, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Lok, K.Y.; Chan, R.; Chan, D.; Li, L.; Leung, G.; Woo, J.; Lightowler, H.J.; Henry, C.J.K. Glycaemic Index and Glycaemic Load Values of a Selection of Popular Foods Consumed in Hong Kong. Br. J. Nutr. 2010, 103, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.S.K.; Tan, W.J.K.; Ponnalagu, S.D.O.; Koecher, K.; Menon, R.; Tan, S.-Y.; Henry, C.J. The Glycaemic Index and Insulinaemic Index of Commercially Available Breakfast and Snack Foods in an Asian Population. Br. J. Nutr. 2018, 119, 1151–1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soong, Y.Y.; Quek, R.Y.C.; Henry, C.J. Glycemic Potency of Muffins Made with Wheat, Rice, Corn, Oat and Barley Flours: A Comparative Study between in Vivo and in Vitro. Eur. J. Nutr. 2015, 54, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wei Jie Tan, K.; Jeyakumar Henry, C. Co-Ingestion of Essence of Chicken to Moderate Glycaemic Response of Bread. Int. J. Food Sci. Nutr. 2015, 66, 931–935. [Google Scholar] [CrossRef]
- Hettiaratchi, U.P.K.; Ekanayake, S.; Welihinda, J. Glycaemic Indices of Three Sri Lankan Wheat Bread Varieties and a Bread-Lentil Meal. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. S4), 21–30. [Google Scholar] [CrossRef]
- Santhi Sirisha, K.; Vijaya Lakshmi, V. Estimation of Glycemic Index of Ragi Recipes Incorporated with Curry Leaf Powder. Int. J. Recent. Adv. Multidiscip. Res. 2016, 3, 1936–1939. [Google Scholar]
- Nurjanah, N.; Mahani; Muchtadi, D.; Palupi, N.S.; Widowati, S. Chemical Characteristics and Glycemic Index of Processed Products from Corn Starch Modified with Green Tea Polyphenols. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 012029. [Google Scholar] [CrossRef]
- Molnar, C.; Gair, J. Concepts of Biology—1st Canadian Edition; BCcampus: Victoria, BC, Canada, 2015; ISBN 978-1-989623-99-2. [Google Scholar]
- Pereira, L. Edible Seaweeds of the World; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-3050-1. [Google Scholar]
- Chan, K.; Chen, S.; Chen, P. Astaxanthin Attenuated Thrombotic Risk Factors in Type 2 Diabetic Patients. J. Funct. Foods 2019, 53, 22–27. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Ferdouse, F.; Holdt, S.L.; Smith, R.; Murúa, P.; Yang, Z. The Global Status of Seaweed Production, Trade and Utilization. GLOBEFISH Res. Programme 2018, 124, 7. [Google Scholar]
- Cai, J.; Lovatelli, A.; Aguilar-Manjarrez, J.; Cornish, L.; Dabbadie, L.; Desrochers, A.; Diffey, S.; Garrido Gamarro, E.; Geehan, J.; Hurtado, A.; et al. Seaweeds and Microalgae: An Overview for Unlocking Their Potential in Global Aquaculture Development. FAO Fish. Aquac. Circ. 2021. [Google Scholar] [CrossRef]
- O’Connor, K. Seaweed: A Global History; Reaktion Books: London, UK, 2017; ISBN 978-1-78023-799-2. [Google Scholar]
- Pérez-Lloréns, J.L.; Mouritsen, O.G.; Rhatigan, P.; Cornish, M.L.; Critchley, A.T. Seaweeds in Mythology, Folklore, Poetry, and Life. J. Appl. Phycol. 2020, 32, 3157–3182. [Google Scholar] [CrossRef]
- Fukuda, S.; Saito, H.; Nakaji, S.; Yamada, M.; Ebine, N.; Tsushima, E.; Oka, E.; Kumeta, K.; Tsukamoto, T.; Tokunaga, S. Pattern of Dietary Fiber Intake among the Japanese General Population. Eur. J. Clin. Nutr. 2007, 61, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Ju, S.-Y. Asthma and Dietary Intake of Fish, Seaweeds, and Fatty Acids in Korean Adults. Nutrients 2019, 11, 2187. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, V.; Farfán, M.; Aguilera, J.M. Seaweeds as Novel Foods and Source of Culinary Flavors. Food Rev. Int. 2021, 1–26. [Google Scholar] [CrossRef]
- Pérez-Lloréns, J.L. Seaweed Consumption in the Americas. Gastronomica 2019, 19, 49–59. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional Foods: Product Development, Technological Trends, Efficacy Testing, and Safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef] [Green Version]
- Mendis, E.; Kim, S.-K. Chapter 1—Present and Future Prospects of Seaweeds in Developing Functional Foods. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Marine Medicinal Foods; Academic Press: Cambridge, MA, USA, 2011; Volume 64, pp. 1–15. [Google Scholar]
- Hayes, M. Chapter 14—Seaweeds: A Nutraceutical and Health Food. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 365–387. ISBN 978-0-12-418697-2. [Google Scholar]
- Vidal, A.; Fallarero, A.; De Andrade-Wartha, E.R.S.; De Oliveira e Silva, A.M.; De Lima, A.; Torres, R.P.; Vuorela, P.; Mancini-Filho, J. Composición química y actividad antioxidante del alga marina roja Bryothamnion triquetrum (S.G.Gmelin) Howe. Rev. Bras. Cienc. Farm. 2006, 42, 589–600. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, S.-K. Chapter 2—Nutritional and Digestive Health Benefits of Seaweed. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Marine Medicinal Foods; Academic Press: San Diego, CA, USA, 2011; Volume 64, pp. 17–28. [Google Scholar]
- Schiener, P.; Black, K.D.; Stanley, M.S.; Green, D.H. The Seasonal Variation in the Chemical Composition of the Kelp Species Laminaria Digitata, Laminaria Hyperborea, Saccharina Latissima and Alaria Esculenta. J. Appl. Phycol. 2015, 27, 363–373. [Google Scholar] [CrossRef]
- Praiboon, J.; Palakas, S.; Noiraksa, T.; Miyashita, K. Seasonal Variation in Nutritional Composition and Anti-Proliferative Activity of Brown Seaweed, Sargassum Oligocystum. J. Appl. Phycol. 2018, 30, 101–111. [Google Scholar] [CrossRef]
- Nelson, M.M.; Phleger, C.F.; Nichols, P.D. Seasonal Lipid Composition in Macroalgae of the Northeastern Pacific Ocean. Bot. Mar. 2002, 45, 58–65. [Google Scholar] [CrossRef]
- García-Poza, S.; Leandro, A.; Cotas, C.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. The Evolution Road of Seaweed Aquaculture: Cultivation Technologies and the Industry 4.0. Int. J. Environ. Res. Public Health 2020, 17, 6528. [Google Scholar] [CrossRef] [PubMed]
- Pak, N.; Araya, H. Macroalgas Marinas Comestibles de Chile Como Fuente de Fibra Dietética: Efecto en la Digestibilidad Aparente de Proteínas, Fibra y Energía y Peso de Deposiciones en Ratas. Available online: http://www.alanrevista.org/ediciones/ediciones/1996/1/art-9/ (accessed on 4 February 2022).
