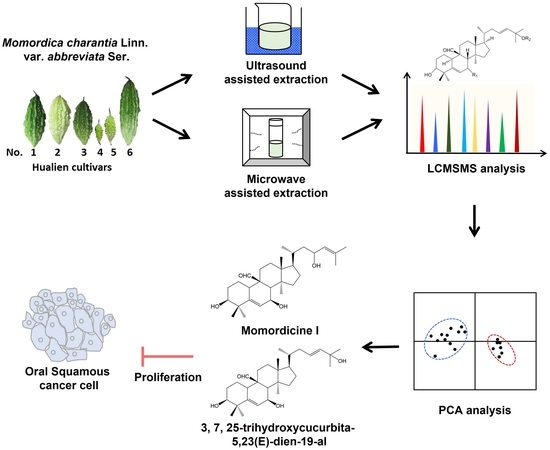
Microwave- and Ultrasound-Assisted Extraction of Cucurbitane-Type Triterpenoids from Momordica charantia L. Cultivars and Their Antiproliferative Effect on SAS Human Oral Cancer Cells
Abstract
:1. Introduction
2. Materials and Method
2.1. Chemicals and Reagents
2.2. Sample Preparation and Extraction
2.2.1. Plant Materials
2.2.2. Ultrasound-Assisted Extraction
2.2.3. Microwave-Assisted Extraction
2.3. Solid Phase Extraction Process Test
2.4. UHPLC–MS/MS Analysis
2.5. Method Validation Assay
2.6. Cell Culture
2.7. Cell Proliferation Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Solid Phase Extraction Process
3.2. Method Validation
3.3. Comparison of Ultrasound-Assisted Extraction and Microwave-Assisted Extraction
3.4. Application of Microwave-Assisted Extraction on Six Different Wild Bitter Melons
3.5. 3β,7β,25-Trihydroxycucurbita-5,23(E)-dien-19-al and Momordicine I Suppressed the Proliferation of SAS Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Yi, C.; Chung, H.R.; Wang, D.J.; Chang, W.C.; Lee, S.Y.; Lin, C.T.; Yang, Y.C.; Yang, W.C.V. Potential biomarkers in saliva for oral squamous cell carcinoma. Oral Oncol. 2010, 46, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Weatherspoon, D.J.; Chattopadhyay, A.; Boroumand, S.; Garcia, I. Oral cavity and oropharyngeal cancer incidence trends and disparities in the United States: 2000–2010. Cancer Epidemiol. 2015, 39, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godsey, J.; Grundmann, O. Review of various herbal supplements as complementary treatments for oral cancer. J. Diet. Suppl. 2016, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.C.; Liaw, C.C.; Hwang, S.Y.; Cheng, H.L.; Zhang, L.J.; Shen, C.C.; Hsu, F.L.; Kuo, Y.H. Antiproliferative and hypoglycemic cucurbitane-type glycosides from the fruits of Momordica charantia. J. Agric. Food Chem. 2013, 61, 2979–2986. [Google Scholar] [CrossRef]
- Hung, C.M.; Chang, C.C.; Lin, C.W.; Ko, S.Y.; Hsu, Y.C. Cucurbitacin E as inducer of cell death and apoptosis in human oral squamous cell carcinoma cell line SAS. Int. J. Mol. Sci. 2013, 14, 17147–17156. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.P.; Kha, T.C.; Parks, S.E.; Roach, P.D. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: A review. Food Rev. Int. 2016, 32, 181–202. [Google Scholar] [CrossRef]
- Nagasawa, H.; Watanabe, K.; Inatomi, H. Effects of bitter melon (Momordica charantia L.) or ginger rhizome (Zingiber offifinale Rosc) on spontaneous mammary tumorigenesis in SHN mice. Am. J. Chin. Med. 2002, 30, 195–205. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, X.H.; Yu, Y.; Poulev, A.; Ribnicky, D.; Floyd, Z.E.; Cefalu, W.T. Bioactives from bitter melon enhance insulin signaling and modulate acyl carnitine content in skeletal muscle in high-fat diet-fed mice. J. Nutr. Biochem. 2011, 22, 1064–1073. [Google Scholar] [CrossRef] [Green Version]
