DSC Phase Transition Profiles Analyzed by Control Charts to Determine Markers for the Authenticity and Deterioration of Flaxseed Oil during Storage
Abstract
:1. Introduction
2. Materials
2.1. Materials
2.2. Methods
2.2.1. Fatty Acid Composition
2.2.2. Chemical Analyses of the Oxidative Stability of Fresh and Stored Flaxseed Oil
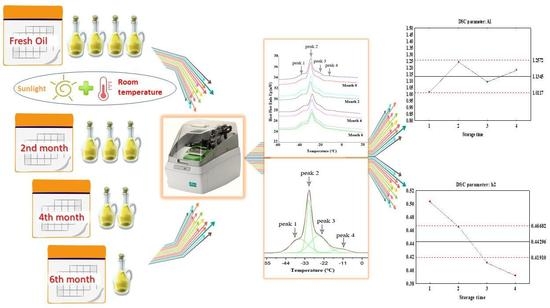
2.2.3. Melting Phase Transition Analysis by Differential Scanning Calorimetry (DSC) of Flaxseed Oil during Storage
2.2.4. Statistical Analysis
3. Results
3.1. Physicochemical Characteristics of Fresh and Stored Flaxseed Oils
3.1.1. Fatty Acid Content
3.1.2. Oxidative Stability Analysis of Flaxseed Oil
3.2. Analysis of the DSC Melting Profiles of Flaxseed Oils during Shelf Life
DSC Melting Profile Changes during Storage of Flaxseed Oil
4. Discussion
4.1. Determination of DSC Parameters as Markers of Authenticity and Deterioration of Flaxseed Oil
4.1.1. Determination of Stable DSC Parameters as Markers of Oils’ Authenticity during Storage
4.1.2. Detection of Flaxseed Oil Deterioration by Unstable DSC Parameters during Storage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Production/Yield Quantities of Oil of Linseed in World + (Total). Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 23 May 2023).
- Tomaszewska-Gras, J.; Islam, M.; Grzeca, L.; Kaczmarek, A.; Fornal, E. Comprehensive Thermal Characteristics of Different Cultivars of Flaxseed Oil (Linum usittatissimum L.). Molecules 2021, 26, 1958. [Google Scholar] [CrossRef]
- Obranović, M.; Škevin, D.; Kraljić, K.; Pospišil, M.; Neđeral, S.; Blekić, M.; Putnik, P. Influence of Climate, Variety and Production Process on Tocopherols, Plastochromanol-8 and Pigments in Flaxseed Oil. Food Technol. Biotechnol. 2015, 53, 496–504. [Google Scholar] [CrossRef]
- Herchi, W.; Bouali, I.; Bahashwan, S.; Rochut, S.; Boukhchina, S.; Kallel, H.; Pepe, C. Changes in Phospholipid Composition, Protein Content and Chemical Properties of Fl Axseed Oil during Development. Plant Physiol. Biochem. 2012, 54, 1–5. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Zeitoun, M.A. Quality Evaluation of Flaxseed Oil Obtained by Different Extraction Techniques. LWT Food Sci. Technol. 2013, 53, 338–345. [Google Scholar] [CrossRef]
- Komartin, R.S.; Stroescu, M.; Chira, N.; Stan, R.; Stoica-Guzun, A. Optimization of Oil Extraction from Lallemantia Iberica Seeds Using Ultrasound-Assisted Extraction. J. Food Meas. Charact. 2021, 15, 2010–2020. [Google Scholar] [CrossRef]
- Teh, S.S.; Birch, J. Physicochemical and Quality Characteristics of Cold-Pressed Hemp, Flax and Canola Seed Oils. J. Food Compos. Anal. 2013, 30, 26–31. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Salama, Z.; El-Hariri, D.M. Evaluation of Fatty Acids Profile and the Content of Some Secondary Metabolites in Seeds of Different Flax Cultivars (Linum usitatissimum L.). Gen. Appl. Plant Physiol. 2007, 33, 187–202. [Google Scholar]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules 2019, 24, 3729. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, M.A.; Milisich, H.J.; Drago, S.R.; González, R.J. Effect of Cultivars and Planting Date on Yield, Oil Content, and Fatty Acid Profile of Flax Varieties (Linum usitatissimum L.). Int. J. Agron. 2014, 2014, 150570. [Google Scholar] [CrossRef] [Green Version]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Choo, W.S.; Birch, J.; Dufour, J.P. Physicochemical and Quality Characteristics of Cold-Pressed Flaxseed Oils. J. Food Compos. Anal. 2007, 20, 202–211. [Google Scholar] [CrossRef]
