Mechanism of Anti-Diabetic Activity from Sweet Potato (Ipomoea batatas): A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Study Selection
2.5. Data Extraction and Management
3. Results
The Literature Search
4. Discussion
4.1. Varieties of Ipomoea Batatas Developed for Type 2 Diabetes
4.2. Types and Concentrations of Phytochemicals Contained in Ipomoea batatas Which Have Anti-Diabetic Effects
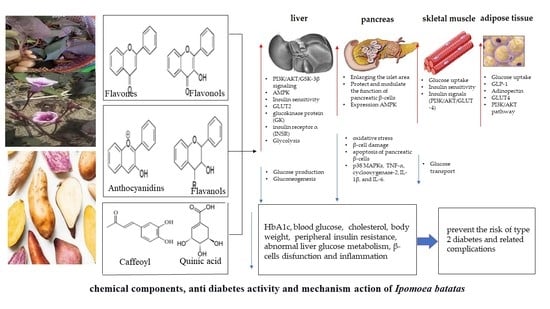
4.3. Mechanism of Action Chemical Components in Ipomoea batatas for Anti-Diabetic Effects
4.3.1. Protects the Integrity of Islet Structures and Modulates Pancreatic β Cell Function
4.3.2. Increased Insulin Secretion and Improved Insulin Sensitivity
4.3.3. Regulation of Carbohydrate Metabolism
4.3.4. Suppression of Glucose Production in the Liver
4.3.5. Inhibition of Glucose Transport in the Intestine and Increased Uptake of Tissue Glucose
4.3.6. Repair of Insulin Signals and Glycogen Synthesis
4.3.7. Inhibition of Inflammatory Pathways
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Diabetes Federation (IDF). Diabetes Atlas 10th Edition. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 11 June 2023).
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Guideline on Clinical Investigation of Medicinal Products in the Treatment or Prevention of Diabetes Mellitus. 2012. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-prevention-diabetes-mellitus-revision_en.pdf (accessed on 11 June 2023).
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; Davidson, M.B.; Einhorn, D.; Garvey, W.T.; et al. AACE comprehensive diabetes management algorithm 2013. Endocr. Pract. 2013, 19, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inzucchi, S.E. Oral antihyperglycemic therapy for type 2 diabetes: Scientific review. JAMA 2002, 287, 360–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Potato Center. Biodiversity for the Future, Potato Agri-Food Systems, Potatoes, Sweetpotato Agri-Food Systems, Sweetpotatoes. Available online: https://cgspace.cgiar.org/handle/10568/108891 (accessed on 11 June 2023).
- Krochmal-Marczak, B.; Zagórska-Dziok, M.; Michalak, M.; Kiełtyka-Dadasiewicz, A. Comparative assessment of phenolic content, cellular antioxidant, antityrosinase and protective activities on skin cells of extracts from three sweet potato (Ipomoea batatas (L.) Lam.) cultivars. J. King Saud Univ. Sci. 2021, 33, 101532. [Google Scholar] [CrossRef]
- Sun, H.; Mu, T.; Xi, L.; Zhang, M.; Chen, J. Sweet potato (Ipomoea batatas L.) leaves as nutritional and functional foods. Food Chem. 2014, 156, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Islam, S. Sweetpotato (Ipomoea batatas L.) leaf: Its potential effect on human health and nutrition. J. Food Sci. 2006, 71, 13–22. [Google Scholar] [CrossRef]
- Yustiantara, P.; Warditiani, N.K.; Sari, P.; Dewi, N.; Ramona, Y.; Jawi, I.M.; Wirasuta, I. Determination of TLC fingerprint biomarker of Ipomoea batatas (L.) Lam leaves extracted with ethanol and its potential as antihyperglycemic agent. Pharmacia 2021, 68, 907–917. [Google Scholar] [CrossRef]
- Ooi, C.P.; Loke, S.C. Sweet potato for type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2013, 2013, Cd009128. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Grewal, A.; Kataria, H.; Dhawan, I. Literature search for research planning and identification of research problem. Indian J. Anaesth. 2016, 60, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Neuffer, B.; Pacini, G. Efficacy of Ipomoea batatas (Caiapo) on diabetes control in type 2 diabetic subjects treated with diet. Diabetes Care 2004, 27, 436–440. [Google Scholar] [CrossRef] [Green Version]