- Lorenzo, J.M.; Agregán, R.; Munekata, P.E.S.; Franco, D.; Carballo, J.; Şahin, S.; Lacomba, R.; Barba, F.J. Proximate Composition and Nutritional Value of Three Macroalgae: Ascophyllum Nodosum, Fucus vesiculosus and Bifurcaria Bifurcata. Mar. Drugs 2017, 15, E360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holdt, S.L. Nutrient Reduction in Aquaculture Waste by Macroalgae Production; SDU Odense: Odense, Denmark, 2010; Available online: https://orbit.dtu.dk/en/publications/nutrient-reduction-in-aquaculture-waste-by-macroalgae-production (accessed on 2 February 2022).
- Agregán, R.; Munekata, P.E.; Domínguez, R.; Carballo, J.; Franco, D.; Lorenzo, J.M. Proximate Composition, Phenolic Content and in Vitro Antioxidant Activity of Aqueous Extracts of the Seaweeds Ascophyllum Nodosum, Bifurcaria Bifurcata and Fucus vesiculosus. Effect of Addition of the Extracts on the Oxidative Stability of Canola Oil under Accelerated Storage Conditions. Food Res. Int. 2017, 99, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Pinteus, S.; Simões, T.; Horta, A.; Silva, J.; Tecelão, C.; Pedrosa, R. Bifurcaria Bifurcata: A Key Macro-Alga as a Source of Bioactive Compounds and Functional Ingredients. Int. J. Food Sci. Technol. 2016, 51, 1638–1646. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Dietary Fibre and Physicochemical Properties of Several Edible Seaweeds from the Northwestern Spanish Coast. Food Res. Int. 2010, 43, 2289–2294. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary Fiber, Amino Acid, Fatty Acid and Tocopherol Contents of the Edible Seaweeds Ulva Lactuca and Durvillaea Antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Herbreteau, F.; Coiffard, L.J.M.; Derrien, A.; Roeck-Holtzhauer, Y.D. The Fatty Acid Composition of Five Species of Macroalgae. Bot. Mar. 1997, 40, 25–28. [Google Scholar] [CrossRef]
- Díaz-Rubio, M.E.; Pérez-Jiménez, J.; Saura-Calixto, F. Dietary Fiber and Antioxidant Capacity in Fucus vesiculosus Products. Int. J. Food Sci. Nutr. 2009, 60, 23–34. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino Acids, Fatty Acids, and Dietary Fibre in Edible Seaweed Products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Marsham, S.; Scott, G.W.; Tobin, M.L. Comparison of Nutritive Chemistry of a Range of Temperate Seaweeds. Food Chem. 2007, 100, 1331–1336. [Google Scholar] [CrossRef]
- Foti, M.C. Antioxidant Properties of Phenols. J. Pharma. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.J. Bioenergy from Brown Seaweeds; Fakultet for Naturvitenskap og Teknologi: Trondheim, Norway, 2000; ISBN 978-82-7984-134-0. [Google Scholar]
- Wen, X.; Peng, C.; Zhou, H.; Lin, Z.; Lin, G.; Chen, S.; Li, P. Nutritional Composition and Assessment of Gracilaria Lemaneiformis Bory. J. Int. Plant Biol. 2006, 48, 1047–1053. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Nutritional Quality of Some Wild and Cultivated Seaweeds: Nutrient Composition, Total Phenolic Content and in Vitro Digestibility. J. Appl. Phycol. 2016, 28, 3575–3585. [Google Scholar] [CrossRef]
- Jiménez-Escrig, A.; Sánchez-Muniz, F.J. Dietary Fibre from Edible Seaweeds: Chemical Structure, Physicochemical Properties and Effects on Cholesterol Metabolism. Nutr. Res. 2000, 20, 585–598. [Google Scholar] [CrossRef]
- Rupérez, P.; Saura-Calixto, F. Dietary Fibre and Physicochemical Properties of Edible Spanish Seaweeds. Eur. Food Res. Technol. 2001, 212, 349–354. [Google Scholar] [CrossRef]
- Cofrades, S.; López-López, I.; Bravo, L.; Ruiz-Capillas, C.; Bastida, S.; Larrea, M.T.; Jiménez-Colmenero, F. Nutritional and Antioxidant Properties of Different Brown and Red Spanish Edible Seaweeds. Food Sci. Technol. Int. 2010, 16, 361–370. [Google Scholar] [CrossRef]
- Simpson, F.J.; Shacklock, P.F. The Cultivation of Chondrus Crispus. Effect of Temperature on Growth and Carrageenan Production. Bot. Mar. 1979, 22, 295–298. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral Content of Edible Marine Seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Denis, C.; Morançais, M.; Li, M.; Deniaud, E.; Gaudin, P.; Wielgosz-Collin, G.; Barnathan, G.; Jaouen, P.; Fleurence, J. Study of the Chemical Composition of Edible Red Macroalgae Grateloupia Turuturu from Brittany (France). Food Chem. 2010, 119, 913–917. [Google Scholar] [CrossRef]
- Khairy, H.M.; El-Shafay, S.M. Seasonal variations in the biochemical composition of some common seaweed species from the coast of Abu Qir Bay, Alexandria, Egypt. Oceanologia 2013, 55, 435–452. [Google Scholar] [CrossRef] [Green Version]
- Mohy El-Din, S.M.; El-Ahwany, A.M.D. Bioactivity and Phytochemical Constituents of Marine Red Seaweeds (Jania Rubens, Corallina Mediterranea and Pterocladia Capillacea). J. Taibah Univ. Sci. 2016, 10, 471–484. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, J.; Uquiche, E.; Robert, P.; Romero, N.; Quitral, V.; Llantén, C. Functional and Nutritional Value of the Chilean Seaweeds Codium Fragile, Gracilaria Chilensis and Macrocystis Pyrifera. Eur. J. Lipid Sci. Technol. 2009, 111, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient Content of Tropical Edible Seaweeds, Eucheuma Cottonii, Caulerpa Lentillifera and Sargassum Polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Ueng, J.-P.; Tsai, G.-J. Proximate Composition, Total Phenolic Content, and Antioxidant Activity of Seagrape (Caulerpa Lentillifera). J. Food Sci. 2011, 76, C950–C958. [Google Scholar] [CrossRef]