- Hasiewicz-Derkacz, K.; Kulma, A.; Czuj, T.; Prescha, A.; Zuk, M.; Grajzer, M.; Łukaszewicz, M.; Szopa, J. Natural Phenolics Greatly Increase Flax (Linum usitatissimum) Oil Stability. BMC Biotechnol. 2015, 15, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.; Muzolf-Panek, M.; Fornal, E.; Tomaszewska-Gras, J. DSC Isothermal and Non-Isothermal Assessment of Thermo-Oxidative Stability of Different Cultivars of Camelina Sativa L. Seed Oils. J. Therm. Anal. Calorim. 2022, 147, 10013–10026. [Google Scholar] [CrossRef]
- Tańska, M.; Roszkowska, B.; Skrajda, M.; Grzegorz, D. Commercial Cold Pressed Flaxseed Oils Quality and Oxidative Stability at the Beginning and the End of Their Shelf Life. J. Oleo Sci. 2016, 65, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.; Bełkowska, L.; Konieczny, P.; Fornal, E.; Tomaszewska-Gras, J. Differential Scanning Calorimetry for Authentication of Edible Fats and Oils—What Can We Learn from the Past to Face the Current Challenges? J. Food Drug Anal. 2022, 30, 185–201. [Google Scholar] [CrossRef]
- Islam, M.; Rajagukguk, Y.V.; Siger, A.; Tomaszewska-Gras, J. Assessment of Hemp Seed Oil Quality Pressed from Fresh and Stored Seeds of Henola Cultivar Using Differential Scanning Calorimetry. Foods 2023, 12, 135. [Google Scholar] [CrossRef]
- Cichocki, W.; Kmiecik, D.; Baranowska, H.M.; Staroszczyk, H.; Sommer, A.; Kowalczewski, P.Ł. Chemical Characteristics and Thermal Oxidative Stability of Novel Cold-Pressed Oil Blends: GC, LF NMR, and DSC Studies. Foods 2023, 12, 2660. [Google Scholar] [CrossRef] [PubMed]
- Rajagukguk, Y.V.; Islam, M.; Tomaszewska-Gras, J. Influence of Seeds’ Age and Clarification of Cold-Pressed Raspberry (Rubus idaeus L.) Oil on the DSC Oxidative Stability and Phase Transition Profiles. Foods 2023, 12, 358. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Li, D.; Zhang, L.X.; Liu, Y.L.; Wang, X.-D. Heating Effect on the DSC Melting Curve of Flaxseed Oil. J. Therm. Anal. Calorim. 2014, 115, 2129–2135. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Preparations of Methyl Esters of Fatty Acids. 1997. Available online: https://www.coursehero.com/file/53583244/AOCS-Official-Method-Ce-2-66-Preparation-of-Methyl-Esters-of-Fatty-Acidspdf/ (accessed on 20 May 2023).
- AOCS. AOCS Official Method Acid Value. Cd 3d-63. 2009. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details/productId/111545 (accessed on 20 May 2023).
- ISO 3960; Animal and Vegetable Fats and Oils—Determination—Iodometric (Visual) Endpoint. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6885; Animal and Vegetable Fats and Oils: Determination of Anisidine Value. ISO: Geneva, Switzerland, 2016.
