Inulin Functionalized “Giuncata” Cheese as a Source of Prebiotic Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
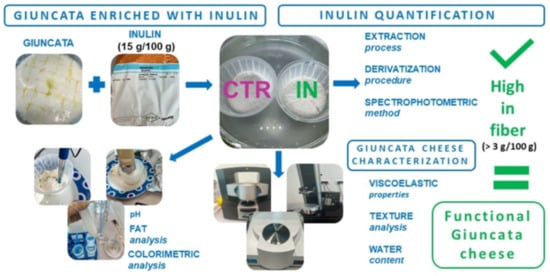
2.2. Fortified Giuncata Cheese Manufacturing
2.3. Inulin Extraction Procedure
2.4. Derivatization and Spectrophotometric Method for Inulin Quantification
2.5. Characterization of Inulin-Fortified Giuncata Cheese
2.5.1. Physical–Chemical Composition
2.5.2. Viscoelastic Properties
2.5.3. Texture Analysis
2.5.4. Thermo Gravimetric (TGA) and Differential Thermal (DTA) Analyses
2.5.5. Total Water Content
2.6. Statistical Analysis
3. Results
3.1. Fortified Giuncata Manufactoring
3.2. Inulin Fortification and Extraction Procedure
3.3. Study of Giuncata Enrichment
3.3.1. Physical–Chemical Composition
3.3.2. Viscoelastic Properties of Giuncata Cheese
3.3.3. Texture Analysis
3.3.4. Water Content and Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apolinário, A.C.; de Lima Damasceno, B.P.G.; de Macêdo Beltrão, N.E.; Pessoa, A.; Converti, A.; da Silva, J.A. Inulin-type fructans: A review on different aspects of biochemical and pharmaceutical technology. Carbohydr. Polym. 2014, 101, 368–378. [Google Scholar] [CrossRef]
- Judprasong, K.; Tanjor, S.; Puwastien, P.; Sungpuag, P. Investigation of Thai plants for potential sources of inulin-type fructans. J. Food Compos. Anal. 2011, 24, 642–649. [Google Scholar] [CrossRef]
- Roberfroid, M.B.; Van Loo, J.A.; Gibson, G.R. The bifidogenic nature of chicory inulin and its hydrolysis products. J. Nutr. 1998, 128, 11–19. [Google Scholar] [CrossRef]
- Jackson PP, J.; Wijeyesekera, A.; Theis, S.; van Harsselaar, J.; Rastall, R.A. Food for thought! Inulin-type fructans: Does the food matrix matter? J. Funct. Foods 2022, 90, 104987. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Vieira-Silva, S.; Wang, J.; Sailer, M.; Theis, S.; Verbeke, K.; Raes, J. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut 2017, 66, 1968–1974. [Google Scholar] [CrossRef]
- Hughes, R.L.; Alvarado, D.A.; Swanson, K.S.; Holscher, H.D. The prebiotic potential of inulin-type fructans: A systematic review. Adv. Nutr. 2022, 13, 492–529. [Google Scholar] [CrossRef]
- Krupa-Kozak, U.; Drabińska, N.; Rosell, C.M.; Piłat, B.; Starowicz, M.; Jeliński, T.; Szmatowicz, B. High-Quality Gluten-Free Sponge Cakes without Sucrose: Inulin-Type Fructans as Sugar Alternatives. Foods 2020, 9, 1735. [Google Scholar] [CrossRef]
- Borges, J.V.; de Souza, J.A.; Fagnani, R.; Costa, G.N.; Dos Santos, J.S. Reduced fat Frescal sheep milk cheese with inulin: A first report about technological aspects and sensory evaluation. J. Dairy Res. 2019, 86, 368–373. [Google Scholar] [CrossRef]
- Popoola-Akinola, O.O.; Raji, T.J.; Olawoye, B. Lignocellulose, dietary fibre, inulin and their potential application in food. Heliyon 2022, 8, e10459. [Google Scholar] [CrossRef]
- Mudannayake, D.C.; Jayasena, D.D.; Wimalasiri, K.M.; Ranadheera, C.S.; Ajlouni, S. Inulin fructans-food applications and alternative plant sources: A review. Int. J. Food Sci. Technol. 2022, 57, 5764–5780. [Google Scholar] [CrossRef]
- Lourencetti, R.E.; Benossi, L.; Marques, D.R.; Joia, B.M.; Monteiro, A.R.G. Development of biscuit type cookie with partial replacement of fat by inulin. Int. J. Nutr. Food Sci. 2013, 2, 261–265. [Google Scholar]
- Mansouripour, S.; Mizani, M.; Rasouli, S.; Gerami, A.; Sharifan, A. Effect of inulin and galactooligosaccharides on particle size distribution and rheological properties of prebiotic ketchup. Int. J. Food Prop. 2017, 20, 157–170. [Google Scholar] [CrossRef]
- Ren, Y.; Song, K.Y.; Kim, Y. Physicochemical and retrogradation properties of low-fat muffins with inulin and hydroxypropyl methylcellulose as fat replacers. J. Food Process. Preserv. 2020, 44, e14816. [Google Scholar] [CrossRef]
- Cardarelli, H.R.; Buriti, F.C.; Castro, I.A.; Saad, S.M. Inulin and oligofructose improve sensory quality and increase the probiotic viable count in potentially synbiotic petit-suisse cheese. LWT-Food Sci. Technol. 2008, 41, 1037–1046. [Google Scholar] [CrossRef]
- Hennelly, P.J.; Dunne, P.G.; O’sullivan, M.; O’riordan, E.D. Textural, rheological and microstructural properties of imitation cheese containing inulin. J. Food Eng. 2006, 75, 388–395. [Google Scholar] [CrossRef]
- Keenan, D.F.; Resconi, V.C.; Kerry, J.P.; Hamill, R.M. Modelling the influence of inulin as a fat substitute in comminuted meat products on their physico-chemical characteristics and eating quality using a mixture design approach. Meat Sci. 2014, 96, 1384–1394. [Google Scholar]
- Koca, N.; Metin, M. Textural, melting and sensory properties of low-fat fresh kashar cheeses produced by using fat replacers. Int. Dairy J. 2004, 14, 365–373. [Google Scholar] [CrossRef]
- Pagliarini, E.; Beatrice, N. Sensory and Rheological properties of low-fat filled ‘pasta filata’cheese. J. Dairy Res. 1994, 61, 299–304. [Google Scholar] [CrossRef]
- Eur-lex.europa. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:02006R1924-20141213&from=IT (accessed on 11 May 2023).
