Impact of Different Oak Chips’ Aging on the Volatile Compounds and Sensory Characteristics of Vitis amurensis Wines
Abstract
:1. Introduction
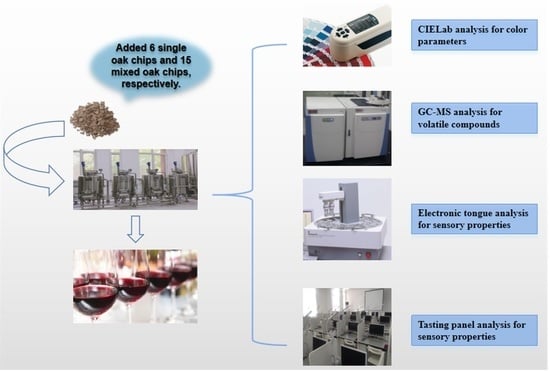
2. Materials and Methods
2.1. Materials
2.2. Preparation of Vitis Amurensis Wine
2.3. Oak-Chip Aging
2.4. Determination of Polyphenols
2.4.1. Determination of Total Phenolic Compounds
2.4.2. Determination of Total Tannins
2.5. Color Evaluation
2.6. Extraction and GC–MS Analysis of Aroma Components
2.7. Sensory Analysis by Electronic Tongue
2.7.1. Principal Component Analysis (PCA)
2.7.2. Radar Graph Analysis
2.8. Sensory Evaluation by Tasting Panel
2.9. Statistical Analysis
3. Results
3.1. Polyphenol Content of V. amurensis Wines
3.2. Color Evaluation
3.3. Aroma Components
3.3.1. Esters
3.3.2. Alcohols
3.3.3. Acids
3.4. Sensory Evaluation by Panelists
3.5. Electronic Tongue (E-Tongue) Evaluation
3.5.1. Principal Component Analysis (PCA)
3.5.2. Radar Graph of V. amurensis Wines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Yang, Y.; Fan, S.; Lu, W. First Report of Brown Rot of fruit on Vitis amurensis Caused by Monilinia polystroma in China. Plant Dis. 2021, 105, 2014. [Google Scholar] [CrossRef] [PubMed]
- Chai, F.; Liu, W.; Xiang, Y.; Meng, X.; Sun, X.; Cheng, C.; Liu, G.; Duan, L.; Xin, H.; Li, S. Comparative metabolic profiling of Vitis amurensis and Vitis vinifera during cold acclimation. Hortic. Res. 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Hua, L. Review: Research progress in amur grape, Vitis amurensis Rupr. Can. J. Plant Sci. 2013, 93, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Yan, X. Research and Utilization of Cold Resistance of Chinese Wild Vitis. J. Anhui Agric. Sci. 2007, 11, 3238–3239. [Google Scholar]
- Zhao, Q.; Duan, C.Q.; Wang, J. Anthocyanins Profile of Grape Berries of Vitis amurensis, Its Hybrids and Their Wines. Int. J. Mol. Sci. 2010, 11, 2212–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.H.; Ma, C.; Liang, X.Q.; Du, L. Measurement and Analysis of the Content of Total Ployphenols and Proanthocyanidins in Carbernet Sauvignon Dry Red Wine from Yun’nan and from Other Regions. Liquor-Making Science & Technology 2011, 04, 29–32. [Google Scholar] [CrossRef]
- Gga, B.; Liu, S.; Sun, X.; Fang, Y. Oenological potential and health benefits of Chinese non- Vitis vinifera species: An opportunity to the revalorization and to breed new varieties. Food Res. Int. 2020, 137, 109443. [Google Scholar] [CrossRef]
- Artem, V.; Antoce, A.O.; Namolosanu, I.; Ranca, A.; Petrescu, A. Horticulture, The influence of the vine cultivation technology on the phenolic composition of red grapes. Sci. Pap. Ser. B Hortic. 2015, 55, 117–122. [Google Scholar]
- Gonzalez-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gandara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Zhang, K.K.; Wang, H.B.; Wang, X.D.; Shi, X.B.; Wang, B.L.; Zheng, X.C.; Liu, F.Z. Studies on Quality Development of ’Italia’Grape During On-vine Storage Under Delayed Cultivation. ACTA Hortic. Sin. 2016, 43, 853–866. [Google Scholar]
- Atanacković, M.; Petrović, A.; Jović, S.; Bukarica, L.G.; Bursać, M.; Cvejić, J. Influence of winemaking techniques on the resveratrol content, total phenolic content and antioxidant potential of red wines. Food Chem. 2012, 131, 513–518. [Google Scholar] [CrossRef]
- Hernández, T.; Estrella, I.; Carlavilla, D.; Martín-álvarez, P.J.; Moreno-Arribas, M.V. Phenolic compounds in red wine subjected to industrial malolactic fermentation and ageing on lees. Anal. Chim. Acta 2006, 563, 116–125. [Google Scholar] [CrossRef]
- Hernández-Orte, P.; Franco, E.; Huerta, C.G.; García, J.M.; Cabellos, M.; Suberviola, J.; Orriols, I.; Cacho, J. Criteria to discriminate between wines aged in oak barrels and macerated with oak fragments. Food Res. Int. 2014, 57, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Bozalongo, R.; Carrillo, J.D.; Torroba, M.; Tena, M.T. Analysis of French and American oak chips with different toasting degrees by headspace solid-phase microextraction-gas chromatography-mass spectrometry. J. Chromatogr. A 2007, 1173, 10–17. [Google Scholar] [CrossRef]
- Kozlovic, G.; Jeromel, A.; Maslov, L.; Pollnitz, A.; Croatia, M. Use of acacia barrique barrels Influence on the quality of Malvazija from Istria wines. Food Chem. 2012, 120, 698–702. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Zhang, H.; Xiao, L.; Tahir, H.E. Aroma profile and sensory characteristics of a sulfur dioxide-free mulberry (Morus nigra) wine subjected to non-thermal accelerating aging techniques. Food Chem. 2017, 232, 89–97. [Google Scholar] [CrossRef]
- Jean-Louis, P.; Philippe, R.; Jérôme, B.-A.; Farid, S.; Michel, M. Determination of ellagitannins in extracts of oak wood and in distilled beverages matured in oak barrels. J. Assoc. Off. Anal. Chem. 2020, 73, 498–501. [Google Scholar]
- Puech, J.-L. Phenolic-Compounds in Oak Wood Extracts Used in the Aging of Brandies. J. Sci. Food Agric. 1988, 42, 165–172. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Puech, J.-L. Wood maturation of distilled beverages. Trends Food Sci. Technol. 1998, 9, 95–101. [Google Scholar] [CrossRef]