- Tomaszewska-Gras, J. Rapid Quantitative Determination of Butter Adulteration with Palm Oil Using the DSC Technique. Food Control 2016, 60, 629–635. [Google Scholar] [CrossRef]
- Anastasiu, A.E.; Chira, N.A.; Banu, I.; Ionescu, N.; Stan, R.; Rosca, S.I. Oil Productivity of Seven Romanian Linseed Varieties as Affected by Weather Conditions. Ind. Crop. Prod. 2016, 86, 219–230. [Google Scholar] [CrossRef]
- Cantele, C.; Bertolino, M.; Bakro, F.; Giordano, M.; Jędryczka, M.; Cardenia, V. Antioxidant Effects of Hemp (Cannabis Sativa L.) Inflorescence Extract in Stripped Linseed Oil. Antioxidants 2020, 9, 1131. [Google Scholar] [CrossRef] [PubMed]
- Malcolmson, L.J.; Przybylski, R.; Daun, J.K. Storage Stability of Milled Flaxseed. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 235–238. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The Antioxidant Activity and Oxidative Stability of Cold-Pressed Oils. JAOCS J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [Green Version]
- Codex Alimentarius. Standard for Named Vegetable Oils Codex Stan 210-1999. In Codex Alimentarius; FAO: Rome, Italy, 1999; pp. 1–13. [Google Scholar]
- Gordon, M.H. The Development of Oxidative Rancidity in Foods; Woodhead Publishing Ltd.: Sawston, UK, 2001. [Google Scholar] [CrossRef]
- Kozub, A.; Nikolaichuk, H.; Przykaza, K.; Tomaszewska-Gras, J.; Fornal, E. Lipidomic Characteristics of Three Edible Cold-Pressed Oils by LC/Q-TOF for Simple Quality and Authenticity Assurance. Food Chem. 2023, 415, 135761. [Google Scholar] [CrossRef] [PubMed]
- Mcelhaney, R.N. The Use of Differential Scanning Calorimetry and Differential Thermal Analysis in Studies of Model and Biological Membranes. Chem. Phys. Lipids 1982, 30, 229–259. [Google Scholar] [CrossRef]
- Tan, C.P.; Che Man, Y.B. Comparative Differential Scanning Calorimetric Analysis of Vegetable Oils: I. Effects of Heating Rate Variation. Phytochem. Anal. 2002, 13, 129–141. [Google Scholar] [CrossRef]
- Minato, A.; Ueno, S.; Yano, J.; Smith, K.; Seto, H.; Amemiya, Y.; Sato, K. Thermal and Structural Properties of Sn-1,3-Dipalmitoyl-2-Oleoylglycerol and Sn-1,3-Dioleoyl-2-Palmitoylglycerol Binary Mixtures Examined with Synchrotron Radiation X-Ray Diffraction. JAOCS J. Am. Oil Chem. Soc. 1997, 74, 1213–1220. [Google Scholar] [CrossRef]
- Pratama, Y.; Burholt, S.; Baker, D.L.; Sadeghpour, A.; Simone, E.; Rappolt, M. Polymorphism of a Highly Asymmetrical Triacylglycerol in Milk Fat: 1-Butyryl 2-Stearoyl 3-Palmitoyl-Glycerol. Cryst. Growth Des. 2022, 22, 6120–6130. [Google Scholar] [CrossRef]