- Peña-Rodríguez, A.; Mawhinney, T.P.; Ricque-Marie, D.; Cruz-Suárez, L.E. Chemical Composition of Cultivated Seaweed Ulva Clathrata (Roth) C. Agardh. Food Chem. 2011, 129, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical Composition and Functional Properties of Ulva Lactuca Seaweed Collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-Proliferative and Potential Anti-Diabetic Effects of Phenolic-Rich Extracts from Edible Marine Algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Brownlee, I.A.; Allen, A.; Pearson, J.P.; Dettmar, P.W.; Havler, M.E.; Atherton, M.R.; Onsøyen, E. Alginate as a Source of Dietary Fiber. Crit. Rev. Food Sci. Nutr. 2005, 45, 497–510. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjåk-Bræk, G.; Smidsrød, O. Alginate Based New Materials. Int. J. Biol. Macromol. 1997, 21, 47–55. [Google Scholar] [CrossRef]
- Hoad, C.L.; Rayment, P.; Spiller, R.C.; Marciani, L.; de Celis Alonso, B.; Traynor, C.; Mela, D.J.; Peters, H.P.F.; Gowland, P.A. In Vivo Imaging of Intragastric Gelation and Its Effect on Satiety in Humans. J. Nutr. 2004, 134, 2293–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solah, V.A.; Kerr, D.A.; Adikara, C.D.; Meng, X.; Binns, C.W.; Zhu, K.; Devine, A.; Prince, R.L. Differences in Satiety Effects of Alginate- and Whey Protein-Based Foods. Appetite 2010, 54, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, S.Y.; Noor Aziah, A.A.; Cheng, L.H. Characteristics of Wheat Dough and Chinese Steamed Bread Added with Sodium Alginates or Konjac Glucomannan. Food Hydrocoll. 2011, 25, 951–957. [Google Scholar] [CrossRef]
- Nishide, E.; Uchida, N. Effects of Ulva Powder on the Ingestion and Excretion of Cholesterol in Rats. In Proceedings of the 17th International Seaweed Symposium, Cape Town, South Africa, 28 January–2 February 2001; Oxford University Press: Oxford, UK, 2003; pp. 165–168. [Google Scholar]
- Kim, H.; Lee, J.-H. Antimicrobial Activities against Methicillin-Resistant Staphylococcus Aureus from Macroalgae. J. Industrial Eng. Chem. 2008, 14, 568–572. [Google Scholar] [CrossRef]
- Murata, M.; Nakazoe, J. Production and Use of Marine AIgae in Japan. Jpn. Agric. Res. Q. 2001, 35, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Torsdottir, I.; Alpsten, M.; Holm, G.; Sandberg, A.-S.; Tölli, J. A Small Dose of Soluble Alginate-Fiber Affects Postprandial Glycemia and Gastric Emptying in Humans with Diabetes. J. Nutr. 1991, 121, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Idota, Y.; Kato, T.; Shiragami, K.; Koike, M.; Yokoyama, A.; Takahashi, H.; Yano, K.; Ogihara, T. Mechanism of Suppression of Blood Glucose Level by Calcium Alginate in Rats. Biol. Pharma. Bull. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idota, Y.; Kogure, Y.; Kato, T.; Yano, K.; Arakawa, H.; Miyajima, C.; Kasahara, F.; Ogihara, T. Relationship between Physical Parameters of Various Metal Ions and Binding Affinity for Alginate. Biol. Pharm. Bull. 2016, 39, 1893–1896. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Wang, Y.; Shafer, R.; Winn, N.C.; Kanaley, J.A.; Vardhanabhuti, B. Glycemic Effects Following the Consumption of Mixed Soy Protein Isolate and Alginate Beverages in Healthy Adults. Food Funct. 2019, 10, 1718–1725. [Google Scholar] [CrossRef]
- El Khoury, D.; Goff, H.D.; Berengut, S.; Kubant, R.; Anderson, G.H. Effect of Sodium Alginate Addition to Chocolate Milk on Glycemia, Insulin, Appetite and Food Intake in Healthy Adult Men. Eur. J. Clin. Nutr. 2014, 68, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Georg Jensen, M.; Kristensen, M.; Astrup, A. Effect of Alginate Supplementation on Weight Loss in Obese Subjects Completing a 12-Wk Energy-Restricted Diet: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2012, 96, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.A.; Lai, C.-S.; Corwin, H.; Ma, Y.; Maki, K.C.; Garleb, K.A.; Wolf, B.W. Inclusion of Guar Gum and Alginate into a Crispy Bar Improves Postprandial Glycemia in Humans. J. Nutr. 2004, 134, 886–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Idota, Y.; Shiragami, K.; Koike, M.; Nishibori, F.; Tomokane, M.; Seki, T.; Itabashi, K.; Hakoda, K.; Takahashi, H.; et al. Randomized, Double-Blind, Crossover Clinical Trial of the Effect of Calcium Alginate in Noodles on Postprandial Blood Glucose Level. Biol. Pharm. Bull. 2018, 41, 1367–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, B.W.; Lai, C.-S.; Kipnes, M.S.; Ataya, D.G.; Wheeler, K.B.; Zinker, B.A.; Garleb, K.A.; Firkins, J.L. Glycemic and Insulinemic Responses of Nondiabetic Healthy Adult Subjects to an Experimental Acid-Induced Viscosity Complex Incorporated into a Glucose Beverage. Nutrition 2002, 18, 621–626. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [Green Version]
- Skriptsova, A.V.; Shevchenko, N.M.; Zvyagintseva, T.N.; Imbs, T.I. Monthly Changes in the Content and Monosaccharide Composition of Fucoidan from Undaria Pinnatifida (Laminariales, Phaeophyta). J. Appl. Phycol. 2010, 22, 79–86. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from Brown Seaweeds: An Update on Structures, Extraction Techniques and Use of Enzymes as Tools for Structural Elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef] [Green Version]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown Seaweed Fucoidan: Biological Activity and Apoptosis, Growth Signaling Mechanism in Cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The Seasonal Variation of Fucoidan within Three Species of Brown Macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Mar. Drugs 2021, 19, 30. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S. Molecular Weight and Sulfate Content Modulate the Inhibition of α-Amylase by Fucoidan Relevant for Type 2 Diabetes Management. Pharma. Nutr. 2015, 3. [Google Scholar] [CrossRef]
