Effect of Thermosonication on the Nutritional Quality of Lapsi (Choerospondias axillaris) Fruit Juice: Application of Advanced Artificial Neural Networks
Abstract
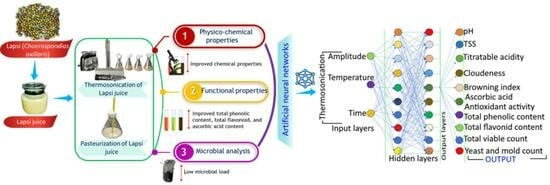
:1. Introduction
2. Materials and Methods
2.1. Lapsi (Choerospondias axillaris) Fruit Sample
2.2. Chemicals
2.3. Juice Preparation
2.4. Pasteurisation of the Juices
2.5. Thermosonication of the Juices
2.6. Determination of Physicochemical Properties
2.6.1. pH, Total Soluble Solids, and Titratable Acidity
2.6.2. Cloudiness and Browning Index
2.7. Determination of Functional Properties
2.7.1. Ascorbic Acid Content
2.7.2. Antioxidant Activity
2.7.3. Total Phenolic Content
2.7.4. Total Flavonoid Content
2.8. Determination of Microbial Attributes
2.9. Experimental Design for Optimisation
Artificial Neural Network Modelling
2.10. Statistical Analysis
3. Results and Discussion
3.1. Impact of Processing on Physicochemical Properties of Lapsi Juices
3.1.1. pH, TSS, and TA
3.1.2. Cloudiness and Browning Index of Lapsi Juice
3.2. Impact of Processing on Functional Properties of Lapsi Juices
3.2.1. Ascorbic Acid
3.2.2. Antioxidant Activity
3.2.3. Total Phenolic Content
3.2.4. Total Flavonoid Content
3.3. Impact of Processing on the Microbial Counts of Lapsi Juices
3.4. The Efficiency and Evaluation of the Established ANN Model
3.5. ANN-Based Process for Parameter Optimisation
4. Conclusions
Industrial Relevance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TA | Titratable acidity |
| TSS | Total soluble solid |
| CI | Cloudiness index |
| BI | Browning index |
| AA | Ascorbic acid |
| AOA | Antioxidant activity |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| YMC | Yeast and mould count |
| R | Coefficient of determination |
| MSE | Mean square error |
| MAE | Mean absolute error |
| TS | Thermosonication |
| PS | Pasteurisation |
| LJ | Lapsi juice |
| RLJ | Raw lapsi juice |
| TSLJ | Thermosonicated lapsi juice |
| PSLJ | Pasteurised lapsi juice |
| ND | Not detected |
| ANN | Artificial neural network |
| GAE | Gallic acid equivalents |
| QE | Quercetin equivalent |
| cfu | Colony forming unit |
References
- Headey, D.D.; Alderman, H.H. The relative caloric prices of healthy and unhealthy foods differ systematically across income levels and continents. J. Nutr. 2019, 149, 2020–2033. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Vanlalhmangaiha; Akoijam, R.S.; Lungmuana; Boopathi, T.; Saha, S. Bioactivity and traditional uses of 26 underutilized ethno-medicinal fruit species of North-East Himalaya, India. J. Food Meas. Charact. 2018, 12, 2503–2514. [Google Scholar] [CrossRef]
- Mann, S.; Chakraborty, D.; Biswas, S. An alternative perspective of an underutilized fruit tree Choerospondias axillaris in health promotion and disease prevention: A review. Food Biosci. 2022, 47, 101609. [Google Scholar] [CrossRef]
- KC, Y.; Dangal, A.; Thapa, S.; Rayamajhi, S.; Chalise, K.; Shiwakoti, L.D.; Katuwal, N. Nutritional, phytochemicals, and sensory analysis of Lapsi (Choerospondias axillaris) fruit leather. Int. J. Food Prop. 2022, 25, 960–975. [Google Scholar] [CrossRef]
- Das, P.; Nayak, P.K.; Kesavan, R.K. Ultrasound assisted extraction of food colorants: Principle, mechanism, ex-traction technique and applications: A review on recent progress. Food Chem. Adv. 2022, 1, 100144. [Google Scholar] [CrossRef]