- Gahler, S.; Otto, K.; Böhm, V. Alterations of Vitamin C, Total Phenolics, and Antioxidant Capacity as Affected by Processing Tomatoes to Different Products. J. Agric. Food Chem. 2003, 51, 7962–7968. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Dhianawaty, D.; Syamsunarno, M.R.A.A.; Dwiwina, R.G.; Indriyanti, R.A. Separation and Quantification of Sinensetin, Imperatorin and Total Tannin Content as Active Phytoconstituents of Methanol Extract of Imperata cylindrica Root from Kendari. Pharmacogn. J. 2021, 13, 1216–1224. [Google Scholar] [CrossRef]
- Yin, J.B.; Fan, W.L.; Yan, X.U. Study on sensory characteristics of volatile organic acids in Cabernet Gernischt wine. Sci. Technol. Food Ind. 2009, 30, 142–144. [Google Scholar]
- Jin, X.; Wu, S.; Yu, W.; Xu, X.; Huang, M.; Tang, Y.; Yang, Z. Wine Authentication Using Integration Assay of MIR, NIR, E-tongue, HS-SPME-GC-MS, and Multivariate Analyses: A Case Study for a Typical Cabernet Sauvignon Wine. J. AOAC Int. 2019, 102. [Google Scholar] [CrossRef] [PubMed]
- Hartung, A. Factors to consider in wine evaluation. Am. Weld. Soc. 1999, 31, 116–121. [Google Scholar]
- Felhofer, M.; Bock, P.; Xiao, N.; Preimesberger, C.; Lindemann, M.; Hansmann, C.; Gierlinger, N. Oak wood drying: Precipitation of crystalline ellagic acid leads to discoloration. Holzforsch 2021, 75, 712–720. [Google Scholar] [CrossRef]
- Puech, J.L.; Feuillat, F.; Mosedale, J.R. The tannins of oak heartwood: Structure, properties, and their influence on wine flavor. Am. J. Enol. Vitic. 1999, 50, 469–478. [Google Scholar]
- Díaz-Maroto, M.C.; Schneider, R.; Baumes, R.J. Formation pathways of ethyl esters of branched short-chain fatty acids during wine aging. J. Agric. Food Chem. 2005, 53, 3503–3509. [Google Scholar] [CrossRef]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J. Olfactory Impact of Higher Alcohols on Red Wine Fruity Ester Aroma Expression in Model Solution. J. Agric. Food Chem. 2015, 63, 9777. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, T.; Zhao, N.; Xu, J.; Fan, M. Technology, Performance of a novel β-glucosidase BGL0224 for aroma enhancement of Cabernet Sauvignon wines. LWT 2021, 144, 111244. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I. S Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Yue, T.X.; Chi, M.; Song, C.Z.; Liu, M.Y.; Meng, J.F.; Zhang, Z.W.; Li, M.H. Aroma Characterization of Cabernet Sauvignon Wine from the Plateau of Yunnan (China) with Different Altitudes Using SPME-GC/MS. Int. J. Food Prop. 2015, 18, 1584–1596. [Google Scholar] [CrossRef]
- Celik, Z.D.; Cabaroglu, T.; Krieger-Weber, S. Impact of malolactic fermentation on the volatile composition of Turkish Kalecik karası red wines. J. Inst. Brew. 2019, 125, 92–99. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Huang, Y.; Lu, J.; Zhang, Y. Volatile aroma compounds in wines from Chinese wild/hybrid species. J. Food Biochem. 2018, 43. [Google Scholar] [CrossRef]
- Fowles, G.W.A. Acids in grapes and wines: A review. J. Wine Res. 1992, 3, 25–41. [Google Scholar] [CrossRef]
- Vázquez-Pateiro, I.; Arias-González, U.; Miras-Avalos, J.M. Evolution of the Aroma of Treixadura Wines during Bottle Aging. Foods 2020, 9, 1419. [Google Scholar] [CrossRef]
- Fan, L.; Guangling, S.; Huanjiao, G.; Kaili, Z.; Jingyuan, L. Effect of Adding Oak Chips on the Quality of Wine. J. Qingdao Agric. Univ. (Nat. Sci.) 2019, 36, 7. [Google Scholar]
- Pérez-Magariño, S.; González-San José, M.L. Polyphenols and colour variability of red wines made from grapes harvested at different ripeness grade. Food Chem. 2006, 96, 197–208. [Google Scholar] [CrossRef]
- Mateus, N.; Pascual-Teresa, S.D.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; Freitas, V.D. Structural diversity of anthocyanin-derived pigments in port wines. Food Chem. 2002, 76, 335–342. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Wei, Z.; Yu, W.; Cui, Y. Effect of tannin addition on chromatic characteristics, sensory qualities and antioxidant activities of red wines. RSC Adv. 2020, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Dumitriu Gabur, G.D.; Teodosiu, C.; Gabur, I.; Cotea, V.V.; Peinado, R.A.; Lopez de Lerma, N. Evaluation of Aroma Compounds in the Process of Wine Ageing with Oak Chips. Foods 2019, 8, 662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, R.C.; Sefton, M.A.; Taylor, D.K.; Elsey, G.M.J. An odour detection threshold determination of all four possible stereoisomers of oak lactone in a white and a red wine. Aust. J. Grape Wine Res. 2006, 12, 115–118. [Google Scholar] [CrossRef]
- Gordillo, B.; Baca-Bocanegra, B.; Rodriguez-Pulído, F.; González-Miret, M.; García Estévez, I.; Quijada-Morín, N.; Heredia, F.; Escribano-Bailón, M.J. Optimisation of an oak chips-grape mix maceration process. Influence of chip dose and maceration time. Food Chem. 2016, 206, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, R. Wine Science: Principles, Practices, Perception; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]



| No. | Samples | Sample Abbreviation | Total Additive Amounts (g/L) | Sample Proportion |
|---|---|---|---|---|
| 1 | Control | Control | ||
| 2 | Non-toasted French oak | NFr | 4 | 1 |
| 3 | Moderately toasted French oak | MFr | 4 | 1 |
| 4 | Heavily toasted French oak | HFr | 4 | 1 |
| 5 | Moderately toasted Chinese oak | MCh | 4 | 1 |
| 6 | Heavily toasted Chinese oak | HCh | 4 | 1 |
| 7 | Moderately toasted American oak | MAm | 4 | 1 |
| 8 | Non-toasted French oak:Moderately toasted French oak | NFr:MFr | 4 | 1:1 |
| 9 | Non-toasted French oak:Heavily toasted French oak | NFr:HFr | 4 | 1:1 |