- Shan, X.; Liu, X.; Hao, J.; Cai, C.; Fan, F.; Dun, Y.; Zhao, X.; Liu, X.; Li, C.; Yu, G. In Vitro and in Vivo Hypoglycemic Effects of Brown Algal Fucoidans. Int. J. Biol. Macromol. 2016, 82, 249–255. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Stortz, C.A. A Comprehensive and Comparative Analysis of the Fucoidan Compositional Data Across the Phaeophyceae. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [Green Version]
- Suprunchuk, V.E. Low-Molecular-Weight Fucoidan: Chemical Modification, Synthesis of Its Oligomeric Fragments and Mimetics. Carbohydr. Res. 2019, 485, 107806. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhao, Y.; Hu, S.; Shi, D.; Xue, C. Fucoidan from Sea Cucumber Cucumaria Frondosa Exhibits Anti-Hyperglycemic Effects in Insulin Resistant Mice via Activating the PI3K/PKB Pathway and GLUT4. J. Biosci. Bioeng. 2016, 121, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria Pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, C.; Abe, S.; Kouzuki, M.; Shimohiro, H.; Ota, Y.; Sakinada, H.; Takeuchi, T.; Okura, T.; Kasagi, T.; Hanaki, K. A Randomized Placebo-Controlled Trial of an Oral Preparation of High Molecular Weight Fucoidan in Patients with Type 2 Diabetes with Evaluation of Taste Sensitivity. Yonago Acta Med. 2019, 62, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-J.; Yoon, K.-Y.; Lee, B.-Y. Fucoidan Regulate Blood Glucose Homeostasis in C57BL/KSJ M+/+db and C57BL/KSJ Db/Db Mice. Fitoterapia 2012, 83, 1105–1109. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structural Dependence of Sulfated Polysaccharide for Diabetes Management: Fucoidan From Undaria Pinnatifida Inhibiting α-Glucosidase More Strongly Than α-Amylase and Amyloglucosidase. Front. Pharm. 2020, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Alpha-Amylase and Alpha-Glucosidase Inhibition Is Differentially Modulated by Fucoidan Obtained from Fucus vesiculosus and Ascophyllum Nodosum. Phytochemistry 2014. [Google Scholar] [CrossRef] [PubMed]
- Daub, C.D.; Mabate, B.; Malgas, S.; Pletschke, B.I. Fucoidan from Ecklonia Maxima Is a Powerful Inhibitor of the Diabetes-Related Enzyme, α-Glucosidase. Int. J. Biol. Macromol. 2020, 151, 412–420. [Google Scholar] [CrossRef] [PubMed]
- January, G.G.; Naidoo, R.K.; Kirby-McCullough, B.; Bauer, R. Assessing Methodologies for Fucoidan Extraction from South African Brown Algae. Algal Res. 2019, 40, 101517. [Google Scholar] [CrossRef]
- Senthil, S.L.; Kumar, T.V.; Geetharamani, D.; Maruthupandi, T. Screening of Seaweeds Collected from Southeast Coastal Area of India for α-Amylase Inhibitory Activity, Antioxidant Activity and Biocompatibility. Int. J. Pharm. Pharm. Sci 2013, 5, 240–244. [Google Scholar]
- Hu, S.; Xia, G.; Wang, J.; Wang, Y.; Li, Z.; Xue, C. Fucoidan from Sea Cucumber Protects against High-Fat High-Sucrose Diet-Induced Hyperglycaemia and Insulin Resistance in Mice. J. Funct. Foods 2014, 10, 128–138. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Unnikrishnan, P.S.; Suthindhiran, K.; Jayasri, M.A. Inhibitory Potential of Turbinaria Ornata against Key Metabolic Enzymes Linked to Diabetes. Biomed. Res. Int. 2014, 2014, 783895. [Google Scholar] [CrossRef] [Green Version]
- Havale, S.H.; Pal, M. Medicinal Chemistry Approaches to the Inhibition of Dipeptidyl Peptidase-4 for the Treatment of Type 2 Diabetes. Bioorg. Med. Chem. 2009, 17, 1783–1802. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.; Dordevic, A.L.; Ryan, L.; Bonham, M.P. The Impact of a Single Dose of a Polyphenol-Rich Seaweed Extract on Postprandial Glycaemic Control in Healthy Adults: A Randomised Cross-Over Trial. Nutrients 2018, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Corona, D.M.; Martínez-Abundis, E.; González-Ortiz, M. Effect of Fucoidan Administration on Insulin Secretion and Insulin Resistance in Overweight or Obese Adults. J. Med. Food 2014, 17, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Extraction, Structure and Biofunctional Activities of Laminarin from Brown Algae. Int. J. Food Sci. Technol. 2015, 50, 24–31. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.-J.; Kim, H.J.; Kim, Y.-S.; Park, W. Immunostimulatory Effect of Laminarin on RAW 264.7 Mouse Macrophages. Molecules 2012, 17, 5404–5411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trigueiro, T.G.; Pereira, D.C.; Martins, A.P.; Colepicolo, P.; Marinho-Soriano, E. Cultivation of Three Color Strains of Gracilaria Domingensis in an Integrated Organic System. Int. Aquat. Res. 2017, 9, 225–233. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.-M.; Zheng, L.; Yan, X.-J. The Preparation and Bioactivity Research of Agaro-Oligosaccharides. Food Technol. Biotechnol. 2005, 43, 29–36. [Google Scholar]
- Enoki, T.; Sagawa, H.; Tominaga, T.; Nishiyama, E.; Koyama, N.; Sakai, T.; Yu, F.-G.; Ikai, K.; Kato, I. Drugs, Foods or Drinks with the Use of Algae-Derived Physiologically Active Substances 1999. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO1999024447 (accessed on 23 January 2022).