- Tan, C.P.; Che Man, Y.B. Differential Scanning Calorimetric Analysis of Edible Oils: Comparison of Thermal Properties and Chemical Composition. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 143–155. [Google Scholar] [CrossRef]
- Konieczny, P.; Tomaszewska-Gras, J.; Andrzejewski, W.; Mikołajczak, B.; Urbańska, M.; Mazurkiewicz, J.; Stangierski, J. DSC and Electrophoretic Studies on Protein Denaturation of Anodonta Woodiana (Lea, 1834). J. Therm. Anal. Calorim. 2016, 126, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Vega, S.; Garcia-Gonzalez, M.A.; Lanas, A.; Velazquez-Campoy, A.; Abian, O. Deconvolution Analysis for Classifying Gastric Adenocarcinoma Patients Based on Differential Scanning Calorimetry Serum Thermograms. Sci. Rep. 2015, 5, 7988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Saito, K.; Ataké, T. Theoretical Analysis of Peak Height in Classical DTA, Power-Compensated DSC and Heat-Flux DSC. Thermochim. Acta 1986, 107, 277–282. [Google Scholar] [CrossRef]
- Lim, S.A.H.; Antony, J.; Albliwi, S. Statistical Process Control (SPC) in the Food Industry—A Systematic Review and Future Research Agenda. Trends Food Sci. Technol. 2014, 37, 137–151. [Google Scholar] [CrossRef]
- Pudjihardjo, I.; Mulyana, I.J.; Asrini, L.J. Autocorrelated Multivariate Control Chart in Cooking Oil Industry. Proc. Int. Conf. Ind. Eng. Oper. Manag. 2019, 2019, 1440–1448. [Google Scholar]







| Seeds Varieties | Time | Chemical Analysis | |||
|---|---|---|---|---|---|
| AV (mg KOH/g) | PV (meq O2/kg) | p-AV | TOTOX | ||
| FL_BU | 0 month | 0.80 ± 0.04 b | 3.95 ± 0.63 c | 0.87 ± 0.35 a | 8.78 ± 1.02 c |
| After 6 months | 2.54 ± 0.02 g | 14.44 ± 0.08 h | 2.02 ± 0.02 c | 30.90 ± 0.15 h | |
| FL_DL | 0 month | 0.41 ± 0.01 a | 2.49 ± 0.15 b | 0.65 ± 0.01 a | 5.63 ± 0.31 b |
| After 6 months | 1.59 ± 0.02 d | 10.64 ± 0.11 f | 1.70 ± 0.07 bc | 22.76 ± 0.21 f | |
| FL_SZA | 0 month | 1.27 ± 0.03 c | 1.41 ± 0.06 a | 0.73 ± 0.13 a | 3.54 ± 0.20 a |
| After 6 months | 2.40 ± 0.02 f | 9.73 ± 0.13 e | 1.49 ± 0.01 b | 21.09 ± 0.28 e | |
| FL_SZB | 0 month | 0.81 ± 0.03 b | 6.90 ± 0.23 d | 0.94 ± 0.12 a | 14.74 ± 0.41 d |
| After 6 months | 1.79 ± 0.02 e | 15.57 ± 0.16 i | 1.63 ± 0.02 bc | 33.61 ± 0.30 i | |
| FL_NN | 0 month | 1.60 ± 0.02 d | 1.21 ± 0.06 a | 0.76 ± 0.25 a | 3.17 ± 0.24 a |
| After 6 months | 3.25 ± 0.10 h | 11.69 ± 0.22 g | 2.47 ± 0.03 d | 25.08 ± 0.50 g | |
| Peak Temperature (°C) | Time | FL_BU | FL_DL | FL_SZA | FL_SZB | FL_NN |
|---|---|---|---|---|---|---|
| T1 | 0 | −36.85 ± 0.11 aA | −36.37 ± 0.12 aAB | −36.65 ± 0.22 aAB | −36.36 ± 0.07 aA | −36.55 ± 0.93 aA |