| 10 | Non-toasted French oak:Moderately toasted Chinese oak | NFr:MCh | 4 | 1:1 |
| 11 | Non-toasted French oak:Heavily toasted Chinese oak | NFr:HCh | 4 | 1:1 |
| 12 | Non-toasted French oak:Moderately toasted American oak | NFr:MAm | 4 | 1:1 |
| 13 | Moderately toasted French oak:Heavily toasted French oak | MFr:HFr | 4 | 1:1 |
| 14 | Moderately toasted French oak:Moderately toasted Chinese oak | MFr:MCh | 4 | 1:1 |
| 15 | Moderately toasted French oak:Heavily toasted Chinese oak | MFr:HCh | 4 | 1:1 |
| 16 | Moderately toasted French oak:Moderately toasted American oak | MFr:MAm | 4 | 1:1 |
| 17 | Heavily toasted French oak:Moderately toasted Chinese oak | HFr:MCh | 4 | 1:1 |
| 18 | Heavily toasted French oak:Heavily toasted Chinese oak | HFr:HCh | 4 | 1:1 |
| 19 | Heavily toasted French oak:Moderately toasted American oak | HFr:MAm | 4 | 1:1 |
| 20 | Moderately toasted Chinese oak:Heavily toasted Chinese oak | MCh:HCh | 4 | 1:1 |
| 21 | Moderately toasted Chinese oak:Moderately toasted American oak | MCh:MAm | 4 | 1:1 |
| 22 | Heavily toasted Chinese oak:Moderately toasted American oak | HCh:MAm | 4 | 1:1 |
| Appearance, 3 Max | Aroma, 6 Max | Taste and Texture, 6 Max | Aftertaste, 3 Max | Overall Impression, 2 Max | Total Scores | |
|---|---|---|---|---|---|---|
| Grades | 3—Excellent -Brilliant with outstanding characteristic color. 2—Good -Clear with characteristic color. 1—Poor -Slight haze and or slightly off-color. 0—Objectionable -Cloudy and/or off-color. | 6—Extraordinary -Unmistakable, characteristic aroma of grape variety or wine type. Outstanding and complex bouquet. Exceptional balance of aroma and bouquet. 5—Excellent -Characteristic aroma. Complex bouquet. Well balanced. 4—Good -Characteristic aroma. Distinguishable bouquet. 3—Acceptable -Slight aroma and bouquet. Pleasant. 2—Deficient -No perceptible aroma or bouquet or with slight off odors. 1—Poor -Off odors. 0—Objectionable -Objectionable or offensive odors. | 6—Extraordinary -Unmistakable, characteristic flavor of grape variety or wine type. Extraordinary balance. Smooth, full-bodied, and overwhelming. 5—Excellent -All of the above, but a little less. Excellent, but not overwhelming. 4—Good -Characteristic grape variety or wine type flavor. Good balance. Smooth. May have minor imperfections. 3—Acceptable -Undistinguished wine but pleasant. May have minor off-flavors. May be slightly out of balance and/or somewhat thin or rough. 2—Deficient -Undistinguished wine with more pronounced faults than above. 1—Poor -Disagreeable flavors, poorly balanced, and/or unpleasant. 0—Objectionable -Objectionable or offensive flavors and/or texture. | 3—Excellent -Lingering, outstanding aftertaste. 2—Good -Pleasant aftertaste. 1-Poor -Little or no distinguishable aftertaste. 0—Objectionable -Unpleasant aftertaste. | 2—Excellent 1—Good 0—Poor | 18–20 Extraordinary 15–17 Excellent 12–14 Good 9–11 Commercially Acceptable 6–8 Deficient 0–5 Poor and objectionable |
| No. | Sample Abbreviation | TP (g/L) | TTA(g/L) |
|---|---|---|---|
| 1 | Control | 7.91 ± 0.03d | 4.57 ± 0.12c |
| 2 | NFr | 8.26 ± 0.04cd | 4.92 ± 0.06bc |
| 3 | MFr | 8.44 ± 0.08cd | 5.09 ± 0.10bc |
| 4 | HFr | 8.17 ± 0.09cd | 4.82 ± 0.42bc |
| 5 | MCh | 8.80 ± 0.04bc | 5.45 ± 0.06b |
| 6 | HCh | 8.95 ± 0.03b | 5.61 ± 0.05ab |
| 7 | Mam | 8.54 ± 0.09c | 5.20 ± 0.14bc |
| 8 | NFr:MFr | 7.89 ± 0.07d | 4.67 ± 0.11c |
| 9 | NFr:HFr | 8.46 ± 0.18c | 5.11 ± 0.37bc |
| 10 | NFr:MCh | 8.66 ± 0.11bc | 5.32 ± 0.17bc |
| 11 | NFr:HCh | 8.91 ± 0.09bc | 5.56 ± 0.14ab |
| 12 | NFr:Mam | 8.36 ± 0.16cd | 5.01 ± 0.23bc |
| 13 | MFr:HFr | 8.43 ± 0.13cd | 5.09 ± 0.19bc |
| 14 | MFr:MCh | 8.35 ± 0.11cd | 5.00 ± 0.17bc |
| 15 | MFr:HCh | 9.36 ± 0.19a | 6.02 ± 0.26ab |
| 16 | MFr:Mam | 8.49 ± 0.13c | 5.15 ± 0.18bc |
| 17 | HFr:MCh | 7.92 ± 0.09d | 4.63 ± 0.31c |
| 18 | HFr:HCh | 8.05 ± 0.04d | 4.70 ± 0.04c |
| 19 | HFr:Mam | 8.07 ± 0.03d | 4.72 ± 0.05c |
| 20 | MCh:HCh | 9.43 ± 0.13a | 6.18 ± 0.17a |
| 21 | MCh:Mam | 8.51 ± 0.05cd | 5.17 ± 0.06bc |
| 22 | HCh:Mam | 8.89 ± 0.09bc | 5.54 ± 0.11bc |
| Sample | L* | a* | b* | c* | h* | ΔE* |
|---|---|---|---|---|---|---|
| Control | 25.63 ± 0.07h | 6.11 ± 0.35a | 2.27 ± 0.03b | 2.31 ± 0.04h | 45.81 ± 0.23a | 31.91 ± 0.36ab |
| NFr | 28.15 ± 0.02a | 4.64 ± 0.01b | 1.83 ± 0.01b | 4.98 ± 0.01cd | 41.84 ± 0.01b | 30.33 ± 0.02ab |
| MFr | 26.32 ± 0.11fg | 1.60 ± 0.21ef | 2.24 ± 0.00b | 2.76 ± 0.12gh | 40.86 ± 0.42d | 32.97 ± 0.24ab |
| HFr | 26.5 ± 0.03ef | 3.20 ± 0.03cd | 2.18 ± 0.03b | 3.89 ± 0.00ef | 39.66 ± 0.07ef | 32.18 ± 0.05ab |
| MCh | 26.41 ± 0.08f | 2.06 ± 0.14ef | 2.20 ± 0.02b | 2.81 ± 0.20g | 39.91 ± 0.28e | 32.71 ± 0.16ab |
| HCh | 24.74 ± 0.02i | 3.80 ± 0.06c | 2.54 ± 0.02ab | 2.57 ± 0.04gh | 45.98 ± 0.18a | 33.38 ± 0.07ab |
| MAm | 26.84 ± 0.02de | 4.63 ± 0.02b | 2.47 ± 0.01ab | 5.24 ± 0.01c | 40.75 ± 0.01d | 31.26 ± 0.03ab |
| NFr:MFr | 25.43 ± 0.17h | 3.25 ± 0.56cd | 2.63 ± 0.01ab | 4.19 ± 0.45ef | 39.36 ± 0.06ef | 32.95 ± 0.59ab |
| NFr:HFr | 26.6 ± 0.01ef | 2.64 ± 0.06de | 2.09 ± 0.06b | 3.37 ± 0.07f | 39.06 ± 0.07f | 32.34 ± 0.09ab |
| NFr:MCh | 27.45 ± 0.04c | 4.25 ± 0.15bc | 2.10 ± 0.01b | 4.74 ± 0.13d | 40.96 ± 0.15cd | 30.99 ± 0.16ab |
| NFr:HCh | 26.76 ± 0.02e | 5.42 ± 0.03a | 2.34 ± 0.01b | 5.90 ± 0.03b | 41.76 ± 0.01c | 31.1 ± 0.04ab |
| NFr:MAm | 25.57 ± 0.09h | 1.38 ± 0.08f | 2.27 ± 0.08b | 2.66 ± 0.03gh | 41.37 ± 0.32cd | 33.69 ± 0.14a |