- Eur-lex.europa. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/?uri=celex%3A02006R1924-20141213 (accessed on 11 May 2023).
- Wang, R.; Wan, J.; Liu, C.; Xia, X.; Ding, Y. Pasting, thermal, and rheological properties of rice starch were partially replaced by inulin with different degrees of polymerization. Food Hydrocoll. 2019, 92, 228–232. [Google Scholar] [CrossRef]
- Li, Y.; Ma, X.; Liu, X. Physicochemical and rheological properties of cross-linked inulin with different degree of polymerization. Food Hydrocoll. 2019, 95, 318–325. [Google Scholar] [CrossRef]
- Teferra, T.F. Possible actions of inulin as prebiotic polysaccharide: A review. Food Front. 2021, 2, 407–416. [Google Scholar] [CrossRef]
- Meyer, D.; Bayarri, S.; Tárrega, A.; Costell, E. Inulin as a texture modifier in dairy products. Food Hydrocoll. 2011, 25, 1881–1890. [Google Scholar] [CrossRef]
- Barclay, T.; Ginic-Markovic, M.; Johnston, M.R.; Cooper, P.D.; Petrovsky, N. Analysis of the hydrolysis of inulin using real time 1H NMR spectroscopy. Carbohydr. Res. 2012, 352, 117–125. [Google Scholar] [CrossRef]
- Petkova, N.I.; Todorova, M.; Vlaseva, R.; Denev, P. Spectrophotometric Method for Determination of Inulin and Fructooligosaccharides in Lactic Acid Fermented Dairy Products. Conf. Pap. 2013. Available online: https://www.researchgate.net/publication/258519310 (accessed on 15 September 2023).
- Besir, A.; Yazici, F.; Mortas, M.; Gul, O. A novel spectrophotometric method based on Seliwanoff test to determine 5-(Hydroxymethyl) furfural (HMF) in honey: Development, in house validation and application. LWT 2021, 139, 110602. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Nzekoue, F.K.; Sagratini, G.; Ricciutelli, M.; Vittori, S.; Caprioli, G. Development and application of a novel analytical method for the determination of 8 plant sterols/stanols in 22 legumes samples. J. Food Compos. Anal. 2023, 118, 105195. [Google Scholar] [CrossRef]
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative study on total lipid determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and modified Bligh Dyer extraction methods. J. Food Compos. Anal. 2001, 14, 93–100. [Google Scholar]
- Caprioli, G.; Kamgang Nzekoue, F.; Fiorini, D.; Scocco, P.; Trabalza-Marinucci, M.; Acuti, G.; Tardella, F.M.; Sagratini, G.; Catorci, A. The effects of feeding supplementation on the nutritional quality of milk and cheese from sheep grazing on dry pasture. Int. J. Food Sci. Nutr. 2020, 71, 50–62. [Google Scholar] [CrossRef]
- Seuvre, A.M.; Turci, C.; Voilley, A. Effect of the temperature on the release of aroma compounds and on the rheological behaviour of model dairy custard. Food Chem. 2008, 108, 1176–1182. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Nzekoue, F.K.; Alesi, A.; Ricciutelli, M.; Sagratini, G.; Suo, X.; Torregiani, E.; Vittori, S.; Caprioli, G. Development of Innovative Vitamin D Enrichment Designs for Two Typical Italian Fresh Cheeses: Burrata and Giuncata. Molecules 2023, 28, 1049. [Google Scholar] [CrossRef]
- Salvatore, E.; Pes, M.; Mazzarello, V.; Pirisi, A. Replacement of fat with long-chain inulin in a fresh cheese made from caprine milk. Int. Dairy J. 2014, 34, 1–5. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Drake, M.A. Invited review: Sensory and mechanical properties of cheese texture. J. Dairy Sci. 2007, 90, 1611–1624. [Google Scholar] [CrossRef]