| 2 | −37.19 ± 0.37 abA | −36.93 ± 0.27 abcA | −37.57 ± 0.36 aA | −36.2 ± 0.18 cA | −36.44 ± 0.32 bcA | |
| 4 | −36.72 ± 0.62 aA | −36.58 ± 0.06 aAB | −36.44 ± 0.18 aB | −35.69 ± 0.46 abA | −34.80 ± 0.17 bB | |
| 6 | −36.55 ± 0.18 aA | −36.12 ± 0.29 abB | −36.38 ± 0.20 aB | −35.59 ± 0.11 bA | −35.59 ± 0.06 bAB | |
| T2 | 0 | −31.67 ± 0.09 aA | −30.10 ± 0.11 abAB | −30.37 ± 0.25 abB | −29.72 ± 0.05 bA | −30.01 ± 0.97a bA |
| 2 | −31.54 ± 0.2 aA | −30.50 ± 0.29 bcA | −31.06 ± 0.21 abA | −29.64 ± 0.23 dA | −30.00 ± 0.25 cdA | |
| 4 | −30.88 ± 0.27 aB | −29.31 ± 0.28 bcB | −29.53 ± 0.12 bC | −28.35 ± 0.20 dB | −28.66 ± 0.03 cdA | |
| 6 | −30.22 ± 0.11 aB | −29.39 ± 0.24 bB | −29.67 ± 0.08 abC | −28.6 ± 0.13 cB | −28.55 ± 0.08 cA | |
| T3 | 0 | −25.12 ± 0.00 aA | −24.94 ± 0.18 abA | −24.96 ± 0.2 abA | −24.19 ± 0.12 bA | −24.58 ± 0.54 abA |
| 2 | −25.01 ± 0.00 aA | −25.05 ± 0.09 aA | −25.14 ± 0.00 aA | −24.85 ± 0.08 abA | −24.55 ± 0.22 bA | |
| 4 | −24.98 ± 0.16 aA | −25.10 ± 0.00 aA | −25.11 ± 0.00 aA | −24.63 ± 0.66 aA | −23.31 ± 0.16 bB | |
| 6 | −25.12 ± 0.00 aA | −24.55 ± 0.77 aA | −25.13 ± 0.00 aA | −24.47 ± 0.04 aA | −22.99 ± 0.07 bB | |
| T4 | 0 | −13.63 ± 0.24 aA | −12.50 ± 0.49 abA | −12.64 ± 0.34 abA | −12.20 ± 0.06 bA | −13.87± 0.18 aA |
| 2 | −13.57 ± 0.16 aA | −12.84 ± 0.18 abA | −13.27 ± 0.20 aA | −11.76 ± 0.42 cA | −12.21 ± 0.61 bcB | |
| 4 | −14.02 ± 0.00 aA | −10.62 ± 0.23 bcB | −11.13 ± 0.24 bB | −10.40 ± 0.29 bcB | −9.94 ± 0.15 cC | |
| 6 | −12.05 ± 0.24 bB | −11.24 ± 0.16 aB | −11.13 ± 0.04 abB | −10.54 ± 0.28 cB | −10.49 ± 0.08 cC |
| Peak Height (W/g) | Time | FL_BU | FL_DL | FL_SZA | FL_SZB | FL_NN |
|---|---|---|---|---|---|---|
| h1 | 0 | 0.15 ± 0.008 bB | 0.13 ± 0.008 abA | 0.14 ± 0.005 abA | 0.12 ± 0.008 aA | 0.08 ± 0.014 aA |
| 2 | 0.17 ± 0.007 cB | 0.15 ± 0.006 bA | 0.15 ± 0.008 bcA | 0.13 ± 0.004 aA | 0.12 ± 0.003 aA | |
| 4 | 0.12 ± 0.004 abcA | 0.14 ± 0.008 bcA | 0.15 ± 0.002 cA | 0.11 ± 0.006 abA | 0.11 ± 0.013 aA | |
| 6 | 0.15 ± 0.006 aB | 0.13 ± 0.024 bA | 0.15 ± 0.011 abA | 0.12 ± 0.000 cA | 0.12 ± 0.001 cA | |
| h2 | 0 | 0.47 ± 0.033 aB | 0.52 ± 0.099 aA | 0.46 ± 0.003 aB | 0.52 ± 0.002 aC | 0.51 ± 0.062 aAB |
| 2 | 0.43 ± 0.016 aB | 0.46 ± 0.008 abA | 0.46 ± 0.012 abB | 0.48 ± 0.013 bB | 0.48 ± 0.031 bB | |
| 4 | 0.45 ± 0.005 cB | 0.37 ± 0.005 abA | 0.37 ± 0.000 aA | 0.40 ± 0.005 bA | 0.46 ± 0.016 cAB | |
| 6 | 0.36 ± 0.007 aA | 0.42 ± 0.040 aA | 0.39 ± 0.013 aA | 0.41 ± 0.010 aA | 0.38 ± 0.000 aA | |
| h3 | 0 | 0.14 ± 0.006 aA | 0.14 ± 0.006a A | 0.14 ± 0.000 aA | 0.15 ± 0.005 aA | 0.13 ± 0.000 aA |