| MFr:HFr | 25.90 ± 0.18gh | 3.52 ± 0.04cd | 2.39 ± 0.05ab | 4.26 ± 0.06e | 39.59 ± 0.05ef | 32.51 ± 0.19ab |
| MFr:MCh | 28.01 ± 0.12ab | 5.42 ± 0.27a | 6.97 ± 6.54a | 5.90 ± 0.23b | 41.80 ± 0.32bc | 28.74 ± 6.55b |
| MFr:HCh | 27.86 ± 0.02b | 6.00 ± 0.04a | 2.12 ± 0.33b | 6.37 ± 0.07a | 42.63 ± 0.55b | 30.02 ± 0.33ab |
| MFr:MAm | 26.83 ± 0.06de | 3.00 ± 0.16d | 2.13 ± 0.08b | 3.69 ± 0.08f | 39.37 ± 0.33ef | 31.99 ± 0.19ab |
| HFr:MCh | 26.54 ± 0.11ef | 4.11 ± 0.13bc | 2.32 ± 0.00b | 4.72 ± 0.11de | 40.41 ± 0.14de | 31.76 ± 0.17ab |
| HFr:HCh | 26.11 ± 0.13g | 2.88 ± 0.47de | 2.22 ± 0.28b | 3.65 ± 0.21f | 39.68 ± 0.23ef | 32.63 ± 0.56ab |
| HFr:MAm | 27.06 ± 0.03d | 2.87 ± 0.22de | 1.91 ± 0.14b | 3.45 ± 0.11f | 39.57 ± 0.59ef | 31.92 ± 0.26ab |
| MCh:HCh | 25.68 ± 0.08h | 2.42 ± 0.04de | 2.39 ± 0.03ab | 3.41 ± 0.01f | 39.70 ± 0.07ef | 33.13 ± 0.09ab |
| MCh:MAm | 28.00 ± 0.11ab | 2.23 ± 0.21e | 1.83 ± 0.16b | 2.89 ± 0.06g | 39.31 ± 0.10ef | 31.42 ± 0.29 ab |
| HCh:MAm | 26.64 ± 0.04ef | 2.95 ± 0.11d | 2.36 ± 0.02b | 3.78 ± 0.09f | 38.94 ± 0.01f | 32.10 ± 0.12 ab |
| Compounds | CON | NFr | MFr | HFr | MCh | HCh | MAm | NFr: MFr | NFr: HFr | NFr: MCh | NFr: HCh | NFr: MAm | MFr: HFr | MFr: MCh | MFr: HCh | MFr: MAm | HFr: MCh | HFr: HCh | HFr: MAm | MCh: HCh | MCh: MAm | HCh: MAm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Esters | ||||||||||||||||||||||
| Ethyl acetate | 1.638 ± 0.15c | - | 3.798 ± 0.54ab | 1.723 ± 0.92c | - | - | - | - | - | 5.345 ± 0.86a | - | - | - | - | - | 2.738 ± 0.28cd | - | - | - | - | 2.927 ± 0.54bc | - |
| Isopropyl acetate | 37.60 ± 4.27gh | 48.74 ± 3.30fg | 52.54 ± 3.05f | 37.80 ± 2.89gh | 53.83 ± 1.65ef | 59.94 ± 2.45ef | 31.17 ± 3.33h | 55.06 ± 0.95ef | 71.89 ± 2.94d | 83.8 ± 1.05c | 43.69 ± 1.53g | 47.13 ± 0.94fg | 125.1 ± 1.45a | 49.74 ± 2.72fg | 48.18 ± 3.47fg | 62.35 ± 3.53e | 95.9± 3.10b | 54.35 ± 0.93ef | 46.5± 2.35fg | 44.24 ± 3.66fg | 57.45 ± 2.15ef | 43.69 ± 2.46g |
| 3-Methylbuty lacetate | 5.635 ± 0.4ef | 6.354 ± 0.81ef | 8.08± 0.45de | 5.513 ± 0.84ef | 7.154 ± 0.42ef | 8.14± 0.3de | 4.847 ± 0.36f | 7.978 ± 0.71de | 9.26± 0.21d | 14.72 ± 0.15c | 5.353 ± 0.52f | 5.943 ± 0.99ef | 18.37 ± 0.54a | 7.269 ± 0.39e | 6.679 ± 0.42ef | 8.87± 0.54de | 15.35± 0.56bc | 8.29± 0.45de | 6.946 ± 0.47ef | 6.213 ± 0.17ef | 7.504 ± 0.96de | 5.353 ± 0.3f |
| Butanoic acid, ethyl ester | 2.271 ± 0.83b | 2.669 ± 0.61b | 2.648 ± 0.47b | 2.694 ± 0.87b | 2.891 ± 0.14b | 2.855 ± 0.29b | 2.042 ± 0.39b | 3.098 ± 0.49b | 3.736 ± 0.77ab | 4.744 ± 0.39ab | 2.458 ± 0.84b | 2.508 ± 0.23b | 5.725 ± 0.98a | 3.201 ± 0.52b | 2.644 ± 0.77b | 3.811 ± 0.22ab | 4.806 ± 0.24ab | 3.048 ± 0.77b | 2.592 ± 0.31b | 2.991 ± 0.95b | 2.618 ± 0.93b | 2.458 ± 0.18b |
| Acetic acid, hydroxy-,ethyl ester | 2.461 ± 0.68cd | 9.77± 0.35a | 3.680 ± 0.43c | 4.680 ± 0.43bc | 8.48± 0.82a | 5.308 ± 0.75bc | 1.240 ± 0.76d | 3.998 ± 0.98c | 4.860 ± 0.23bc | 5.65± 0.21bc | 2.967 ± 0.24cd | 4.384 ± 0.38bc | 6.030 ± 0.44b | 4.382 ± 0.61bc | 3.261 ± 0.29c | 4.370 ± 0.74bc | 4.481 ± 0.32bc | 4.513 ± 0.13bc | 3.814 ± 0.50c | 3.130± 0.88c | 4.243 ± 0.82bc | 2.960 ± 0.17cd |
| Propanoic acid,2-hydroxy-, methyl ester | 5.608 ± 0.28d | 8.34± 0.57bc | 7.092 ± 0.63cd | 5.674 ± 0.59d | 6.709 ± 0.52cd | 7.733 ± 0.18c | 5.691 ± 0.32d | 7.86± 0.57c | 9.50± 0.17b | 10.85 ± 0.25ab | 6.122 ± 0.56d | 6.297 ± 0.22cd | 12.22 ± 0.71a | 8.49± 0.79bc | 7.643 ± 0.54cd | 8.44± 0.21bc | 11.39 ± 0.57ab | 8.19 ± 0.37bc | 6.632 ± 0.38cd | 7.264 ± 0.26cd | 7.091 ± 0.69cd | 6.122 ± 0.55d |
| Propanoic acid,2-hydroxy-, ethyl ester | 2.264 ± 0.33bc | - | 3.134 ± 0.12bc | - | - | 2.872 ± 0.15bc | 2.132 ± 0.79c | 3.199 ± 0.18bc | 4.011 ± 0.72b | - | - | - | - | 6.993 ± 0.40a | - | 3.683 ± 0.62bc | 5.707 ± 0.65ab | 3.411 ± 0.74bc | 2.944 ± 0.94bc | - | - | - |
| Ethyl(s)- (-)-lactate | 53.14 ± 1.92c | 71.83 ± 2.36ab | 56.36 ± 4.26bc | 54.96 ± 1.57bc | 56.86 ± 3.25bc | 63.53 ± 3.77c | 58.07 ± 1.86bc | 72.59 ± 3.94ab | 72.94 ± 4.35ab | 71.96 ± 4.37ab | 59.92 ± 2.86bc | 59.55 ± 3.56bc | 75.75 ± 1.24a | 74.06 ± 1.69ab | 67.84 ± 3.64ab | 69.42 ± 1.39ab | 72.01 ± 3.57ab | 70.65 ± 3.25ab | 67.42 ± 3.09ab | 75.79 ± 1.13a | 64.84 ± 3.81c | 59.92 ± 2.13bc |
| Nonanoic acid, ethyl ester | 3.545 ± 0.71d | 5.006 ± 0.26d | 5.051 ± 0.66d | 4.353 ± 0.28d | 4.303 ± 0.57d | 5.876 ± 0.15cd | 3.641 ± 0.33d | 4.883 ± 0.77d | 7.003 ± 0.47c | 11.22 ± 0.57ab | 3.938 ± 0.65d | 4.183 ± 0.29d | 12.47 ± 0.46a | 5.811 ± 0.68cd | 5.138 ± 0.42d | 6.441 ± 0.14cd | 9.98± 0.72b | 5.197 ± 0.84d | 4.317 ± 0.84d | 3.731 ± 0.49d | 4.548 ± 0.13d | 3.938 ± 0.49d |
| Trans-2-hexenyl-acetate | 11.69 ± 3.14c | 16.51 ± 0.64bc | 16.39 ± 0.97bc | 13.52 ± 1.13c | 14.30 ± 3.25c | 18.69 ± 1.05bc | 12.83 ± 3.46c | 16.11 ± 0.89c | 23.25 ± 2.16b | 37.93 ± 0.97a | 12.20 ± 2.31c | 12.95 ± 2.18c | 40.79 ± 2.03a | 17.41 ± 1.87bc | 15.89 ± 1.07c | 20.74 ± 0.91bc | 35.05 ± 3.30a | 17.35 ± 2.49bc | 14.15 ± 3.08c | 11.79 ± 3.39c | 15.41 ± 1.50c | 12.21 ± 0.88c |
| Propanoic acid,3-methoxyl-, ethyl ester | 34.11 ± 2.72b | 9.30 ± 2.28de | 37.27 ± 1.12b | 14.43 ± 1.12de | 14.89 ± 1.62d | 9.19 ± 1.42de | 5.203 ± 0.79e | 9.42 ± 1.03de | 54.07 ± 2.42a | 11.52 ± 1.46de | 24.71 ± 2.02c | 8.33 ± 1.98e | 13.79 ± 2.17de | 10.07 ± 2.55de | 7.531 ± 1.49e | 9.64 ± 1.39de | 12.82 ± 3.03de | 13.06 ± 1.94de | 8.19 ± 1.79e | 7.660 ± 2.11e | 34.54 ± 0.94b | 24.71 ± 1.31c |