- Kusano, S.; Abe, H.; Tamura, H. Isolation of antidiabetic components from white-skinned sweet potato (Ipomoea batatas L.). Biosci. Biotechnol. Biochem. 2001, 65, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Mu, T.; Sun, H. Profiling of phenolic acids and flavonoids in sweet potato (Ipomoea batatas L.) leaves and evaluation of their anti-oxidant and hypoglycemic activities. Food Biosci. 2021, 39, 100801. [Google Scholar] [CrossRef]
- Li, F.; Li, Q.; Gao, D.; Peng, Y. The optimal extraction parameters and anti-diabetic activity of flavonoids from Ipomoea batatas leaf. Afr. J. Tradit. Complement Altern. Med. 2009, 6, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Zhao, R.; Li, Q.; Long, L.; Li, J.; Yang, R.; Gao, D. Antidiabetic activity of flavone from Ipomoea batatas leaf in non-insulin dependent diabetic rats. Int. J. Food Sci. Technol. 2007, 42, 80–85. [Google Scholar] [CrossRef]
- Novrial, D.; Soebowo, S.; Widjojo, P. Protective Effect of Ipomoea batatas L Leaves Extract on Histology of Pancreatic Langerhans Islet and Beta Cell Insulin Expression of Rats Induced by Streptozotocin. Molekul 2020, 15, 48–55. [Google Scholar] [CrossRef]
- Jiang, T.; Shuai, X.; Li, J.; Yang, N.; Deng, L.; Li, S.; He, Y.; Guo, H.; Li, Y.; He, J. Protein-bound anthocyanin compounds of purple sweet potato ameliorate hyperglycemia by regulating hepatic glucose metabolism in high-fat diet/streptozotocin-induced diabetic mice. J. Agric. Food Chem. 2020, 68, 1596–1608. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Akram, M.; Daniyal, M.; Ahmad, S. Evaluation of antidiabetic activity of Ipomoea batatas L. extract in alloxan-induced diabetic rats. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418814678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.L.; Lee, S.L.; Chen, C.J.; Chen, H.C.; Kao, M.C.; Liu, C.H.; Chen, J.Y.; Lai, Y.T.; Wu, Y.C. Characterization of secondary metabolites from purple Ipomoea batatas leaves and their effects on glucose uptake. Molecules 2016, 21, 745. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.K.; Chen, C.M.; Varga, V.; Shih, L.C.; Chen, P.R.; Lo, S.F.; Shyur, L.F.; Li, S.C. White sweet potato ameliorates hyperglycemia and regenerates pancreatic islets in diabetic mice. Food Nutr. Res. 2020, 64, 3609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludvik, B.; Waldhäusl, W.; Prager, R.; Kautzky-Willer, A.; Pacini, G. Mode of action of Ipomoea batatas (Caiapo) in type 2 diabetic patients. Metabolism 2003, 64, 875–880. [Google Scholar] [CrossRef]
- Nagamine, R.; Ueno, S.; Tsubata, M.; Yamaguchi, K.; Takagaki, K.; Hira, T.; Hara, H.; Tsuda, T. Dietary sweet potato (Ipomoea batatas L.) leaf extract attenuates hyperglycaemia by enhancing the secretion of glucagon-like peptide-1 (GLP-1). Food Funct. 2014, 5, 2309–2316. [Google Scholar] [CrossRef]
- Kurata, R.; Yahara, S.; Yamakawa, O.; Yoshimoto, M. Simple High-yield purification of 3,4,5-Tri-O-caffeoylquinic acid from sweetpotato (Ipomoea batatas L.) leaf and Its inhibitory effects on Aldose reductase. Food Sci. Technol. Res. 2011, 17, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Niwa, A.; Tajiri, T.; Higashino, H. Ipomoea batatas and Agarics blazei ameliorate diabetic disorders with therapeutic antioxidant potential in streptozotocin-induced diabetic rats. J. Clin. Biochem. Nutr. 2011, 48, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Ludvik, B.; Hanefeld, M.; Pacini, G. Improved metabolic control by Ipomoea batatas (Caiapo) is associated with increased adiponectin and decreased fibrinogen levels in type 2 diabetic subjects. Diabetes Obes. Metab. 2008, 10, 586–592. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, Z.C.; Yuan, T.; Wang, H.; Xie, X.; Fu, Z.F. Antioxidants and α-glucosidase inhibitors from Ipomoea batatas leaves identified by bioassay-guided approach and structure-activity relationships. Food Chem. 2016, 208, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Oki, N.; Nonaka, S.; Ozaki, S. The effects of an arabinogalactan-protein from the white-skinned sweet potato (Ipomoea batatas L.) on blood glucose in spontaneous diabetic mice. Biosci. Biotechnol. Biochem. 2011, 75, 596–598. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, A.; Nagata, T.; Furuya, F.; Nishizawa, M.; Mukai, E. White-skinned sweet potato (Ipomoea batatas L.) acutely suppresses postprandial blood glucose elevation by improving insulin sensitivity in normal rats. Heliyon 2023, 9, e14719. [Google Scholar] [CrossRef]
- Sakuramata, Y.; Oe, H.; Kusano, S.; Aki, O. Effects of combination of Caiapo with other plant-derived substance on anti-diabetic efficacy in KK-Ay mice. Biofactors 2004, 22, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Itishom, R.; Wafa, I.; Setyo Budi, D.; Pratama, N. Oral Delivery of Purple Sweet Potato (Ipomoea batatas L.) extract-loaded carboxymethyl chitosan and alginate nanocapsule in streptozotocin induced diabetic mice. Indian J. Pharm. Edu. Res. 2021, 55, 709–714. [Google Scholar] [CrossRef]
- Kusano, S.; Tamasu, S.; Nakatsugawa, S. Effects of the white-skinned sweet potato (Ipomoea batatas L.) on the expression of adipocytokine in adipose tissue of genetic type 2 diabetic mice. Food Sci. Technol. Res. 2005, 11, 369–372. [Google Scholar] [CrossRef] [Green Version]
- Sui, X.; Zhang, Y.; Zhou, W. In vitro and in silico studies of the inhibition activity of anthocyanins against porcine pancreatic α-amylase. J. Funct. Foods 2016, 21, 50–57. [Google Scholar] [CrossRef]
- Shyur, L.F.; Varga, V.; Chen, C.M.; Mu, S.C.; Chang, Y.C.; Li, S.C. Extract of white sweet potato tuber against TNF-α-induced insulin resistance by activating the PI3K/Akt pathway in C2C12 myotubes. Bot. Stud. 2021, 62, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, J.L.; Shen, L.H.; Feng, L.J.; Zhou, Q. Inhibition mechanism of diacylated anthocyanins from purple sweet potato (Ipomoea batatas L.) against α-amylase and α-glucosidase. Food Chem. 2021, 359, 129934. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Ramachela, K.; Mukwevho, E. Aqueous-methanol extracts of orange-fleshed sweet potato (Ipomoea batatas) ameliorate oxidative stress and modulate type 2 diabetes associated genes in insulin resistant C2C12 cells. Molecules 2018, 23, 2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.H.; Kim, H.W.; Kim, S.Y.; Kim, S.M.; Kim, J.B.; Lee, Y.M. In vitro and in vivo hypoglycemic effects of cyanidin 3-caffeoyl-p-hydroxybenzoylsophoroside-5-glucoside, an anthocyanin isolated from purple-fleshed sweet potato. Food Chem. 2019, 272, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Mu, T.; Xi, L.; Song, Z. Effects of domestic cooking methods on polyphenols and antioxidant activity of sweet potato leaves. J. Agric. Food Chem. 2014, 62, 8982–8989. [Google Scholar] [CrossRef]
- Tran, N.; Pham, B.; Le, L. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Suen, C.L.; Yang, C.; Quek, S.Y. Antioxidant capacity and major polyphenol composition of teas as affected by geographical location, plantation elevation and leaf grade. Food Chem. 2018, 244, 109–119. [Google Scholar] [CrossRef]
- Jia, R.; Tang, C.; Chen, J.; Zhang, X.; Wang, Z. Total phenolics and anthocyanins contents and antioxidant activity in four different aerial parts of leafy sweet potato (Ipomoea batatas L.). Molecules 2022, 27, 3117. [Google Scholar] [CrossRef] [PubMed]