| 2 | 0.14 ± 0.002 aA | 0.15 ± 0.006 bAB | 0.15 ± 0.001 bB | 0.16 ± 0.006 bA | 0.16 ± 0.002 bB | |
| 4 | 0.14 ± 0.003 aA | 0.17 ± 0.003 bB | 0.16 ± 0.008 abB | 0.15 ± 0.010 abA | 0.14 ± 0.008 aA | |
| 6 | 0.14 ± 0.001 aA | 0.14 ± 0.002 aA | 0.15 ± 0.001 aB | 0.15 ± 0.008 aA | 0.14 ± 0.001 aA | |
| h4 | 0 | 0.02 ± 0.001 cAB | 0.01 ± 0.001 aA | 0.02 ± 0.001 bAB | 0.02 ± 0.001 bcB | 0.02 ± 0.002 bcB |
| 2 | 0.03 ± 0.002 cB | 0.02 ± 0.001 abA | 0.02 ± 0.001 bcB | 0.02 ± 0.001 aB | 0.02 ± 0.003 abB | |
| 4 | 0.02 ± 0.002 bA | 0.02 ± 0.001 abA | 0.02 ± 0.000 abA | 0.01 ± 0.002 bA | 0.01 ± 0.002 bA | |
| 6 | 0.02 ± 0.000 bA | 0.01 ± 0.004 abA | 0.01 ± 0.003 abA | 0.01 ± 0.003 abA | 0.01 ± 0.000 aA |
| Peak Area | Time | FL_BU | FL_DL | FL_SZA | FL_SZB | FL_NN |
|---|---|---|---|---|---|---|
| A1 | 0 | 1.39 ± 0.07 bB | 1.18 ± 0.07 abA | 1.19 ± 0.04 abA | 1.03 ± 0.07 aA | 0.86 ± 0.12 aA |
| 2 | 1.45 ± 0.06 cB | 1.27 ± 0.05 bA | 1.33 ± 0.07 bcA | 1.12 ± 0.04 aA | 1.04 ± 0.02 aA | |
| 4 | 1.09 ± 0.03 abA | 1.14 ± 0.1 abA | 1.30 ± 0.02 bA | 0.10 ± 0.07 aA | 0.93 ± 0.11 aA | |
| 6 | 1.36 ± 0.05 aB | 1.12 ± 0.21 aA | 1.30 ± 0.04 aA | 1.07 ± 0.00 aA | 1.08 ± 0.01 aA | |
| A2 | 0 | 2.50 ± 0.20 aB | 2.68 ± 0.73 aB | 2.22 ± 0.01 aB | 2.70 ± 0.05 aC | 2.42 ± 0.04 aA |
| 2 | 2.20 ± 0.08 aB | 2.19 ± 0.04 aAB | 2.28 ± 0.03 aB | 2.21 ± 0.14 aB | 2.42 ± 0.26 aA | |
| 4 | 2.29 ± 0.04 bB | 1.23 ± 0.11 aA | 1.40 ± 0.09 aA | 1.61 ± 0.05 aA | 2.31 ± 0.20 bA | |
| 6 | 1.41 ± 0.06 aA | 1.74 ± 0.16 abAB | 1.62 ± 0.18 abA | 1.72 ± 0.05 abA | 1.89 ± 0.02 bA | |
| A3 | 0 | 1.20 ± 0.00 aA | 1.35 ± 0.06 abA | 1.32 ± 0.00 abA | 1.44 ± 0.05 bA | 1.25 ± 0.00 aA |
| 2 | 1.29 ± 0.03 aB | 1.44 ± 0.06 bAB | 1.41 ± 0.06 abA | 1.50 ± 0.06 bA | 1.48 ± 0.02 bB | |
| 4 | 1.29 ± 0.02 aB | 1.58 ± 0.02 bB | 1.49 ± 0.08 abA | 1.44 ± 0.09 abA | 1.32 ± 0.08 bA | |
| 6 | 1.38 ± 0.01 aC | 1.38 ± 0.02 aA | 1.47 ± 0.01 aA | 1.41 ± 0.08 aA | 1.34 ± 0.01 aA | |
| A4 | 0 | 0.21 ± 0.01 bB | 0.14 ± 0.02 aAB | 0.18 ± 0.02 abB | 0.18 ± 0.02 abB | 0.22 ± 0.02 bB |
| 2 | 0.22 ± 0.01 bB | 0.19 ± 0.01 abB | 0.23 ± 0.01 bC | 0.15 ± 0.02 aB | 0.20 ± 0.04 abB | |
| 4 | 0.18 ± 0.02 bAB | 0.12 ± 0.01 aA | 0.13 ± 0.00 abA | 0.08 ± 0.02 aA | 0.08 ± 0.02 aA | |
| 6 | 0.14 ± 0.00 bA | 0.11 ± 0.02 abA | 0.11 ± 0.02 abA | 0.09 ± 0.02 abA | 0.06 ± 0.00 aA | |
| % peak area | ||||||
| P A1 | 0 | 26.21 ± 0.07 bB | 22.18 ± 2.33 abA | 24.25 ± 0.57 abA | 19.23 ± 0.99 aA | 18.16 ± 2.17 aA |
| 2 | 28.16 ± 1.30 cB | 25.02 ± 0.20 bA | 25.33 ± 1.09 bcA | 22.54 ± 0.74 abB | 20.33 ± 1.62 aA | |