| Pentadecanoic acid, 3-methybutyl ester | 80.3 ± 1.24f | 131.1 ± 2.93cd | 119.1 ± 4.28d | 105.8 ± 3.03e | 121.8 ± 2.02d | 118.5 ± 3.15d | 146.9 ± 1.28b | 140.1 ± 2.29bc | 157.2 ± 2.44a | 137.9 ± 3.26bc | 113.8 ± 1.09de | 107.2 ± 2.49e | 148.2 ± 2.17ab | 140.3 ± 4.24bc | 127.4 ± 3.98cd | 138.3 ± 4.43bc | 142.8 ± 1.19bc | 142.7 ± 4.12bc | 127.1 ± 1.09cd | 143.2 ± 3.68b | 133.6 ± 1.49c | 113.8 ± 2.87de |
| Butanoic acid, diethyl ester | 1.406 ± 0.22a | 1.632 ± 0.30a | 1.458 ± 0.44a | 1.451 ± 0.33a | - | 1.427 ± 0.57a | 1.311 ± 0.22a | 1.735 ± 0.41a | 1.875 ± 0.32a | - | - | - | 1.853 ± 0.64a | 1.763 ± 0.94a | - | 1.550 ± 0.41a | 1.903 ± 0.41a | 1.832 ± 0.77a | 1.521 ± 0.63a | - | - | - |
| Benzoic acid, ethyl ester | 2.729 ± 0.16b | 2.647 ± 0.29bc | 3.391 ± 0.42a | 1.859 ± 0.21bc | 2.749 ± 0.43a | 2.963 ± 0.39a | 1.293 ± 0.35c | 3.148 ± 0.48a | 4.814 ± 0.88a | 4.046 ± 0.58a | 1.812 ± 0.72c | 1.997 ± 0.34bc | 4.989 ± 0.87a | 2.906 ± 0.92a | 3.031 ± 0.85a | 2.921 ± 0.79a | 3.699 ± 0.37a | 2.393 ± 0.89bc | 1.052 ± 0.22c | 2.068 ± 0.48bc | 2.294 ± 0.63bc | 1.812 ± 0.98c |
| Propanoic acid, 2-hydroxy-, ethyl ester | 9.47 ± 1.87e | 10.20 ± 1.99e | 53.03 ± 3.39b | 17.07 ± 1.19de | 18.01 ± 2.26de | 11.77 ± 1.14e | 6.365 ± 1.46e | 11.12 ± 2.13e | 75.16 ± 3.33a | 22.79 ± 1.12de | 29.96 ± 1.24c | 9.88 ± 2.55e | 24.97 ± 2.88cd | 10.55 ± 2.37e | 8.21 ± 2.16e | 11.63 ± 0.58e | 22.06 ± 1.41d | 12.50 ± 1.67e | 9.12 ± 1.75e | 6.691 ± 2.83e | 47.82 ± 1.42b | 29.96 ± 2.97c |
| Butanedioic acid, diethyl ester | 47.76 ± 1.36b | 51.31 ± 2.38b | 0.921 ± 1.53d | 44.47 ± 3.51bc | 46.13 ± 1.48bc | 0.980 ± 2.25d | 39.75 ± 4.17c | 57.98 ± 2.78ab | 1.491 ± 1.09d | 60.31 ± 2.24ab | 46.14 ± 1.04bc | 42.12 ± 3.58c | 60.88 ± 3.87a | 57.46 ± 2.86ab | 49.81 ± 2.08b | 56.42 ± 1.32ab | 2.154 ± 0.34d | 60.69 ± 4.19a | 0.6981 ± 0.95d | 58.03 ± 1.94ab | 53.87 ± 2.97ab | 46.14 ± 1.97b |
| 2-Phenethyl acetate | 14.41 ± 0.66e | 16.20 ± 0.94de | 16.82 ± 0.90d | 11.45 ± 0.36f | 13.36 ± 0.17ef | - | 10.50 ± 0.72f | 16.85 ± 0.65d | 19.77 ± 0.54c | 26.09 ± 0.53b | 14.85 ± 0.47e | - | 40.47 ± 0.33a | - | 13.53 ± 0.13ef | - | - | 15.84 ± 0.57de | - | 12.28 ± 0.51f | 15.54 ± 0.32de | 14.85 ± 0.34e |
| Methyl dihydrojasmonate | 6.025 ± 0.83de | 6.585 ± 0.20d | 5.150 ± 0.27ef | 6.817 ± 0.99d | 3.679 ± 0.23f | 4.973 ± 0.73f | 5.725 ± 0.84de | 4.867 ± 0.74f | 5.567 ± 0.67de | 7.129 ± 0.11c | 3.693 ± 0.19f | 3.885 ± 0.94f | 7.738 ± 0.76a | 5.151 ± 0.78ef | 4.593 ± 0.49f | 5.646 ± 0.16de | 7.179 ± 0.94b | 6.132 ± 0.89de | 5.435 ± 0.32e | 4.582 ± 0.77f | 5.144 ± 0.48f | 3.693 ± 0.62f |
| Butanoic acid, hydroxy-, diethyl ester | 90.4 ± 4.45e | 1.591 ± 3.92g | 1.741 ± 2.09g | 90.1 ± 4.11e | 92.7 ± 2.46e | 92.4 ± 0.47e | 126.5 ± 1.79ab | 110.1 ± 2.50c | 2.413 ± 0.89g | 124.1 ± 1.43ab | - | 81.9 ± 0.96f | 128.2 ± 2.63a | 111.2 ± 3.84c | 101.1 ± 4.14d | 109.9 ± 1.54c | 6.183 ± 1.15g | 120.9 ± 3.77b | 1.701 ± 1.04g | 110.5 ± 1.66c | 108.1 ± 1.74cd | 124.0 ± 0.75ab |
| Ethyl 3-methylbutyl succinate | 38.05 ± 1.04cd | 50.13 ± 1.54bc | 43.06 ± 1.06c | 37.99 ± 2.99cd | 43.51 ± 2.16c | 42.77 ± 3.22c | 32.99 ± 1.00d | 52.62 ± 1.16ab | 59.45 ± 1.82a | 54.76 ± 2.09ab | 43.56 ± 2.10c | 38.53 ± 1.23cd | 58.63 ± 1.13a | 55.05 ± 2.07ab | 46.85 ± 1.82bc | 50.68 ± 2.28bc | 56.06 ± 2.27ab | 55.49 ± 3.02ab | 48.18 ± 0.94bc | 54.33 ± 3.05ab | 51.01 ± 3.44b | 43.56 ± 3.45c |
| Tetradecanoic acid, ethyl ester | 27.76 ± 1.19ab | 30.25 ± 2.04ab | 23.55 ± 1.82b | 26.04 ± 1.42b | 25.89 ± 2.28b | 22.84 ± 3.08b | 19.56 ± 3.01b | 27.15 ± 1.73b | 30.99 ± 1.96ab | 26.58 ± 4.23b | 22.63 ± 2.45b | 19.72 ± 1.65b | 29.71 ± 1.54ab | 26.96 ± 2.77b | 24.73 ± 0.91b | 27.03 ± 3.82b | 30.23 ± 2.40ab | 35.14 ± 1.80a | 26.39 ± 1.23b | 26.17 ± 1.67b | 29.47 ± 3.02ab | 22.61 ± 1.92b |
| Hexadecanoic acid, methyl ester | 2.023 ± 0.62a | 1.732 ± 0.69a | 1.345 ± 0.89a | 1.894 ± 0.10a | 1.361 ± 0.41a | 1.382 ± 0.12a | 1.343 ± 0.36a | 1.813 ± 0.61a | 1.708 ± 0.45a | 2.156 ± 0.18a | 1.561 ± 0.41a | 1.064 ± 0.59a | 2.407 ± 0.88a | 1.633 ± 0.38a | 1.531 ± 0.86a | 2.254 ± 0.42a | 2.677 ± 0.98a | 2.069 ± 0.69a | 1.564 ± 0.18a | 2.928 ± 0.51a | 1.792 ± 0.41a | 1.561 ± 0.59a |
| Ethyl hydrogen succinate | 116.0 ± 1.23j | 161.8 ± 2.78h | 157.1 ± 2.59hi | 112.1 ± 1.63j | 172.8 ± 3.96g | 151.7 ± 1.77i | 109.4 ± 4.03j | 218.3 ± 2.51de | 280.2 ± 1.98a | 222.3 ± 1.06d | 218.6 ± 3.89d | 154.7 ± 2.46hi | 206.7 ± 1.43e | 250.6 ± 1.25bc | 193.5 ± 4.35f | 209.2 ± 3.85e | 223.9 ± 3.23d | 246.6 ± 3.13c | 169.8 ± 2.33gh | 248.8 ± 2.62c | 246.2 ± 4.17c | 218.6 ± 1.76d |
| Hexadecanoic acid, 2-hydroxyethyl ester | 18.38 ± 1.01ab | 22.98 ± 2.68ab | 8.67 ± 3.54bc | 21.84 ± 4.22ab | 11.53 ± 2.05b | 10.53 ± 4.46bc | 23.68 ± 2.64ab | 0.6435 ± 3.47c | 13.80 ± 1.04b | 27.39 ± 2.64a | 18.79 ± 4.09ab | 12.33 ± 3.42b | 13.78 ± 2.38b | 0.7617 ± 1.64c | 19.99 ± 2.57ab | 0.6411 ± 0.23c | 18.93 ± 2.19ab | 1.273 ± 1.14c | 12.46 ± 2.08b | 19.61 ± 4.26ab | 16.68 ± 1.37b | 18.79 ± 3.93ab |
| Total | 614.7 | 666.7 | 631.4 | 624.2 | 722.9 | 646.4 | 652.2 | 831 | 915 | 973 | 676.8 | 624.6 | 1039 | 852 | 759.1 | 817 | 785.3 | 892 | 568.5 | 852 | 913 | 801 |
| Alcohols | ||||||||||||||||||||||
| 1-Propanol | 97.9 ± 2.84h | 141.4 ± 1.41g | 125.2 ± 2.19fg | 104.4 ± 2.17h | 125.3 ± 3.21f | 131.1 ± 1.09ef | 82.7 ± 4.02i | 154.1 ± 1.70cd | 169.1 ± 3.21b | 153.9 ± 3.43cd | 121.3 ± 1.69fg | 114.3 ± 3.32h | 182.6 ± 4.21a | 150.5 ± 1.84d | 130.7 ± 2.89ef | 158.9 ± 3.61c | 165.9 ± 3.29b | 136.2 ± 2.58e | 126.8 ± 3.14ef | 163.2 ± 1.66b | 142.7 ± 3.31de | 121.4 ± 3.76fg |