- Lii, C.K.; Chen, H.W.; Yun, W.T.; Liu, K.L. Suppressive effects of wild bitter gourd (Momordica charantia Linn. var. abbreviata ser.) fruit extracts on inflammatory responses in RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 122, 227–233. [Google Scholar] [CrossRef]
- Beloin, N.; Gbeassor, M.; Akpagana, K.; Hudson, J.; de Soussa, K.; Koumaglo, K.; Arnason, J.T. Ethnomedicinal uses of Momordica charantia (Cucurbitaceae) in Togo and relation to its phytochemistry and biological activity. J. Ethnopharmacol. 2005, 96, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat. Prod. Rep. 2005, 22, 386–399. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Cao, J.; Qu, H.; Bi, X.; Zhao, Y. Structures of new triterpenoids and cytotoxicity activities of the isolated major compounds from the fruit of Momordica charantia L. J. Agric. Food Chem. 2012, 60, 3927–3933. [Google Scholar] [CrossRef]
- Weng, J.R.; Bai, L.Y.; Chiu, C.F.; Hu, J.L.; Chiu, S.J.; Wu, C.Y. Cucurbitane triterpenoid from Momordica charantia induces apoptosis and autophagy in breast cancer cells, in part, through peroxisome proliferator-activated receptor γ activation. Evid. Based Complement. Altern. Med. 2013, 2013, 935675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Lin, L.; Chau, F.T. Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrason. Sonochem. 2001, 8, 347–352. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Vongsangnak, W.; Gua, J.; Chauvatcharin, S.; Zhong, J.J. Towards efficient extraction of notoginseng saponins from cultured cells of Panax notoginseng. Biochem. Eng. J. 2004, 18, 115–120. [Google Scholar] [CrossRef]
- Tian, X.J.; Wang, Y.; Li, H.; Fan, J.P. Microwave Extracting Process of Total Saponins from Momordica charantia. Packag. Food Mach. 2013, 1, 20–23. [Google Scholar] [CrossRef]
- Ma, J.; Krynitsky, A.J.; Grundel, E.; Rader, J.I. Quantitative determination of cucurbitane-type triterpenes and triterpene glycosides in dietary supplements containing bitter melon (Momordica charantia) by HPLC-MS/MS. J. AOAC Int. 2012, 95, 1597–1608. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chiou, S.H.; Huang, C.Y.; Jan, C.I.; Lin, S.C.; Hu, W.Y.; Chou, S.H.; Liu, C.J.; Lo, J.F. Tid1 functions as a tumour suppressor in head and neck squamous cell carcinoma. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2009, 219, 347–355. [Google Scholar] [CrossRef]
- Wang, S.; Tang, L.; Guo, Y.; Yan, F.; Chen, F. Rapid analysis of momordicoside A in bitter melon by high performance liquid chromatography following solid phase extraction. Se Pu= Chin. J. Chromatogr. 2001, 19, 128–131. [Google Scholar] [PubMed]
- Liu, Y.J.; Lai, Y.J.; Wang, R.; Lo, Y.C.; Chiu, C.H. The Effect of Thermal Processing on the Saponin Profiles of Momordica charantia L. J. Food Qual. 2020, 2020, 8862020. [Google Scholar] [CrossRef]