- Brown, J.A.; Foegeding, E.A.; Daubert, C.R.; Drake, M.A.; Gumpertz, M. Relationships among rheological and sensorial properties of young cheeses. J. Dairy Sci. 2003, 86, 3054–3067. [Google Scholar]
- Diaz-Bustamante, M.L.; Reyes, L.H.; Solano, O.A.A. Application of a Multiscale Approach in the Substitution and Reduction of NaCl in Costeño-Type Artisan Cheese. Appl. Sci. 2020, 10, 9008. [Google Scholar] [CrossRef]
- Dimitreli, G.; Thomareis, A.S. Instrumental textural and viscoelastic properties of processed cheese as affected by emulsifying salts and in relation to its apparent viscosity. Int. J. Food Prop. 2009, 12, 261–275. [Google Scholar] [CrossRef]
- Sołowiej, B.; Glibowski, P.; Muszyński, S.; Wydrych, J.; Gawron, A.; Jeliński, T. The effect of fat replacement by inulin on the physicochemical properties and microstructure of acid casein processed cheese analogues with added whey protein polymers. Food Hydrocoll. 2015, 44, 1–11. [Google Scholar] [CrossRef]
- Karimi, R.; Azizi, M.H.; Ghasemlou, M.; Vaziri, M. Application of inulin in cheese as prebiotic, fat replacer and texturizer: A review. Carbohydr. Polym. 2015, 119, 85–100. [Google Scholar] [CrossRef]
- Saldo, J.; Sendra, E.; Guamis, B. Changes in water binding in high-pressure treated cheese, measured by TGA (thermogravimetrical analysis). Innov. Food Sci. Emerg. Technol. 2002, 3, 2013–2207. [Google Scholar]
- Chen, Y.; MacNaughtan, W.; Jones, P.; Yang, Q.; Foster, T. The state of water and fat during the maturation of Cheddar cheese. Food Chem. 2020, 303, 125390. [Google Scholar] [CrossRef]






| Inulin Added to Milk (g) | Milk (g) | Produced Cheese (g) | Concentration of Inulin (g/100 g) |
|---|---|---|---|
| 10 | 500 | 137.1 ± 12.0 a | 3.6 ± 0.3 a |
| 15 | 500 | 122.6 ± 4.5 b | 4.0 ± 0.1 b |
| Giuncata IN | Giuncata CTR | |
|---|---|---|
| pH | 6.6 ± 0.0 a | 6.5 ± 0.0 a |
| Color | ||
| 95.6 ± 0.6 a | 93.9 ± 0.2 b |
| −2.0 ± 0.3 a | −1.8 ± 0.2 a |
| 7.5 ± 0.6 a | 7.8 ± 0.7 b |
| Fats (Soxhlet extraction) Fats (Folch extraction) | 11.72 ± 0.6% a 11.91± 0.2% a | 12.28 ± 0.7% b 12.52± 0.3% b |
| Positive Fmax “Compressive Force” (N) | Negative Fmax (N) | Positive Area “Stiffness” (N mm) | Negative Area “Adhesiveness” (N mm) | |
|---|---|---|---|---|
| Giuncata CTR | 1.055 ± 0.291 a | 0.384 ± 0.083 a | 10.03 ± 3.24 a | 3.88 ± 1.43 a |
| Giuncata IN | 0.611 ± 0.127 b | 0.296 ± 0.041 b | 6.57 ± 1.10 b | 3.39 ± 0.46 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perinelli, D.R.; Santanatoglia, A.; Caprioli, G.; Bonacucina, G.; Vittori, S.; Maggi, F.; Sagratini, G. Inulin Functionalized “Giuncata” Cheese as a Source of Prebiotic Fibers. Foods 2023, 12, 3499. https://doi.org/10.3390/foods12183499
Perinelli DR, Santanatoglia A, Caprioli G, Bonacucina G, Vittori S, Maggi F, Sagratini G. Inulin Functionalized “Giuncata” Cheese as a Source of Prebiotic Fibers. Foods. 2023; 12(18):3499. https://doi.org/10.3390/foods12183499
Chicago/Turabian StylePerinelli, Diego Romano, Agnese Santanatoglia, Giovanni Caprioli, Giulia Bonacucina, Sauro Vittori, Filippo Maggi, and Gianni Sagratini. 2023. "Inulin Functionalized “Giuncata” Cheese as a Source of Prebiotic Fibers" Foods 12, no. 18: 3499. https://doi.org/10.3390/foods12183499
APA StylePerinelli, D. R., Santanatoglia, A., Caprioli, G., Bonacucina, G., Vittori, S., Maggi, F., & Sagratini, G. (2023). Inulin Functionalized “Giuncata” Cheese as a Source of Prebiotic Fibers. Foods, 12(18), 3499. https://doi.org/10.3390/foods12183499