- Kidgell, J.T.; Magnusson, M.; De Nys, R.; Glasson, C.R.K. Ulvan: A Systematic Review of Extraction, Composition and Function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Skoler-Karpoff, S.; Ramjee, G.; Ahmed, K.; Altini, L.; Plagianos, M.G.; Friedland, B.; Govender, S.; Kock, A.D.; Cassim, N.; Palanee, T.; et al. Efficacy of Carraguard for Prevention of HIV Infection in Women in South Africa: A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2008, 372, 1977–1987. [Google Scholar] [CrossRef]
- Cáceres, P.J.; Carlucci, M.J.; Damonte, E.B.; Matsuhiro, B.; Zúñiga, E.A. Carrageenans from Chilean Samples of Stenogramme Interrupta (Phyllophoraceae): Structural Analysis and Biological Activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef]
- Groult, H.; Cousin, R.; Chot-Plassot, C.; Maura, M.; Bridiau, N.; Piot, J.-M.; Maugard, T.; Fruitier-Arnaudin, I. λ-Carrageenan Oligosaccharides of Distinct Anti-Heparanase and Anticoagulant Activities Inhibit MDA-MB-231 Breast Cancer Cell Migration. Mar. Drugs 2019, 17, 140. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Feferman, L.; Unterman, T.; Tobacman, J.K. Exposure to Common Food Additive Carrageenan Alone Leads to Fasting Hyperglycemia and in Combination with High Fat Diet Exacerbates Glucose Intolerance and Hyperlipidemia without Effect on Weight. J. Diabetes Res. 2015, 2015, 513429. [Google Scholar] [CrossRef] [Green Version]
- Paradis, M.-E.; Couture, P.; Lamarche, B. A Randomised Crossover Placebo-Controlled Trial Investigating the Effect of Brown Seaweed (Ascophyllum Nodosum and Fucus vesiculosus) on Postchallenge Plasma Glucose and Insulin Levels in Men and Women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Mitamura, R. Effects of Undaria Pinnatifida (Wakame) on Postprandial Glycemia and Insulin Levels in Humans: A Randomized Crossover Trial. Plant Foods Hum. Nutr. 2019, 74, 461–467. [Google Scholar] [CrossRef]
- Tanemura, Y.; Yamanaka-Okumura, H.; Sakuma, M.; Nii, Y.; Taketani, Y.; Takeda, E. Effects of the Intake of Undaria Pinnatifida (Wakame) and Its Sporophylls (Mekabu) on Postprandial Glucose and Insulin Metabolism. J. Med. Investig. 2014, 61, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A Sustainable Functional Food for Complementary and Alternative Therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the Search of New Functional Food Ingredients from Algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Xia, E.-Q.; Zhu, S.-S.; He, M.-J.; Luo, F.; Fu, C.-Z.; Zou, T.-B. Marine Peptides as Potential Agents for the Management of Type 2 Diabetes Mellitus—A Prospect. Mar. Drugs 2017, 15, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Huertas, E. Health Effects of Oleic Acid and Long Chain Omega-3 Fatty Acids (EPA and DHA) Enriched Milks. A Review of Intervention Studies. Pharmacol. Res. 2010, 61, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical Marine Macroalgae as Potential Sources of Nutritionally Important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty Acids, Total Lipid, Protein and Ash Contents of Processed Edible Seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Sirot, V.; Dumas, C.; Desquilbet, L.; Mariotti, F.; Legrand, P.; Catheline, D.; Leblanc, J.-C.; Margaritis, I. A Restricted Cubic Spline Approach to Assess the Association between High Fat Fish Intake and Red Blood Cell EPA + DHA Content. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 318–326. [Google Scholar] [CrossRef]
- Lu, H.; Nielsen, N.; Baron, C.; Jacobsen, C. Marine Phospholipids: The Current Understanding of Their Oxidation Mechanisms and Potential Uses for Food Fortification. Crit. Rev. Food Sci. Nutr. 2015, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payghami, N.; Jamili, S.; Rustaiyan, A.; Saeidnia, S.; Nikan, M.; Gohari, A.R. Alpha-Amylase Inhibitory Activity and Sterol Composition of the Marine Algae, Sargassum Glaucescens. Pharma. Res. 2015, 7, 314. [Google Scholar]
- Jung, H.A.; Islam, M.N.; Lee, C.M.; Oh, S.H.; Lee, S.; Jung, J.H.; Choi, J.S. Kinetics and Molecular Docking Studies of an Anti-Diabetic Complication Inhibitor Fucosterol from Edible Brown Algae Eisenia Bicyclis and Ecklonia Stolonifera. Chem. Biol. Int. 2013, 206, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yang, C.; Liu, B.; Lin, L.; Sarker, S.D.; Nahar, L.; Yu, H.; Cao, H.; Xiao, J. Bioactive Compounds from Marine Macroalgae and Their Hypoglycemic Benefits. Trends Food Sci. Technol. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Gabbia, D.; De Martin, S. Brown Seaweeds for the Management of Metabolic Syndrome and Associated Diseases. Molecules 2020, 25, 4182. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Barbosa, M.; Andrade, P.B.; Valentão, P. Phlorotannins from Fucales: Potential to Control Hyperglycemia and Diabetes-Related Vascular Complications. J. Appl. Phycol. 2019, 31, 3143–3152. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in Marine Algae and Their Bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Jeon, Y.-J. Anti-Diabetic Effects of Brown Algae Derived Phlorotannins, Marine Polyphenols through Diverse Mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- Roy, M.-C.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a Commercially-Available Algal Phlorotannins Extract on Digestive Enzymes and Carbohydrate Absorption in Vivo. Food Res. Int. 2011, 44, 3026–3029. [Google Scholar] [CrossRef]
- Wright, S.W.; Jeffrey, S.W. Pigment Markers for Phytoplankton Production. In Marine Organic Matter: Biomarkers, Isotopes and DNA; Springer: Berlin, Germany, 2006; pp. 71–104. [Google Scholar]