| 1-Butanol | 72.43 ± 2.33d | 30.83 ± 2.06gh | 84.8 ± 1.68c | 46.04 ± 3.69fg | 185.1 ± 3.87a | 38.65 ± 3.61gh | 41.88 ± 2.62fg | 52.91 ± 3.61ef | 105.2 ± 4.46b | 46.86 ± 0.97fg | 48.88 ± 4.07f | 30.14 ± 1.49h | 39.65 ± 2.35g | 49.04 ± 4.37f | 40.72 ± 2.02fg | 53.41 ± 2.19ef | 48.42 ± 1.15fg | 45.69 ± 1.46fg | 44.81 ± 1.57fg | 60.85 ± 0.92e | 84.3 ± 0.99c | 48.88 ± 3.29f |
| 1-Butanol,3-methyl- | 167.4 ± 7.64d | 201.3 ± 5.04bc | 192.3 ± 6.76c | 170.4 ± 8.49d | 177.9 ± 5.90cd | 195.1 ± 6.57bc | 167.9 ± 9.49d | 225.0 ± 5.21ab | 237.3 ± 6.90a | 203.4 ± 6.23bc | 196.9 ± 5.64bc | 188.2 ± 8.02cd | 215.2 ± 6.54b | 219.5 ± 5.85ab | 201.1 ± 6.37bc | 212.0 ± 8.12bc | 170 ± 3.87d | 202.9 ± 5.19bc | 173 ± 3.37cd | 215.9 ± 6.31ab | 206.7 ± 6.83bc | 196.9 ± 8.32bc |
| 2-Pentanol | 24.62 ± 2.57e | 28.89 ± 4.08e | 35.47 ± 3.27de | 24.79 ± 2.08e | 30.72 ± 3.93de | 35.64 ± 2.42de | 21.84 ± 3.28e | 36.45 ± 1.65de | 47.61 ± 4.32cd | 67.74 ± 1.96b | 23.54 ± 2.42e | 26.61 ± 4.29e | 81.3 ± 1.99a | 32.78 ± 3.96de | 30.19 ± 2.85de | 39.76 ± 1.32d | 55.61 ± 3.87c | 37.16 ± 1.54de | 31.81 ± 1.71de | 25.37 ± 3.69e | 33.86 ± 2.32de | 23.54 ± 3.51e |
| 1-Pentanol | 57.68 ± 1.73gh | 68.49 ± 3.35fg | 82.3 ± 1.35ef | 57.71 ± 2.91gh | 70.66 ± 2.89fg | 83.3 ± 1.56e | 50.76 ± 3.01h | 83.6± 2.68de | 110.5 ± 2.74c | 150.5 ± 4.24b | 56.99 ± 4.27gh | 62.45 ± 1.31g | 177.4 ± 1.27a | 75.81 ± 2.93ef | 70.77 ± 2.50fg | 92.8 ± 2.92d | 156.1 ± 1.06b | 88.7 ± 2.34de | 73.47 ± 3.37f | 61.15 ± 3.93g | 79.81 ± 3.74ef | 56.99 ± 2.31gh |
| 4-Methylpentanol | 2.987 ± 0.20b | - | 3.309 ± 0.39ab | - | - | 3.261 ± 0.41ab | 2.951 ± 0.88b | 3.936 ± 0.41ab | 4.599 ± 0.63ab | - | - | - | 4.823 ± 0.78a | 3.639 ± 0.37ab | - | 3.623 ± 0.21ab | 4.339 ± 0.64ab | 4.002 ± 0.60ab | 3.242 ± 0.35ab | - | - | - |
| 1-Hexanol | 63.23 ± 1.39e | 83.6 ± 1.65cd | 70.61 ± 2.85de | 67.18 ± 3.98e | 76.43 ± 0.94d | 79.73 ± 0.93cd | 64.37 ± 2.67e | 98.1 ± 2.65b | 109.2 ± 1.94a | 99.7 ± 1.33b | 76.95 ± 2.55d | 71.91 ± 1.38de | 107.0 ± 1.88ab | 94.2 ± 4.15bc | 85.8 ± 4.29c | 94.6 ± 2.47b | 104.9 ± 2.06ab | 96.9 ± 3.33b | 86.0 ± 2.36c | 98.5 ± 1.15b | 81.3 ± 2.95cd | 76.95 ± 2.57d |
| 1-Hexen-3-ol | 1.306 ± 0.82a | - | 1.087 ± 0.56a | 1.261 ± 0.43a | - | 1.648 ± 0.85a | 1.336 ± 0.47a | 1.968 ± 0.88a | 1.585 ± 0.16a | - | - | - | 1.964 ± 0.59a | 1.514 ± 0.73a | - | 1.373 ± 0.38a | 1.539 ± 0.17a | 1.883 ± 0.59a | 1.643 ± 0.59a | - | - | - |
| 1-Propanol,3-ethoxy- | 12.71 ± 0.81bc | 11.79 ± 0.39cd | 11.43 ± 0.88cd | 9.74 ± 0.23d | 10.34 ± 0.85cd | 11.89 ± 0.87cd | 9.16 ± 0.57d | 13.72 ± 0.86bc | 16.25 ± 0.74ab | 16.59 ± 0.59a | 11.56 ± 0.22cd | 10.06 ± 0.48d | 17.76 ± 0.60a | 13.23 ± 0.28bc | 12.15 ± 0.74c | 13.72 ± 0.73bc | 17.13 ± 0.68a | 14.26 ± 0.25b | 12.01 ± 0.39cd | 13.52 ± 0.64bc | 12.93 ± 0.31bc | 11.56 ± 0.56cd |
| 2-Hexen-1-ol | 2.017 ± 0.12bc | - | 2.737 ± 0.13bc | 1.764 ± 0.92c | - | 2.543 ± 0.67bc | 1.539 ± 0.57c | 2.493 ± 0.45bc | 3.649 ± 0.19b | - | 1.873 ± 0.51c | - | 5.684 ± 0.65a | 2.448 ± 0.69bc | - | 2.717 ± 0.58bc | 5.424 ± 0.82a | 2.524 ± 0.16bc | 2.168 ± 0.56bc | 1.856 ± 0.29c | - | 1.873 ± 0.26c |
| 3-Hexen-1-ol, (E)- | 3.771 ± 0.46cd | 4.635 ± 0.73bc | 5.086 ± 0.91bc | 4.095 ± 0.81c | 4.227 ± 0.95c | 5.325 ± 0.94bc | 3.468 ± 0.68cd | 5.505 ± 0.23bc | 6.981 ± 0.15b | 10.56 ± 0.92a | 3.474 ± 0.93cd | 1.230 ± 0.80d | 11.58 ± 0.49a | 1.459 ± 0.48d | 4.681 ± 0.46bc | 5.793 ± 0.77bc | 10.68 ± 0.86a | 5.829 ± 0.39bc | 4.834 ± 0.62bc | 3.921 ± 0.54c | 4.856 ± 0.98bc | 3.474 ± 0.96cd |
| 1-Octen-3-ol | 17.82 ± 3.27cd | 21.59 ± 2.52cd | 23.58 ± 1.10c | 18.04 ± 3.01cd | 17.57 ± 0.28cd | 25.31 ± 0.88bc | 15.68 ± 2.56d | 23.25 ± 3.14c | 31.79 ± 2.74b | 47.65 ± 2.13a | 19.13 ± 2.36cd | 19.21 ± 1.56cd | 49.46 ± 2.81a | 23.67 ± 3.23c | 21.93 ± 1.29cd | 27.53 ± 1.22bc | 45.83 ± 2.34a | 25.30 ± 0.71bc | 20.86 ± 2.69cd | 18.63 ± 2.39cd | 23.36 ± 1.59c | 19.13 ± 1.31cd |
| 3-Hexen-1-ol, (Z)- | 52.22 ± 3.03a | 72.60 ± 2.10b | 52.81 ± 1.97b | 56.01 ± 1.74c | 49.91 ± 1.08cd | 74.79 ± 3.66cd | 361.3 ± 3.89cd | 84.2 ± 2.12cd | 86.1 ± 3.62d | 64.29 ± 4.13d | 99.7 ± 1.72de | 67.43 ± 3.43de | 77.88 ± 1.16de | 75.75 ± 2.06de | 65.11 ± 3.59de | 79.01 ± 2.49e | 80.7 ± 1.83eg | 61.28 ± 1.25eg | 71.15 ± 0.97eg | 69.75 ± 3.03eg | 72.11 ± 3.97eg | 99.7 ± 2.25g |
| 1-Heptanol | 5.210 ± 0.97a | - | 1.398 ± 0.61b | - | - | 0.7570 ± 0.88 bc | - | 7.385 ± 0.54bc | 14.05 ± 0.49bc | - | 0.7511 ± 0.65 c | 4.731 ± 0.17c | - | 3.373 ± 0.32d | 9.62 ± 0.85d | 9.55 ± 0.58de | 10.28 ± 0.96e | 7.684 ± 0.73e | 0.833 ± 0.29e | 0.4410 ± 0.47 e | 8.25 ± 0.91e | 0.7514 ± 0.19 e |
| 2,3- Butanediol, [R-(R*, R*)]- | 33.63 ± 4.40a | 43.82 ± 2.32a | 40.21 ± 3.91ab | 28.65 ± 3.71ab | 39.52 ± 2.20ab | 44.36 ± 1.62b | 23.60 ± 2.21b | 54.90 ± 2.69b | 59.29 ± 3.23b | 41.59 ± 2.01b | 47.90 ± 1.26b | 32.37 ± 0.97b | 40.44 ± 2.80bc | 51.34 ± 3.20bc | 43.63 ± 4.14bc | 54.02 ± 1.35bc | 45.45 ± 3.31bc | 60.23 ± 3.13bc | 47.49 ± 2.71c | 42.88 ± 4.32cd | 38.64 ± 4.17cd | 47.91 ± 3.27d |
| 2-Octen-1-ol, (E) | 13.76 ± 3.12a | 16.44 ± 2.98a | 19.79 ± 3.26a | 13.90 ± 3.35b | 15.69 ± 0.51bc | 19.71 ± 2.94bc | 11.53 ± 2.11c | 19.17 ± 1.12c | 27.66 ± 0.94c | 40.64 ± 2.25cd | 13.99 ± 2.81cd | 14.27 ± 1.03cd | 42.05 ± 3.18cd | 17.82 ± 0.31cd | 16.58 ± 1.14cd | 21.51 ± 3.11cd | 41.48 ± 3.41cd | 21.58 ± 0.77cd | 16.83 ± 1.53cd | 14.33 ± 1.21cd | 19.65 ± 3.25cd | 13.99 ± 2.41d |