| 4 | 22.49 ± 0.13 aA | 28.01 ± 1.06 bcA | 30.20 ± 0.25 cB | 24.11 ± 1.49 abB | 20.13 ± 2.37 aA | |
| 6 | 31.65 ± 0.36 bC | 25.58 ± 2.89 aA | 28.97 ± 0.47 abB | 24.93 ± 0.04 aB | 24.67 ± 0.15 aA | |
| P A2 | 0 | 48.21 ± 1.32 aC | 50.03 ± 5.48 aC | 45.91 ± 0.42 aB | 52.65 ± 1.66 aC | 51.71 ± 1.92 aA |
| 2 | 42.67 ± 1.12 aB | 43.03 ± 0.59 aBC | 43.40 ± 0.70 aB | 44.35 ± 0.95 abB | 47.08 ± 2.32 bA | |
| 4 | 47.15 ± 0.22 bcC | 30.07 ± 1.38 aA | 32.45 ± 1.88 aA | 38.94 ± 0.73 abA | 49.70 ± 4.3 cA | |
| 6 | 32.99 ± 0.62 aA | 40.01 ± 0.65 bcB | 35.88 ± 2.1 abA | 40.12 ± 1.26 bcA | 43.37 ± 0.45 cA | |
| P A3 | 0 | 22.68 ± 1.15 aA | 25.49 ± 3.05 aA | 26.85 ± 0.41 aA | 26.85 ± 0.45 aA | 26.36 ± 0.49 aA |
| 2 | 24.87 ± 0.47 aAB | 28.21 ± 0.24 bcA | 26.87 ± 1.32 abA | 30.03 ± 0.41 cAB | 28.80 ± 1.28 abAB | |
| 4 | 26.63 ± 0.26 aB | 38.92 ± 2.47 cB | 34.43 ± 2.14 abcB | 34.95 ± 2.58 bcB | 28.35 ± 1.59 abAB | |
| 6 | 32.18 ± 0.98 aC | 31.80 ± 2.79 aAB | 32.77 ± 1.96 aB | 32.86 ± 1.88 aB | 30.68 ± 0.24 aB | |
| P A4 | 0 | 3.94 ± 0.10 bcAB | 2.61 ± 0.11 aA | 3.67 ± 0.26 bcB | 3.45 ± 0.22 abB | 4.55 ± 0.25 cB |
| 2 | 4.30 ± 0.24 bB | 3.74 ± 0.16 abA | 4.40 ± 0.14 bC | 3.07 ± 0.20 aAB | 3.78 ± 0.61 abB | |
| 4 | 3.73 ± 0.36 cAB | 2.99 ± 0.03 bcA | 2.92 ± 0.01 abcAB | 1.99 ± 0.39 abA | 1.81 ± 0.34 aA | |
| 6 | 3.17 ± 0.01 bA | 2.61 ± 0.76 abA | 2.37 ± 0.33 abA | 2.09 ± 0.58 abA | 1.27 ± 0.06 aA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.; Kaczmarek, A.; Grygier, A.; Tomaszewska-Gras, J. DSC Phase Transition Profiles Analyzed by Control Charts to Determine Markers for the Authenticity and Deterioration of Flaxseed Oil during Storage. Foods 2023, 12, 2954. https://doi.org/10.3390/foods12152954
Islam M, Kaczmarek A, Grygier A, Tomaszewska-Gras J. DSC Phase Transition Profiles Analyzed by Control Charts to Determine Markers for the Authenticity and Deterioration of Flaxseed Oil during Storage. Foods. 2023; 12(15):2954. https://doi.org/10.3390/foods12152954
Chicago/Turabian StyleIslam, Mahbuba, Anna Kaczmarek, Anna Grygier, and Jolanta Tomaszewska-Gras. 2023. "DSC Phase Transition Profiles Analyzed by Control Charts to Determine Markers for the Authenticity and Deterioration of Flaxseed Oil during Storage" Foods 12, no. 15: 2954. https://doi.org/10.3390/foods12152954
APA StyleIslam, M., Kaczmarek, A., Grygier, A., & Tomaszewska-Gras, J. (2023). DSC Phase Transition Profiles Analyzed by Control Charts to Determine Markers for the Authenticity and Deterioration of Flaxseed Oil during Storage. Foods, 12(15), 2954. https://doi.org/10.3390/foods12152954