- Zhang, Y.; Fang, H.; Xie, Q.; Sun, J.; Liu, R.; Hong, Z.; Yi, R.; Wu, H. Comparative Evaluation of the Radical-Scavenging Activities of Fucoxanthin and Its Stereoisomers. Molecules 2014, 19, 2100–2113. [Google Scholar] [CrossRef] [Green Version]
- Bae, M.; Kim, H. The Role of Vitamin C, Vitamin D, and Selenium in Immune System against COVID-19. Molecules 2020, 25, 5346. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary Combination of Fucoxanthin and Fish Oil Attenuates the Weight Gain of White Adipose Tissue and Decreases Blood Glucose in Obese/Diabetic KK-Ay Mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Miyashita, T.; Nishikawa, S.; Emi, S.; Tsukui, T.; Beppu, F.; Okada, T.; Miyashita, K. Fucoxanthin Regulates Adipocytokine MRNA Expression in White Adipose Tissue of Diabetic/Obese KK-Ay Mice. Arch. Biochem. Biophys. 2010, 504, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zaharudin, N.; Staerk, D.; Dragsted, L.O. Inhibition o.of α-Glucosidase Activity by Selected Edible Seaweeds and Fucoxanthin. Food Chem. 2019, 270, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-Obesity and Anti-Diabetic Effects of Fucoxanthin on Diet-Induced Obesity Conditions in a Murine Model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef]
- Laleg, K.; Barron, C.; Santé-Lhoutellier, V.; Walrand, S.; Micard, V. Protein Enriched Pasta: Structure and Digestibility of Its Protein Network. Food Funct. 2016, 7, 1196–1207. [Google Scholar] [CrossRef]
- Lu, Z.-H.; Donner, E.; Yada, R.Y.; Liu, Q. Physicochemical Properties and in Vitro Starch Digestibility of Potato Starch/Protein Blends. Carbohydr. Polym. 2016, 154, 214–222. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, F.; Douglas Goff, H.; Li, Y. Study on Starch-Protein Interactions and Their Effects on Physicochemical and Digestible Properties of the Blends. Food Chem. 2019, 280, 51–58. [Google Scholar] [CrossRef]
- Echave, J.; Fraga-Corral, M.; Garcia-Perez, P.; Popović-Djordjević, J.; Avdović, E.H.; Radulović, M.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Seaweed Protein Hydrolysates and Bioactive Peptides: Extraction, Purification, and Applications. Mar. Drugs 2021, 19, 500. [Google Scholar] [CrossRef]
- Badmus, U.O.; Taggart, M.A.; Boyd, K.G. The Effect of Different Drying Methods on Certain Nutritionally Important Chemical Constituents in Edible Brown Seaweeds. J. Appl. Phycol. 2019, 31, 3883–3897. [Google Scholar] [CrossRef] [Green Version]
- Debet, M.R.; Gidley, M.J. Three Classes of Starch Granule Swelling: Influence of Surface Proteins and Lipids. Carbohydr. Polym. 2006, 64, 452–465. [Google Scholar] [CrossRef]
- Ai, Y.; Hasjim, J.; Jane, J. Effects of Lipids on Enzymatic Hydrolysis and Physical Properties of Starch. Carbohydr. Polym. 2013, 92, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.; Björck, I.; Ostrowska, S.; Eliasson, A.-C.; Asp, N.-G.; Larsson, K.; Lundquist, I. Digestibility of Amylose-Lipid Complexes in-Vitro and in-Vivo. Starch 1983, 35, 294–297. [Google Scholar] [CrossRef]
- Lau, E.; Zhou, W.; Henry, C.J. Effect of Fat Type in Baked Bread on Amylose-Lipid Complex Formation and Glycaemic Response. Br. J. Nutr. 2016, 115, 2122–2129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, L.W.; Kasapis, S.; Lim, K.M.; Foo, C.W. Structural Enhancement Leading to Retardation of in Vitro Digestion of Rice Dough in the Presence of Alginate. Food Hydrocoll. 2009, 23, 1458–1464. [Google Scholar] [CrossRef]
- Marcano, J.; Hernando, I.; Fiszman, S. In Vitro Measurements of Intragastric Rheological Properties and Their Relationships with the Potential Satiating Capacity of Cheese Pies with Konjac Glucomannan. Food Hydrocoll. 2015, 51. [Google Scholar] [CrossRef]
- Regand, A.; Chowdhury, Z.; Tosh, S.M.; Wolever, T.M.S.; Wood, P. The Molecular Weight, Solubility and Viscosity of Oat Beta-Glucan Affect Human Glycemic Response by Modifying Starch Digestibility. Food Chem. 2011, 129, 297–304. [Google Scholar] [CrossRef]
- Dhital, S.; Gidley, M.J.; Warren, F.J. Inhibition of α-Amylase Activity by Cellulose: Kinetic Analysis and Nutritional Implications. Carbohydr. Polym. 2015, 123, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Islam, M.N.; Lee, C.M.; Jeong, H.O.; Chung, H.Y.; Woo, H.C.; Choi, J.S. Promising Antidiabetic Potential of Fucoxanthin Isolated from the Edible Brown Algae Eisenia Bicyclis and Undaria Pinnatifida. Fish. Sci. 2012, 78, 1321–1329. [Google Scholar] [CrossRef]
- Harden, C.J.; Craig Richardson, J.; Dettmar, P.W.; Corfe, B.M.; Paxman, J.R. An Ionic-Gelling Alginate Drink Attenuates Postprandial Glycaemia in Males. J. Funct. Foods 2012, 4, 122–128. [Google Scholar] [CrossRef]
- Clegg, M.E.; Pratt, M.; Markey, O.; Shafat, A.; Henry, C.J.K. Addition of Different Fats to a Carbohydrate Food: Impact on Gastric Emptying, Glycaemic and Satiety Responses and Comparison with in Vitro Digestion. J. Funct. Foods 2012, 48, 91–97. [Google Scholar] [CrossRef]
- Henry, C.J.K.; Lightowler, H.J.; Newens, K.J.; Pata, N. The Influence of Adding Fats of Varying Saturation on the Glycaemic Response of White Bread. Int. J. Food Sci. Nutr. 2008, 59, 61–69. [Google Scholar] [CrossRef]