| À-terpineol | 25.86 ± 1.48a | 26.14 ± 2.29ab | 25.31 ± 1.93ab | 19.82 ± 4.44ab | 29.97 ± 2.47ab | 24.27 ± 1.04ab | 23.64 ± 1.62ab | 30.19 ± 1.26ab | 29.87 ± 2.42ab | 28.28 ± 1.50ab | 22.33 ± 2.24ab | 15.86 ± 3.62ab | 38.72 ± 1.28ab | 34.55 ± 1.20b | 31.52 ± 3.57b | 24.91 ± 4.42b | 20.58 ± 1.01b | 21.03 ± 1.29b | 12.19 ± 2.24bc | 26.12 ± 0.96bc | 20.17 ± 2.57c | 22.33 ± 1.31c |
| 3-(Methylthio) propanol | 37.72 ± 1.45a | 50.09 ± 2.41ab | 52.88 ± 3.86ab | 36.75 ± 1.62b | 43.51 ± 1.66bc | 48.16 ± 4.06bc | 36.73 ± 3.60bc | 60.26 ± 1.73bc | 73.88 ± 1.21c | 63.67 ± 1.31c | 1.880 ± 2.54 | 44.77 ± 4.24cd | 59.04 ± 1.87cd | 53.81 ± 2.33cd | 50.48 ± 3.69cd | 59.39 ± 0.96d | 67.66 ± 2.49d | 67.09 ± 1.70d | 54.48 ± 4.23d | 61.82 ± 2.41d | 54.94 ± 1.29e | 1.884 ± 1.31e |
| Benzyl Alcohol | 1.012 ± 0.82a | 1.672 ± 0.62ab | 1.228 ± 0.53ab | 1.194 ± 0.88ab | 1.151 ± 0.63ab | 1.822 ± 0.37ab | 1.405 ± 0.2ab | 2.411 ± 0.41ab | 1.649 ± 0.72b | 2.415 ± 0.31b | 1.921 ± 0.73b | 1.732 ± 0.39b | 2.331 ± 0.67b | - | 2.060 ± 0.38b | 2.235 ± 0.68b | 4.172 ± 1.33b | 2.573 ± 0.52b | 0.3870 ± 0.59 b | 2.334 ± 0.94b | 1.519 ± 0.66b | 1.923 ± 0.26b |
| Phenylethyl Alcohol | 127.9 ± 1.40c | 137.9 ± 3.39bc | 133.5 ± 3.76c | 131.3 ± 2.84c | 133.6 ± 1.32c | 134.6 ± 1.12bc | 129.4 ± 1.35c | 141.0 ± 4.35bc | 1439 ± 3.55a | 140.9 ± 4.37bc | 133.7 ± 1.55c | 131.1 ± 1.10c | 141.5 ± 1.32bc | 141.1 ± 0.96bc | 136.9 ± 2.32bc | 140.7 ± 4.14bc | 135.9 ± 0.71bc | 142.8 ± 2.67b | 110.3 ± 4.39d | 141.8 ± 2.55bc | 138.1 ± 2.68bc | 133.8 ± 1.81c |
| Benzeneethanol,4-hydroxy- | 65.33 ± 2.60f | 94.5 ± 1.28d | 78.53 ± 2.54e | 80.0 ± 2.26e | 84.6 ± 1.52e | 92.4 ± 3.52de | 65.70 ± 0.95f | 120.9 ± 4.13bc | 116.5 ± 3.83bc | 111.6 ± 3.68c | 115.3 ± 2.75bc | 82.8 ± 3.12e | 104.6 ± 2.33cd | 116.5 ± 2.78bc | 96.7 ± 4.36d | 119.8 ± 4.12bc | 116.8 ± 1.61bc | 141.1 ± 1.52a | 103.6 ± 4.39cd | 122.4 ± 2.54b | 111.7 ± 2.84c | 115.3 ± 2.05bc |
| Total | 886.5 | 1035.7 | 1043.6 | 873 | 1096.2 | 1054.4 | 1116.9 | 1221.4 | 1396.7 | 1290.3 | 998.1 | 919.2 | 1401 | 1162 | 1050.6 | 1217.4 | 1308.9 | 1186.7 | 998.4 | 1144.8 | 1134.9 | 998.3 |
| Acids | ||||||||||||||||||||||
| Acetic acid | 345.0 ± 2.68b | 234.8 ± 2.32e | 256.9 ± 1.11cd | 205.1 ± 1.69h | 260.8 ± 2.05cd | 252.7 ± 2.59d | 159.7 ± 4.05j | 213.3 ± 3.68gh | 170.5 ± 2.15i | 223.8 ± 1.12f | 201.4 ± 4.11h | 153.5 ± 2.87j | 244.1 ± 2.96d | 264.6 ± 2.97c | 218.2 ± 4.47fg | 204.8 ± 3.42h | 218.1 ± 2.55fg | 4243 ± 4.35a | 234.8 ± 1.04e | 214.5 ± 2.29g | 214.9 ± 1.53fg | 201.4 ± 2.72h |
| Butyric acid | 7.647 ± 0.16de | 9.61 ± 0.68d | 10.25 ± 0.25cd | 7.573 ± 0.95e | 8.50 ± 0.46de | - | 6.408 ± 0.26e | 10.89 ± 0.50cd | 14.66 ± 0.49b | 21.40 ± 0.89a | 7.861 ± 0.44de | - | 21.91 ± 0.10a | - | 9.27 ± 0.47de | - | - | 11.43 ± 0.97c | - | 8.26 ± 0.45de | 11.23 ± 0.46cd | 7.861 ± 0.22de |
| Pentanoic acid | 46.83 ± 3.22d | 62.12 ± 3.98bc | 53.61 ± 2.83c | 48.75 ± 1.31c | 50.98 ± 2.13c | - | 46.18 ± 3.37c | 72.96 ± 4.39b | 75.31 ± 3.46ab | 77.64 ± 2.78ab | 58.19 ± 4.14c | - | 5.750 ± 1.37c | - | 63.84 ± 4.20bc | - | - | 77.81 ± 3.59a | - | 73.43 ± 2.99b | 66.16 ± 1.13bc | 58.19 ± 2.91c |
| Hexanoic acid | 15.99 ± 0.69a | - | - | 9.36 ± 0.67d | - | - | - | 14.88 ± 0.34b | - | - | - | - | - | - | - | 12.55 ± 0.33d | 14.27 ± 0.67b | 13.31 ± 0.95c | 2.030 ± 0.66d | - | - | - |
| n-Decanoic acid | 7.605 ± 0.16c | 7.952 ± 0.51b | 5.402 ± 0.76cd | 5.748 ± 0.84cd | 6.456 ± 0.19cd | 5.681 ± 0.71cd | 4.151 ± 0.41d | 4.837 ± 0.64cd | 11.24 ± 0.31b | 7.849 ± 0.66bc | 2.982 ± 0.13cd | 3.771 ± 0.76d | 11.69 ± 0.17a | 4.746 ± 0.35cd | 5.641 ± 0.33cd | 7.841 ± 0.33bc | 5.178 ± 0.46cd | 10.43 ± 0.88b | 4.642 ± 0.77d | - | 5.282 ± 0.48cd | 2.982 ± 0.95cd |
| n-Hexadecanoic acid | 11.77 ± 1.19b | 12.53 ± 1.91bc | 13.04 ± 1.86bc | 12.43 ± 0.90bc | 13.27 ± 3.26b | - | 6.534 ± 0.75b | 13.92 ± 3.46b | 21.30 ± 0.74b | 12.39 ± 1.14bc | 22.26 ± 2.13ab | - | 13.32 ± 2.16b | - | 12.05 ± 2.41bc | - | - | 19.08 ± 1.29b | - | 11.83 ± 0.86c | 15.27 ± 1.17b | 22.26 ± 2.41a |
| Total | 434.8 | 327.0 | 339.2 | 289.0 | 340.0 | 258.4 | 223.0 | 330.8 | 293.0 | 343.1 | 292.7 | 157.3 | 296.8 | 269.3 | 309.0 | 225.2 | 237.5 | 356.4 | 241.5 | 308.0 | 312.8 | 292.7 |
| Aldehydes | ||||||||||||||||||||||
| Furfural | - | 1.092 ± 0.35a | 0.915 ± 0.18a | 0.885 ± 0.22a | 0.930 ± 0.18a | 0.845 ± 0.14a | 1.088 ± 0.19a | 0.880 ± 0.78a | 1.312 ± 0.76a | 1.816 ± 0.21a | 0.7282 ± 0.64a | 0.7009 ± 0.63a | 0.842 ± 0.17a | 1.097 ± 0.91a | 0.7858 ± 0.73a | 1.016 ± 0.15a | 1.829 ± 0.19a | 1.108 ± 0.35a | 0.872 ± 0.76a | 0.7372 ± 0.44a | 1.004 ± 0.54a | 0.7282 ± 0.89a |
| 2-Furancarboxaldehyde,5-methyl- | 2.101 ± 0.47a | 2.342 ± 0.86a | 1.829 ± 0.12a | 2.030 ± 0.90a | 1.936 ± 0.65a | 1.898 ± 0.33a | 1.773 ± 0.93a | 2.496 ± 0.37a | 2.745 ± 0.68a | 2.931 ± 0.84a | 2.221 ± 0.14a | 1.778 ± 0.23a | 2.571 ± 0.28a | 2.398 ± 0.22a | 2.188 ± 0.81a | 2.445 ± 0.63a | 3.033 ± 0.56a | 2.779 ± 0.97a | 2.158 ± 0.41a | 2.471 ± 0.57a | 2.343 ± 1.00a | 2.221 ± 0.35a |
| Total | 2.101 | 3.376 | 2.744 | 2.836 | 2.751 | 2.743 | 2.681 | 3.376 | 4.057 | 4.747 | 2.949 | 2.479 | 3.599 | 3.495 | 2.974 | 3.461 | 3.033 | 3.887 | 2.096 | 3.208 | 3.347 | 2.949 |
| Volatile phenols | ||||||||||||||||||||||