- Krochmal-Marczak, B.; Cebulak, T.; Kapusta, I.; Oszmiański, J.; Kaszuba, J.; Żurek, N. The content of phenolic acids and flavonols in the leaves of nine varieties of sweet potatoes (Ipomoea batatas L.) depending on their development, grown in Central Europe. Molecules 2020, 25, 3473. [Google Scholar] [CrossRef]
- Ji, H.; Zhang, H.; Li, H.; Li, Y. Analysis on the nutrition composition and antioxidant activity of different types of sweet potato cultivars. Food Nutr. Sci. 2015, 6, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Lee, S.Y.; Yang, J.W.; Lee, J.S.; Oh, S.D.; Oh, S.; Lee, S.M.; Lim, M.H.; Park, S.K.; Jang, J.S.; et al. Comparative analysis of phytochemicals and polar metabolites from colored sweet potato (Ipomoea batatas L.) tubers. Food Sci. Biotechnol. 2016, 25, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Mu, T.; Sun, H. Sweet potato (Ipomoea batatas L.) leaf polyphenols ameliorate hyperglycemia in type 2 diabetes mellitus mice. Food Funct. 2021, 12, 4117–4131. [Google Scholar] [CrossRef]
- Ogunrinola, O.; Fajana, O.; Olaitan, S.; Adu, O.; Akinola, M.O. Anti-diabetic activity of Ipomoea batatas leaves extract: Effects on hepatic nzymes in Alaloxan-induced diabetic rats. Res. J. Med. Plant 2015, 9, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Chen, L.; Zheng, J.; Zeng, T.; Li, H.; Xiao, H.; Deng, X.; Hu, X. The protective effect of resveratrol on islet insulin secretion and morphology in mice on a high-fat diet. Diabetes Res. Clin. Pract. 2012, 97, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Kazeem, M.I.; Akanji, M.A.; Yakubu, M.T. Amelioration of pancreatic and renal derangements in streptozotocin-induced diabetic rats by polyphenol extracts of Ginger (Zingiber officinale) rhizome. Pathophysiology 2015, 22, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Cai, E.P.; Lin, J.K. Epigallocatechin gallate (EGCG) and rutin suppress the glucotoxicity through activating IRS2 and AMPK signaling in rat pancreatic beta cells. J. Agric. Food Chem. 2009, 57, 9817–9827. [Google Scholar] [CrossRef]
- Ludvik, B.H.; Mahdjoobian, K.; Waldhaeusl, W.; Hofer, A.; Prager, R.; Kautzky-Willer, A.; Pacini, G. The Effect of Ipomoea batatas (Caiapo) on Glucose Metabolism and Serum Cholesterol in Patients with Type 2 Diabetes: A randomized study. Diabetes Care 2002, 25, 239–240. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhang, M.; Hu, M.Y.; Guo, H.F.; Li, J.; Yu, Y.L.; Jin, S.; Wang, X.T.; Liu, L.; Liu, X.D. Increased glucagon-like peptide-1 secretion may be involved in antidiabetic effects of ginsenosides. J. Endocrinol. 2013, 217, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Chakraborti, C.K. Role of adiponectin and some other factors linking type 2 diabetes mellitus and obesity. World J. Diabetes 2015, 6, 1296–1308. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Abotaleb, M.; Kubatka, P.; Kajo, K.; Büsselberg, D. Flavonoids and their anti-diabetic effects: Cellular mechanisms and effects to improve blood sugar levels. Biomolecules 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Russo, B.; Picconi, F.; Malandrucco, I.; Frontoni, S. Flavonoids and insulin-resistance: From molecular evidences to clinical trials. Int. J. Mol. Sci. 2019, 20, 2061. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Murtaza, G.; Liu, G.; Rahu, N.; Saleem Kalhoro, M.; Hussain Kalhoro, D.; Adebowale, T.O.; Usman Mazhar, M.; Rehman, Z.u.; et al. Flavonoids and type 2 diabetes: Evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol. Res. 2020, 152, 104629. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltiel, A.R. Insulin signaling in health and disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef]