- Hätönen, K.A.; Virtamo, J.; Eriksson, J.G.; Sinkko, H.K.; Sundvall, J.E.; Valsta, L.M. Protein and Fat Modify the Glycaemic and Insulinaemic Responses to a Mashed Potato-Based Meal. Br. J. Nutr. 2011, 106, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, T.; Luhovyy, B.L.; Panahi, S.; Kubant, R.; Brown, P.H.; Anderson, G.H. Mechanism of Action of Pre-Meal Consumption of Whey Protein on Glycemic Control in Young Adults. J. Nutr. Biochem. 2014, 25, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Cui, J.; Dalgleish, D.; Singh, H. The Formation and Breakdown of Structured Clots from Whole Milk during Gastric Digestion. Food Funct. 2016, 7, 4259–4266. [Google Scholar] [CrossRef]
- Quek, R.; Bi, X.; Henry, C.J. Impact of Protein-Rich Meals on Glycaemic Response of Rice. Br. J. Nutr. 2016, 115, 1194–1201. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, M.; Tang, A.C.; Wakaki, Y.; Koyama, W. Glycemic Index of Single and Mixed Meal Foods among Common Japanese Foods with White Rice as a Reference Food. Eur. J. Clin. Nutr. 2003, 57, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Desterke, C.; Turhan, A.G.; Bennaceur-Griscelli, A.; Griscelli, F. PPARγ Cistrome Repression during Activation of Lung Monocyte-Macrophages in Severe COVID-19. iScience 2020, 23, 101611. [Google Scholar] [CrossRef] [PubMed]
- Quitral, V.; Sepúlveda, M.; Gamero-Vega, G.; Jiménez, P. Seaweeds in Bakery and Farinaceous Foods: A Mini-Review. Int. J. Food Sci. 2021, 100403. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N. Incorporation of Himanthalia Elongata Seaweed to Enhance the Phytochemical Content of Breadsticks Using Response Surface Methodology (RSM). Int. Food Res. J. 2013, 40, 1537–1545. [Google Scholar] [CrossRef]


| Food Items | Serving Size (g) | GI Value (Mean ± SEM) | Reference |
|---|---|---|---|
| High-farinaceous food | |||
| White rice | 194 | 96 ± 6.6 | [34] |
| Brown rice | 176 | 66.21 ± 7.78 | [35] |
| Jasmine rice | 180.3 | 78.7 ± 11.6 | [36] |
| Parboiled white rice | 259 | 77 ± 4 | [37] |
| Parboiled brown rice | 167 | 50.1 ± 5.37 | [35] |
| Basmati rice | 188.3 | 50 ± 5.8 | [36] |
| Glutinous rice | 109 | 89 ± 8 | [38] |
| Bario celum (black rice) | 50 | 60.9 ± 7.2 | [39] |
| Beras merah (red rice) | 50 | 78.3 ± 9.9 | [39] |
| White rice porridge | 290 | 98.4 ± 8.1 | [40] |
| Sorghum (coarse) | 232 | 53 ± 2.84 | [41] |
| Sorghum (fine) | 252 | 56 ± 9.83 | [41] |
| Maize (steamed) | 164 | 74.7 ± 6.5 | [42] |
| Millet (steamed) | 169 | 64.4 ± 8.5 | [43] |
| Millet (porridge) | 550 | 93.6 ± 11.3 | [43] |
| Barley powder | 67 | 69.8 ± 6.7 | [44] |
| Processed carbohydrate foods | |||
| Buckwheat noodles | 70.2 | 59.6 ± 13.3 | [44] |
| Wheat noodles | 91.5 | 48.2 ± 4.9 | [44] |
| Wheat pasta | 330 | 72 ± 6.51 | [41] |
| Puffed rice grains | 56.2 | 72.4 ± 6.6 | [44] |
| Rice vermicelli | 63.3 | 56 ± 7 | [45] |
| Rice cakes | 93.8 | 80.7 ± 8.5 | [44] |
| Rice balls | 100 | 96.9 ± 15.1 | [44] |
| Rice dosa | 193 | 76 ± 5 | [46] |
| Rice idli | 162 | 85 ± 4 | [46] |
| Sorghum pasta | 330 | 46 ± 6.47 | [41] |
| Buckwheat jelly | 318.5 | 65.7 ± 11.8 | [44] |
| Bakery foods | |||
| Rice flour muffin | 119.4 | 79.1 ± 6.3 | [47] |
| Rice bread | 116.6 | 73.4 ± 7.6 | [44] |
| White wheat bread | 91.4 | 83 ± 8.8 | [48] |
| Wholemeal wheat bread | 128 | 77 ± 6 | [49] |
| Wheat pancakes | 102.8 | 57 ± 9.7 | [44] |
| White wheat roti | 119 | 64 ± 9.24 | [41] |
| Brown wheat roti | 69.44 | 61 ± 5.77 | [50] |
| Sorghum multigrain roti | 119 | 68 ± 8.63 | [41] |
| Sorghum flakes poha | 277 | 45 ± 5.27 | [41] |
| Wheat flour muffin | 126.1 | 74.4 ± 8.1 | [47] |
| Bagel | 104.1 | 77.4 ± 11.5 | [44] |
| Rye bread | 109.4 | 64.9 ± 18.4 | [44] |
| Corn flour cake | 54 | 85.02 ± 11.21 | [51] |
| Corn flour cookie | 71 | 52.23 ± 6.78 | [51] |
| Corn flour muffin | 136.9 | 74.4 ± 5.4 | [47] |
| Castella cake | 114.2 | 59.9 ± 13.3 | [44] |
| Buckwheat pancakes | 169.4 | 49.9 ± 8.9 | [44] |
| Sorghum biscuits | 75 | 54 ± 6.3 | [41] |
| Processed breakfast cereal | |||
| All-Bran (Kellogg’s Inc., Seol, South Korea) | 57.5 | 51.4 ± 11.1 | [44] |
| Cornflakes (Kellogg’s Inc., Seol, South Korea) | 56.2 | 51.6 ± 10.7 | [44] |
| Rice flakes poha | 277 | 74 ± 4.87 | [41] |
| Wheat biscuits | 75 | 57 ± 11.4 | [41] |
| Seaweed Species | Moisture (% of WW) | Dietary Fiber | Protein | Lipids | Total Polyphenols | Reference | ||
|---|---|---|---|---|---|---|---|---|
| Soluble (% DW) | Insoluble (% DW) | Maximum Protein (% DW) | Total (% DW) | EPA (% DW) | ||||
| Brown seaweed (Phaeophyceae) | ||||||||
| Ascophyllum nodosum | 67–87 | 42–64 | NA | 8.7 | 3.62 | 7.24 | 960 mg PGE/100 g DW | [74,75,76,77] |
| Bifurcaria bifurcata | 73 | 15 | 23 | 8.92 | 6.54 | 4.09 | 1990 mg PGE/100 g DW | [76,77,78,79] |
| Durvillaea antarctica | NA | 28 | 44 | 11.6 | 0.8 | 4.95 | NA | [80] |
| Fucus vesiculosus | 67–82 | 11 | 49 | 12.99 | 3.75 | 9.94 | 1150 mg PGE/100 g DW | [76,77,81,82] |
| Laminaria spp. | 73–94 | 36 | 10 | 21 | 0.8 | 16.2 | NA | [79,83,84,85,86] |
| Saccharina latissima | 73–94 | 17.12 | 13.11 | 25.7 | 0.8 | NA | 11.1 mg GAE/100 g DW | [79,84,86,87,88] |
| Sargassum fusiforme | 61 | 32.9 | 16.3 | 20 | 1.4 | 42.4 | NA | [81,85,89,90] |
| Undaria pinnatifida | 88 | 30.0 | 5.3 | 24 | 4.5 | 13.2 | 4460 mg GAE/100 g DW | [81,85,90,91] |
| Red seaweed (Rhyodophyta) | ||||||||
| Chondrus crispus | 72–78 | 22.25 | 12.04 | 27.2 | 2 | NA | NA | [74,89,92,93] |
| Garateloupiaturuturu | 85 | 48.1 | 12.3 | 22.9 | 3 | NA | NA | [84,94] |
| Jania rubens | NA | NA | NA | 11.28 | 2 | NA | 56 mg GAE/100 g DW | [95,96] |
| Porphyra/Pyropia spp. | 77–91 | 17.9 | 16.8 | 44 | 1.0 | 10.4 | 5530 mg GAE/100 g DW | [84,85,86,87,90,91,97] |
| Pterocladiella capillacea | NA | NA | NA | 20.67 | 2 | NA | 93 mg GAE/100 g DW | [95,96] |
| Green seaweed (Chlorophyta) | ||||||||
| Caulerpa lentillifera | NA | 17.21 | 15.78 | 9.26 | 1.11 | 0.86 | NA | [98,99] |