| Phenol,2-methoxy- | 2.788 ± 0.28c | - | 3.426 ± 0.25bc | 2.548 ± 0.81c | 2.848 ± 0.17c | 3.118 ± 0.78bc | 2.057 ± 0.29c | 3.323 ± 0.20bc | 4.326 ± 0.14b | - | - | - | - | 2.825 ± 0.22c | - | 3.454 ± 0.29bc | 6.492 ± 0.17a | 3.626 ± 0.51bc | 2.773 ± 0.36c | - | - | - |
| Phenol, 4-ethyl- | 2.792 ± 0.88c | 4.586 ± 0.67bc | 2.962 ± 0.54c | 4.550 ± 0.53bc | 2.351 ± 0.47c | 5.648 ± 0.93b | 4.415 ± 0.48bc | 7.429 ± 0.40ab | 4.991 ± 0.62bc | 7.867 ± 0.47ab | 4.376 ± 0.34bc | 4.691 ± 0.88bc | 6.946 ± 0.48ab | 6.264 ± 0.19b | 5.688 ± 0.49b | 7.221 ± 0.98ab | 8.63 ± 0.59a | 7.588 ± 0.14ab | 6.684 ± 0.83ab | 6.289 ± 0.58b | 3.448 ± 0.97c | 4.376 ± 0.36bc |
| Phenol, 4-ethyl-2-methoxy- | 8.36 ± 3.02c | 20.17 ± 2.02bc | 8.75 ± 0.63c | 12.99 ± 3.40c | 8.33 ± 2.83c | 22.99 ± 2.61ab | 21.93 ± 3.38bc | 30.99 ± 0.97a | 13.65 ± 1.99c | 30.14 ± 1.68ab | 16.58 ± 1.38bc | 19.69 ± 2.28bc | 22.81 ± 2.13b | 25.65 ± 2.48ab | 25.03 ± 2.72ab | 30.42 ± 1.01ab | 28.26 ± 1.68ab | 30.44 ± 3.06ab | 29.25 ± 2.68ab | 30.21 ± 2.41ab | 10.72 ± 3.43c | 16.58 ± 2.87bc |
| 4-Viny phenol | 2.548 ± 0.86a | 1.363 ± 0.93a | 1.701 ± 0.84a | 1.584 ± 0.89a | 2.198 ± 0.19a | 1.162 ± 0.28a | 1.132 ± 0.18a | 1.337 ± 0.37a | 1.832 ± 0.44a | 1.808 ± 0.62a | 0.900 ± 0.47a | 0.7819 ± 0.78a | 2.186 ± 0.82a | 1.246 ± 0.21a | 1.150 ± 0.66a | 1.650 ± 0.43a | 2.183 ± 0.61a | 1.520 ± 0.88a | 0.998 ± 0.27a | 0.895 ± 0.33a | 1.467 ± 0.39a | 0.899 ± 0.14a |
| Phenol, 2,6-dimenthoxy- | 13.58 ± 0.59a | 6.170 ± 0.14cd | 5.574 ± 0.91cd | 8.65 ± 0.34b | 6.320 ± 0.76c | 5.513 ± 0.34cd | 5.460 ± 0.75cd | 4.410 ± 0.14cd | 6.238 ± 0.63c | 5.321 ± 0.18cd | 2.817 ± 0.75d | 3.160 ± 0.51d | 8.70 ± 0.83b | 4.730 ± 0.89cd | 4.145 ± 0.67d | 5.240 ± 0.65cd | 6.831 ± 0.97bc | 5.199 ± 0.91cd | 5.440 ± 0.27cd | 3.830 ± 0.17d | 3.843 ± 0.81d | 2.813 ± 0.15d |
| DL-a-Phenyllactic acid | 5.826 ± 0.13c | 6.587 ± 0.56bc | 5.144 ± 0.16c | 6.666 ± 0.82bc | 5.206 ± 0.87c | 5.375 ± 0.14c | 4.529 ± 0.42c | 5.809 ± 0.19c | 6.601 ± 0.51bc | 7.034 ± 0.24b | 5.046 ± 0.48c | 4.639 ± 0.59c | 10.21 ± 0.59a | 6.044 ± 0.64c | 5.095 ± 0.81c | 6.310 ± 0.25c | 8.32 ± 0.25ab | 6.414 ± 0.83c | 5.391 ± 0.65c | 5.832 ± 0.41c | 6.086 ± 0.99c | 5.046 ± 0.75c |
| 2,4-Di-tert-butylphenol | 45.22 ± 3.50cd | 47.52 ± 1.31cd | 45.86 ± 4.12cd | 38.57 ± 2.45de | 42.33 ± 2.51cd | 40.36 ± 2.85d | 34.91 ± 1.45de | 52.61 ± 3.42bc | 59.99 ± 3.18b | 67.84 ± 3.07ab | 40.11 ± 3.03d | 33.49 ± 4.45de | 69.80 ± 2.62a | 49.21 ± 1.84cd | 51.16 ± 0.99bc | 47.95 ± 1.07cd | 50.82 ± 1.04c | 50.86 ± 1.85c | 30.34 ± 1.81e | 46.01 ± 4.48cd | 47.67 ± 1.95cd | 40.11 ± 1.96d |
| 2,3-Dimethylphenol | 12.55 ± 1.36ab | 10.32 ± 1.51ab | 9.69 ± 0.66ab | 14.38 ± 3.09a | 11.40 ± 2.74ab | 7.762 ± 1.49ab | - | 7.320 ± 0.51ab | 10.83 ± 0.54ab | 10.02 ± 2.64ab | 6.287 ± 3.01b | 4.583 ± 2.36b | 13.82 ± 2.88ab | 8.09 ± 2.85ab | 5.913 ± 2.32b | 10.67 ± 2.75ab | 10.84 ± 2.66ab | 9.38 ± 2.65ab | 7.141 ± 0.96b | 5.700 ± 1.28b | 13.20 ± 3.40ab | 6.281 ± 0.58b |
| Total | 93.7 | 96.7 | 83.1 | 89.9 | 81.0 | 91.9 | 74.43 | 113.2 | 108.5 | 130.0 | 76.12 | 71.03 | 134.5 | 104.1 | 98.2 | 112.9 | 122.4 | 115.0 | 88.0 | 98.8 | 86.4 | 76.11 |
| Sample | Appearance | Aroma | Taste | Typicality | Clarity | Total Scores |
|---|---|---|---|---|---|---|
| 3 | 6 | 6 | 3 | 2 | 20 | |
| Control | 2.1 | 4.4 | 4.3 | 1.5 | 1.4 | 13.7 |
| NFr | 2.4 | 4.8 | 4.6 | 1.4 | 1.6 | 14.8 |
| MFr | 2.3 | 4.8 | 4.5 | 1.7 | 1.5 | 14.8 |
| HFr | 2.4 | 4.5 | 4.4 | 1.5 | 1.6 | 14.4 |
| MCh | 2.5 | 4.5 | 4.5 | 1.8 | 1.7 | 15.0 |
| HCh | 2.5 | 4.2 | 4.3 | 1.9 | 1.5 | 14.4 |
| MAm | 2.3 | 4.6 | 4.4 | 1.5 | 1.6 | 14.4 |
| NFr:MFr | 2.4 | 4.7 | 4.8 | 1.4 | 1.6 | 14.9 |
| NFr:HFr | 2.5 | 4.8 | 4.4 | 2.0 | 1.4 | 15.1 |
| NFr:MCh | 2.6 | 4.8 | 4.4 | 1.8 | 1.5 | 15.1 |
| NFr:HCh | 2.3 | 4.7 | 4.2 | 1.7 | 1.6 | 14.5 |
| NFr:MAm | 2.3 | 4.6 | 4.7 | 1.8 | 1.5 | 14.9 |
| MFr:HFr | 2.2 | 4.9 | 4.5 | 1.9 | 1.5 | 15.0 |
| MFr:MCh | 2.5 | 4.4 | 4.3 | 1.7 | 1.8 | 14.7 |
| MFr:HCh | 2.3 | 4.6 | 4.5 | 1.6 | 1.5 | 14.5 |
| MFr:MAm | 2.6 | 4.5 | 4.5 | 1.9 | 1.6 | 15.1 |
| HFr:MCh | 2.2 | 4.8 | 4.4 | 1.8 | 1.4 | 14.6 |
| HFr:HCh | 2.6 | 5 | 4.2 | 1.4 | 1.4 | 14.6 |
| HFr:MAm | 2.4 | 4.8 | 4.1 | 1.8 | 1.5 | 14.6 |
| MCh:HCh | 2.5 | 4.7 | 4.3 | 1.7 | 1.5 | 14.7 |
| MCh:MAm | 2.2 | 4.7 | 4.8 | 1.7 | 1.6 | 15.0 |
| HCh:MAm | 2.4 | 4.6 | 4.3 | 1.6 | 1.7 | 14.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Li, L.; Xue, R.; Wang, C.; Chen, M.; Ramos, J.; Zhang, S.; Sun, B. Impact of Different Oak Chips’ Aging on the Volatile Compounds and Sensory Characteristics of Vitis amurensis Wines. Foods 2022, 11, 1126. https://doi.org/10.3390/foods11081126
Yu Y, Li L, Xue R, Wang C, Chen M, Ramos J, Zhang S, Sun B. Impact of Different Oak Chips’ Aging on the Volatile Compounds and Sensory Characteristics of Vitis amurensis Wines. Foods. 2022; 11(8):1126. https://doi.org/10.3390/foods11081126
Chicago/Turabian StyleYu, Yanxia, Lingxi Li, Ruowei Xue, Chen Wang, Mengying Chen, João Ramos, Shuting Zhang, and Baoshan Sun. 2022. "Impact of Different Oak Chips’ Aging on the Volatile Compounds and Sensory Characteristics of Vitis amurensis Wines" Foods 11, no. 8: 1126. https://doi.org/10.3390/foods11081126
APA StyleYu, Y., Li, L., Xue, R., Wang, C., Chen, M., Ramos, J., Zhang, S., & Sun, B. (2022). Impact of Different Oak Chips’ Aging on the Volatile Compounds and Sensory Characteristics of Vitis amurensis Wines. Foods, 11(8), 1126. https://doi.org/10.3390/foods11081126