- Virly Chiu, C.-H.; Tsai, T.-Y.; Yeh, Y.-C.; Wang, R. Encapsulation of β-glucosidase within PVA fibers by CCD-RSM-guided coelectrospinning: A novel approach for specific mogroside sweetener production. J. Agric. Food Chem. 2020, 68, 11790–11801. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, Y.H.; Avula, B.; Wang, M.; Khan, I.A. Separation of cucurbitane triterpenoids from bitter melon drinks and determination of partition coefficients using vortex-assisted dispersive liquid-phase microextraction followed by UHPLC analysis. J. Sep. Sci. 2017, 40, 2238–2245. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-H.; Avula, B.; Liu, Y.; Khan, I.A. Determination and quantitation of five cucurbitane triterpenoids in Momordica charantia by reversed-phase high-performance liquid chromatography with evaporative light scattering detection. JChS 2008, 46, 133–136. [Google Scholar] [CrossRef]
- Shirsath, S.; Sonawane, S.; Gogate, P. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Mandal, S.C.; Mandal, V.; Das, A.K. Essentials of Botanical Extraction: Principles and Applications; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Zheng, S.; Zhang, W.; Liu, S. Optimization of ultrasonic-assisted extraction of polysaccharides and triterpenoids from the medicinal mushroom Ganoderma lucidum and evaluation of their in vitro antioxidant capacities. PLoS ONE 2020, 15, e0244749. [Google Scholar] [CrossRef]
- Hemwimon, S.; Pavasant, P.; Shotipruk, A. Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep. Purif. Technol. 2007, 54, 44–50. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Microwave assisted extraction of soy isoflavones. Anal. Chim. Acta 2007, 588, 274–282. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.Y.; Gong, X.F. Microwave-assisted extraction used for the isolation of total triterpenoid saponins from Ganoderma atrum. J. Food Eng. 2007, 81, 162–170. [Google Scholar] [CrossRef]
- Kwon, J.H.; Bélanger, J.M.; Paré, J.J. Optimization of microwave-assisted extraction (MAP) for ginseng components by response surface methodology. J. Agric. Food Chem. 2003, 51, 1807–1810. [Google Scholar] [CrossRef]
- Tsai, C.H.; Chen, E.C.F.; Tsay, H.S.; Huang, C.J. Wild bitter gourd improves metabolic syndrome: A preliminary dietary supplementation trial. Nutr. J. 2012, 11, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, C.; Tsai, T.H.; Li, Y.Y.; Wu, W.H.; Huang, C.J.; Tsai, P.J. Wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) extract and its bioactive components suppress Propionibacterium acnes-induced inflammation. Food Chem. 2012, 135, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Tseng, H.C.; Liu, C.T.; Huang, C.J.; Chyuan, J.H.; Sheen, L.Y. Wild bitter gourd protects against alcoholic fatty liver in mice by attenuating oxidative stress and inflammatory responses. Food Funct. 