| Ulva clathrata | 78–80 | 21.9 | 18.7 | 44 | 1.5 | NA | NA | [80,86,100] |
| Ulva lactuca | 78–80 | 20.53 | 34.37 | 44 | 1.27 | 0.87 | 2.86 mg GAE/100 g DW | [80,84,86,101,102] |
| First Author, Year (Reference) | Study Design | Subjects | Intervention | Source | Dose | Duration | Effect |
|---|---|---|---|---|---|---|---|
| El Khoury 2014a [115] | Randomized, placebo-controlled, crossover design study | 24 Healthy adults | Low sodium alginate extract vs. placebo chocolate milk | Laminaria hyperborea | Study 1: 4.06 g | 2 h | ↓ Cmax by 6% |
| El Khoury 2014b [115] | Randomized, placebo-controlled, crossover design study | 24 Healthy adults | High sodium alginate extract vs. low sodium alginate chocolate milk | Laminaria hyperborea | Study 2: 8.13 g | 2 h | ↓ Cmax by 13% ↓ peak insulin by 46% |
| Jensen 2012a [116] | Randomized, double-blind, placebo-controlled, 4-way, crossover design study | 19 Healthy adults | Sodium alginate extract vs. control preload beverage without sodium alginate | Laminaria hyperborea, Lessonia trabeculata | Study 1: 9.9 g | 3.5 h | No significant difference |
| Jensen 2012b [116] | Randomized, double-blind, placebo-controlled, 4-way, crossover design study | 20 Healthy adults | Sodium alginate extract vs. control preload beverage without sodium alginate | Laminaria hyperborea, Lessonia trabeculata | Study 2: 15.0 g | 3.5 h | ↓ iAUC glucose by 40% |
| Huang 2019 [114] | Randomized, double-blind, placebo-controlled, crossover design study | 12 Healthy adults | Sodium alginate extract + 172 kcal sugar beverage with soy protein isolate at pH7 vs. control sugar beverage | N/A | 0.625 g | 2 h | ↓ Cmax by 53.2% |
| Wolf 2002 [119] | Randomized, double-blind, placebo-controlled, crossover design study | 30 Healthy adults | Sodium alginate extract vs. control glucose-based beverage of similar total dietary fiber level | N/A | 3.75 g | 2 h | ↓ iAUC glucose by 75% |
| Williams 2004 [117] | Randomized, double-blind, placebo-controlled, crossover design study | 48 Healthy adults | Sodium alginate extract and guar gum vs. placebo in crispy bar (containing 50g available carbohydrate) | N/A | 1.6 g | 3 h | ↓ Cmax concentration by 30% ↓ iAUC glucose by 33% |
| Kato 2018 [118] | Randomized, double-blind, placebo-controlled, crossover design study | 15 Healthy adults | Calcium alginate extract vs. control meal without calcium alginate | N/A | Study 1: 3.2 g | 2 h | ↓ Cmax by 11% ↓ iAUC glucose by 15% |
| Kato 2018 [118] | Randomized, double-blind, placebo-controlled, crossover design study | 15 Healthy adults | Calcium alginate extract vs. control meal without calcium alginate | N/A | Study 1: 5.0 g | 2 h | ↓ Cmax by 15% ↓ iAUC glucose by 21% |
| Hernández-Corona et al. 2014 [145] | Randomized, double-blind, placebo-controlled, parallel design study | 25 overweight/obese adults | Fucoidan extract vs. placebo | N/A | 0.5 g | 2 h | No significant difference |
| Murray et al. 2018a [144] | Randomized, double-blind, placebo controlled, crossover design study | 38 healthy adults | Fucus vesiculosus vs. placebo (cellulose) preload before 50 g of available carbohydrate from white bread | Fucus vesiculosus | Study 1: 0.5 g | 2 h | No significant difference |
| Murray et al. 2018b [144] | Randomized, double-blind, placebo controlled, crossover design study | 38 healthy adults | Fucus vesiculosus vs. placebo (cellulose) preload before 50 g of available carbohydrate from white bread | Fucus pediculosis | Study 2: 2.0 g | 2 h | No significant difference |
| Paradis et al. 2011 [156] | Randomized, double-blind, placebo-controlled, crossover design study | 23 healthy adults | Ascophyllum nodosum + Fucus vesiculosus vs. placebo preload before 50 g of available carbohydrate from bread | Ascophyllum nodosum + Fucus vesiculosus | 0.5 g | 3 h | ↓ iAUC insulin by 12.1% No significant effect on postprandial glucose |
| Yoshinaga and Mitamura 2019 [157] | Randomized, open-label, 2-period, crossover design | 26 adults with pre-diabetes | Undaria pinnatifida vs. placebo with 200 g rice | Undaria pinnatifida | 4.0 g | 2 h | ↓ Postprandial glucose at 30 min by 7% ↓ iAUC glucose by 8% |
| Tanemura et al. 2014a [158] | Randomized, placebo (as test meal) controlled, crossover design study | 12 healthy adults | Undaria pinnatifida vs. control meal with no extract | Undaria pinnatifida | 70.0 g | 3 h | No significant difference |
| Tanemura et al. 2014b [158] | Randomized, placebo (as test meal) controlled, crossover design study | 12 healthy adults | Undaria pinnatifida sporophylls vs. control meal with no extract | Undaria pinnatifida sporophylls | 70.0 g | 3 h | ↓ Postprandial glucose at 30 min ↓ iAUC glucose 0–30 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, L.W.; Chen, J.-H. Seaweeds as Ingredients to Lower Glycemic Potency of Cereal Foods Synergistically—A Perspective. Foods 2022, 11, 714. https://doi.org/10.3390/foods11050714
Lu LW, Chen J-H. Seaweeds as Ingredients to Lower Glycemic Potency of Cereal Foods Synergistically—A Perspective. Foods. 2022; 11(5):714. https://doi.org/10.3390/foods11050714
Chicago/Turabian StyleLu, Louise Weiwei, and Jie-Hua Chen. 2022. "Seaweeds as Ingredients to Lower Glycemic Potency of Cereal Foods Synergistically—A Perspective" Foods 11, no. 5: 714. https://doi.org/10.3390/foods11050714
APA StyleLu, L. W., & Chen, J. -H. (2022). Seaweeds as Ingredients to Lower Glycemic Potency of Cereal Foods Synergistically—A Perspective. Foods, 11(5), 714. https://doi.org/10.3390/foods11050714