- Miao, R.; Fang, X.; Wei, J.; Wu, H.; Wang, X.; Tian, J. Akt: A Potential drug target for metabolic syndrome. Front. Physiol. 2022, 13, 822333. [Google Scholar] [CrossRef]
- Mehmood, A.; Li, J.; Rehman, A.U.; Kobun, R.; Llah, I.U.; Khan, I.; Althobaiti, F.; Albogami, S.; Usman, M.; Alharthi, F.; et al. Xanthine oxidase inhibitory study of eight structurally diverse phenolic compounds. Front. Nutr. 2022, 9, 966557. [Google Scholar] [CrossRef]
- Wu, X.; Hu, M.; Hu, X.; Ding, H.; Gong, D.; Zhang, G. Inhibitory mechanism of epicatechin gallate on α-amylase and α-glucosidase and its combinational effect with acarbose or epigallocatechin gallate. J. Mol. Liq. 2019, 290, 111202. [Google Scholar] [CrossRef]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, S.; Chen, J.; Su, Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front. Endocrinol. 2018, 9, 802. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.M.; Clark, S.F.; Tucker, D.F.; Birnbaum, M.J.; James, D.E.; Macaulay, S.L. A role for protein kinase Bbeta/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol. Cell Biol. 1999, 19, 7771–7781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewi, C.; Fristiohady, A.; Amalia, R.; Bunggulawa, E.J.; Muchtaridi, M. Alpha-mangostin as an inhibitor of GSK3β in triple-negative breast cancer. J. Biomol. Struct. Dyn. 2023, 41, 4515–4521. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, Z.; Mi, Y. Purple sweet potato color attenuates high fat-induced neuroinflammation in mouse brain by inhibiting MAPK and NF-κB activation. Mol. Med. Rep. 2018, 17, 4823–4831. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Type/Cultivar | Part of Material | Detected Phytochemical Compound | Predicted Bioactive Compound | Type of Study | Dosage | Action and Mechanism | Refs. |
|---|---|---|---|---|---|---|---|
| White sweet potatoes | Powdered white sweet potatoes (Caiapo) | - | Acidic glycoprotein | In vivo | 4 g/day for 12 weeks. |
| [14] |
| White sweet potato | Lyophilized powder of skin or flesh and combination skin and flesh aqueous extracts |
| Acidic glycoprotein | In vivo | 20–2000 mg/kg BW/day for 3 weeks |
| [15] |
| White sweet potato cultivar Simon No. 1 |
|
|
| In vitro | (0.25–100 µg) |
| [16] |
| Sweet potatoes were collected from the Hebei province district in October | 40 to 90% ethanolic viscous leaves extract | Flavonoids | - | In vivo | 50–150 mg/kg BW for 28 days |
| [17] |
| Sweet potatoes were obtained by a local farmer (Hebei province) in autumn | Flavone extracts | Flavones | - | In vivo | 25–100 mg/kg BW for 2 weeks |
| [18] |
| Sweet potato (family of clones B 0059- 3) were harvested in July from the Bandungan, Central Java Indonesia. | Evaporated petroleum ether leave extract |
| - | In vivo | 0.25–0.8, 2.5 g/kg BW for 14 days |
| [19] |
| Purple sweet potatoes (Cultivar Eshu No.12) from the Institute of Food Crops, Hubei Academy of Agricultural Sciences (Wuhan, China) |
| Total anthocyanin content and protein |
| In vivo | p-BAC-PSP (500 mg/kg BW) FAC-PSP (200 mg/kg BW) with total anthocyanin content in FAC-PSP = 40.74 ± 2.88 mgC3G/g |
| [20] |
| The sweet potato was purchased from the local market of Faisalabad (Pakistan). | Evaporated methanolic extract | - |
| In vivo | 4 g/kg BW/day for 14 days |
| [21] |
| Purple sweet potato leaves were collected in Luzhu District, Taoyuan City, Taiwan | Crude extracts, including n-hexane- (IBH), 95% MeOH- (IBM), n-BuOH- (IBB), and H2O-soluble (IBW) fractions | Twenty-four pure compounds |
| In vitro | Crude extract (0.1 mg/mL) pure compounds (0.01 mg/mL) were |
| [22] |
| White sweet potato Tainung No. 10 | Lyophilized powder of leave and tuber | - | Flavonoids, terpenoids, tannins, saponins, glycosides, alkaloids, steroids, and phenolic acids in tuber | In vivo | Powdered leaf: 5–50 mg/kg BW Powdered tuber: 100–300 mg/kg BW |