2014, 5, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Y.; Chiu, C.F.; Chu, P.C.; Lin, W.Y.; Chiu, S.J.; Weng, J.R. A triterpenoid from wild bitter gourd inhibits breast cancer cells. Sci. Rep. 2016, 6, 22419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sur, S.; Steele, R.; Isbell, T.S.; Venkata, K.N.; Rateb, M.E.; Ray, R.B. Momordicine-I, a Bitter Melon Bioactive Metabolite, Displays Anti-Tumor Activity in Head and Neck Cancer Involving c-Met and Downstream Signaling. Cancers 2021, 13, 1432. [Google Scholar] [CrossRef]




| Compound | Molecular Weight (g/mol) | Quantitative | Qualitative | ||||
|---|---|---|---|---|---|---|---|
| Parent Ions * > Product Ions (m/z) | Cone Voltage (V) | Collision Energy (eV) | Parent Ions * > Product Ions (m/z) | Cone Voltage (V) | Collision Energy (eV) | ||
| Momordicoside A | 817.01 | 839 > 839 | 91 | 44 | 839 > 365 | 91 | 57 |
| Momordicoside L | 634.40 | 657 > 477 | 78 | 41 | 657 > 203 | 78 | 43 |
| 3β,7β,25-trihydroxycucurbita-5,23(E)-dien-19-al | 472.40 | 495 > 495 | 93 | 13 | 495 > 477 | 93 | 35 |
| Momordicoside K | 648.40 | 671 > 203 | 87 | 43 | 671 > 491 | 87 | 43 |
| Momordicine Ⅰ | 472.70 | 495 > 495 | 61 | 3 | 495 > 477 | 61 | 33 |
| Momordicoside I | 618.40 | 641 > 337 | 94 | 46 | 641 > 203 | 94 | 52 |
| Momordicoside F2 | 618.40 | 641 > 337 | 94 | 46 | 641 > 203 | 94 | 52 |
| Momordicoside I aglycone | 456.72 | 479 > 479 | 50 | 18 | - | - | - |
| Momordicoside G | 632.42 | 655 > 625 | 68 | 42 | 655 > 337 | 68 | 38 |
| Momordicoside F1 | 632.42 | 655 > 625 | 17 | 51 | 655 > 337 | 17 | 57 |
| Compound | Linear Range (ng/mL) | Retention Time (min) | r | Calibration Curve | LOD (ng/mL) | LOQ (ng/mL) | Interday Recovery (%) | Interday (CV %) |
|---|---|---|---|---|---|---|---|---|
| Momordicoside A | 2–200 | 1.04 | 0.9989 | y = 142.41 x + 148.41 | 0.125 | 0.25 | 100.2 ± 4.7 | 7.77 |
| Momordicoside L | 2–200 | 3.01 | 0.9973 | y = 204.67 x − 242.72 | 0.50 | 1.00 | 113.1 ± 5.7 | 12.19 |
| 3β,7β,25-trihydroxycucurbita-5,23(E)-dien-19-al | 2–200 | 4.26 | 0.9977 | y = 11,142.59 x − 14,020.99 | 0.50 | 1.00 | 110.9 ± 6.3 | 14.52 |
| Momordicoside K | 2–200 | 6.24 | 0.9991 | y = 213.17 x − 571.18 | 1.50 | 2.00 | 95.5 ± 7.3 | 13.09 |
| Momordicine Ⅰ | 20–200 | 6.72 | 0.9941 | y = 1444.18 x + 4685.53 | 10.00 | 15.00 | 101.5 ± 10.6 | 14.21 |
| Momordicoside I | 20–200 | 6.88 | 0.9961 | y = 27.70 x + 62.06 | 3.00 | 10.00 | 99.1 ± 6.1 | 12.33 |
| Momordicoside F2 | 20–200 | 7.11 | 0.9921 | y = 29.99 x − 189.06 | 3.00 | 10.00 | 85.5 ± 10.7 | 14.36 |
| Momordicoside I aglycone | 20–200 | 8.54 | 0.9917 | y = 652.41 x + 479.97 | 3.00 | 10.00 | 103.0 ± 4.0 | 14.69 |
| Momordicoside G | 2–200 | 9.42 | 0.9976 | y = 30.60 x + 3.14 | 1.50 | 2.00 | 106.5 ± 6.6 | 14.23 |
| Momordicoside F1 | 2–200 | 9.31 | 0.9965 | y = 107.45 x + 184.30 | 0.25 | 0.50 | 115.3 ± 3.7 | 12.82 |
| Compound | Contents (μg/g) | |||||
|---|---|---|---|---|---|---|
| No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | |
| Momordicoside A | 358.59 ± 18.12 A,x | 339.61 ± 31.92 A,x | 195.55 ± 6.88 A,y | 379.37 ± 27.53 B,x | 1261.6 ± 51.54 A,w | 11.64 ± 0.60 B,z |
| Momordicine I | 24.78 ± 4.93 B,z | 94.08 ± 16.81 B,y | 141.35 ± 6.34 B,x | 470.01 ± 25.03 A,w | 155.83 ± 11.20 B,x | 43.93 ± 3.60 A,z |
| 3β,7β,25-trihydroxycucurbita-5,23(E)-dien-19-al | 1.89 ± 0.13 D,z | 4.03 ± 0.14 C,z | 6.94 ± 0.38 D,E,z | 189.84 ± 7.80 C,x | 37.67 ± 1.36 C,y | 4.15 ± 0.12 E,z |
| Momordicoside K | 4.17 ± 1.31 C,D,y,z | 5.31 ± 1.33 C,y,z | 9.71 ± 2.05 D,x | 5.71 ± 0.47 E,y | 3.40 ± 0.52 D,z | 3.95 ± 0.65 E,y,z |
| Momordicoside L | 10.15 ± 2.03 C,x,y | 12.32 ± 3.15 C,x | 61.15 ± 5.32 C,w | 7.16 ± 1.12 E,z | 14.54 ± 2.26 D,x | 10.74 ± 1.90 B,x,y |
| Momordicoside I aglycone | N. D. * | N. D. | N. D. | N. D. | N. D. | N. D. |
| Momordicoside I | 1.15 ± 0.18 D,z | 0.60 ± 0.71 C,z | 3.56 ± 0.62 E,F,y | 9.84 ± 0.73 E,x | 17.66 ± 0.85 D,w | 1.47 ± 0.38 F,z |
| Momordicoside F2 | 3.06 ± 0.42 D,z | 3.30 ± 0.30 C,z | 6.14 ± 0.28 D,E,y | 75.63 ± 3.33 D,w | 11.44 ± 0.81 D,x | 6.27 ± 0.32 D,y |
| Momordicoside F1 | 0.43 ± 0.10 D,y,z | 0.42 ± 0.15 C,y,z | 0.65 ± 0.18 F,y | 1.27 ± 0.24 E,x | 1.44 ± 0.23 D,x | 0.24 ± 0.16 F,z |
| Momordicoside G | 6.68 ± 2.57 C,D,z | 6.36 ± 2.68 C,z | 7.74 ± 1.51 D,z | 11.49 ± 1.74 E,y | 6.11 ± 0.63 D,z | 8.20 ± 2.11 C,z |
| Total | 410.91 ± 21.08 y | 466.04 ± 48.91 y | 432.79 ± 16.52 y | 1150.31 ± 52.06 x | 1509.71 ± 55.97 w | 90.58 ± 7.57 z |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-T.; Pao, L.-H.; Chen, C.-Y.; Huang, S.-Q.; Kumaran, A.; Chyuan, J.-H.; Chiu, C.-H. Microwave- and Ultrasound-Assisted Extraction of Cucurbitane-Type Triterpenoids from Momordica charantia L. Cultivars and Their Antiproliferative Effect on SAS Human Oral Cancer Cells. Foods 2022, 11, 729. https://doi.org/10.3390/foods11050729
Lee Y-T, Pao L-H, Chen C-Y, Huang S-Q, Kumaran A, Chyuan J-H, Chiu C-H. Microwave- and Ultrasound-Assisted Extraction of Cucurbitane-Type Triterpenoids from Momordica charantia L. Cultivars and Their Antiproliferative Effect on SAS Human Oral Cancer Cells. Foods. 2022; 11(5):729. https://doi.org/10.3390/foods11050729
Chicago/Turabian StyleLee, Yu-Tsung, Li-Heng Pao, Chi-Yuan Chen, Sui-Qing Huang, Alaganandam Kumaran, Jong-Ho Chyuan, and Chun-Hui Chiu. 2022. "Microwave- and Ultrasound-Assisted Extraction of Cucurbitane-Type Triterpenoids from Momordica charantia L. Cultivars and Their Antiproliferative Effect on SAS Human Oral Cancer Cells" Foods 11, no. 5: 729. https://doi.org/10.3390/foods11050729
APA StyleLee, Y. -T., Pao, L. -H., Chen, C. -Y., Huang, S. -Q., Kumaran, A., Chyuan, J. -H., & Chiu, C. -H. (2022). Microwave- and Ultrasound-Assisted Extraction of Cucurbitane-Type Triterpenoids from Momordica charantia L. Cultivars and Their Antiproliferative Effect on SAS Human Oral Cancer Cells. Foods, 11(5), 729. https://doi.org/10.3390/foods11050729