| [23] |
| White sweet potato | Powdered white sweet potatoes (Caiapo) | - | - | In vivo | 2–4 g/d for 6 weeks |
| [24] |
| Leaves and stems of ‘Suioh’ which was harvested in the summer of 2009 in Kumamoto prefecture, Japan | Lyophilised powder of 60% ethanolic extract | CQA derivatives Mono-CQAs Di-CQAs Tri-CQAs | Total polyphenols CQA derivatives g Mono-CQAs In-CQAs Tri-CQAs | In vitro and in vivo | 2 g/kg BW/day for 5 weeks |
| [25] |
| Genotypes raised in the National Agricultural Research Center for Kyushu Okinawa Region in Japan | Purification of 3,4,5-triCQA from sweet potato leaves | Caffeoylquinic acid derivatives | 3,4,5-tri-O-caffeoylquinic acid | In vitro | 100–500 µM |
| [26] |
| The sweet potato was grown in Kagawa Prefecture (Japan) | Powdered white sweet potatoes (Caiapo) |
| - | In vivo | 5 g/kg of BW/day for 4 weeks |
| [27] |
| White-skinned sweet potato | Powdered white sweet potatoes (Caiapo) | - | - | In vivo | Once daily 4 g for 5 months |
| [28] |
| Fresh orange-fleshed (Jishu No. 16) sweet potato | Ethanolic fraction of distillated water extract |
| Glucosidase inhibition:
| In vitro | 50 µL |
| [29] |
| The white-skinned sweet potato | Arabinogalactanprotein | arabinogalactanprotein | arabinogalactanprotein | In vivo | 20 mg/kg BW of for 8 weeks |
| [30] |
| White-skinned sweet potato | White-skinned sweet potato powder | Three fractions of WSSP (≤10, 10–50, and >50 kDa | - | In vivo | 180–230 g/kg BW for 6–7 weeks |
| [31] |
| Caiapo® |
| - | - | In vivo |
|
| [32] |
| Purple sweet potato | 96% ethanol (96%) and tartaric acid extract | - | - | In vivo | 0.5 cc |
| [33] |
| White-skinned sweet potato | Lyophilized powder of distillated water tuber extract | - | - | In vivo | 400 mg/kg BW/day |
| [34] |
| Purple sweet potato | Commercial anthocyanin |
|
| In vitro and in silico | 2.5, 5, 10, and 15 mg/mL |
| [35] |
| White sweet potato (Simon No. 1) | Tuberous ethanol extract, ethyl-acetate and water fraction |
| - | In vitro | 5–250 μg/mL |
| [36] |
| Purple sweet potato powder cultivar Eshu No. 8 | Anthocyanins |
|
| In vitro and in vivo | 160 mg/kg BW |
| [37] |
| Orange-fleshed sweet potato cultivar ‘Bophelo’ | Aqueous-methanol extracts of tuber (OSPT) and leave (OSPL) |
| Flavonoids
| In vitro | 500 μg/mL and 100 μg/mL of OSPT and OSPL |
| [38] |
| Korean purple sweet potato (ShinzamiSaeungbone9, Saeungyae33, Gyeyae2469, and Gyeyae2258) | 15 individual anthocyanins |
|
| In vitro and in vivo | 80 mg/kg BW |
| [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arisanti, C.I.S.; Wirasuta, I.M.A.G.; Musfiroh, I.; Ikram, E.H.K.; Muchtaridi, M. Mechanism of Anti-Diabetic Activity from Sweet Potato (Ipomoea batatas): A Systematic Review. Foods 2023, 12, 2810. https://doi.org/10.3390/foods12142810
Arisanti CIS, Wirasuta IMAG, Musfiroh I, Ikram EHK, Muchtaridi M. Mechanism of Anti-Diabetic Activity from Sweet Potato (Ipomoea batatas): A Systematic Review. Foods. 2023; 12(14):2810. https://doi.org/10.3390/foods12142810
Chicago/Turabian StyleArisanti, Cokorda Istri Sri, I. Made Agus Gelgel Wirasuta, Ida Musfiroh, Emmy Hainida Khairul Ikram, and Muchtaridi Muchtaridi. 2023. "Mechanism of Anti-Diabetic Activity from Sweet Potato (Ipomoea batatas): A Systematic Review" Foods 12, no. 14: 2810. https://doi.org/10.3390/foods12142810
APA StyleArisanti, C. I. S., Wirasuta, I. M. A. G., Musfiroh, I., Ikram, E. H. K., & Muchtaridi, M. (2023). Mechanism of Anti-Diabetic Activity from Sweet Potato (Ipomoea batatas): A Systematic Review. Foods, 12(14), 2810. https://doi.org/10.3390/foods12142810