- Mandha, J.; Shumoy, H.; Matemu, A.O.; Raes, K. Characterization of fruit juices and effect of pasteurization and storage conditions on their microbial, physicochemical, and nutritional quality. Food Biosci. 2023, 51, 102335. [Google Scholar] [CrossRef]
- Aneja, K.R.; Dhiman, R.; Aggarwal, N.K.; Aneja, A. Emerging preservation techniques for controlling spoilage and pathogenic microorganisms in fruit juices. Int. J. Microbiol. 2014, 2014, 758942. [Google Scholar] [CrossRef]
- Hurtado, A.; Picouet, P.; Jofré, A.; Guàrdia, M.D.; Ros, J.M.; Bañón, S. Application of high-pressure processing for obtain-ing “fresh-like” fruit smoothies. Food Bioprocess Technol. 2015, 8, 2470–2482. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Petruzzi, L.; Perricone, M.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Nonthermal Technologies for Fruit and Vegetable Juices and Beverages: Overview and Advances. Compr. Rev. Food Sci. Food Saf. 2018, 17, 2–62. [Google Scholar] [CrossRef]
- Chavan, P.; Sharma, P.; Sharma, S.R.; Mittal, T.C.; Jaiswal, A.K. Application of high-intensity ultrasound to improve food processing efficiency: A review. Foods 2022, 11, 122. [Google Scholar] [CrossRef]
- Basumatary, B.; Nayak, P.K.; Chandrasekar, C.M.; Nath, A.; Nayak, M.; Kesavan, R.K. Impact of thermo sonication and pasteurization on the physicochemical, microbiological and anti-oxidant properties of pomelo (Citrus maxima) juice. Int. J. Fruit Sci. 2020, 20, S2056–S2073. [Google Scholar] [CrossRef]
- Kesavan, R.K.; Gogoi, S.; Nayak, P.K. Influence of thermosonication and pasteurization on the quality attributes of kutkura (Meyna spinosa) juice. Appl. Food Res. 2023, 3, 100268. [Google Scholar] [CrossRef]
- Kalathingal, M.S.H.; Basak, S.; Mitra, J. Artificial neural network modeling and genetic algorithm optimization of process parameters in fluidized bed drying of green tea leaves. J. Food Process. Eng. 2020, 43, e13128. [Google Scholar] [CrossRef]
- Gupta, T.K.; Raza, K. Optimization of ANN architecture: A review on nature-inspired techniques. In Machine Learning in Bio-Signal Analysis and Diagnostic Imaging; Springer: Amsterdam, The Netherlands, 2019; pp. 159–182. [Google Scholar] [CrossRef]
- Abdullah, S.; Pradhan, R.C.; Aflah, M.; Mishra, S. Efficiency of tannase enzyme for degradation of tannin from cashew apple juice: Modeling and optimization of process using artificial neural network and response surface methodology. J. Food Process. Eng. 2020, 43, e13499. [Google Scholar] [CrossRef]
- Sonawane, A.; Pathak, S.S.; Pradhan, R.C. Optimization of a process for the enzymatic extraction of nutrient enriched bael fruit juice using artificial neural network (ANN) and response surface methodology (RSM). Int. J. Fruit Sci. 2020, 20 (Suppl. S3), S1845–S1861. [Google Scholar] [CrossRef]
- Nourbakhsh, H.; Emam-Djomeh, Z.; Omid, M.; Mirsaeedghazi, H.; Moini, S. Prediction of red plum juice permeate flux during membrane processing with ANN optimized using RSM. Comput. Electron. Agric. 2014, 102, 1–9. [Google Scholar] [CrossRef]
- Cruz, J.M.d.A.; Ramos, A.S.; Corrêa, R.F.; Sanches, E.A.; Campelo, P.H.; Kinupp, V.F.; Bezerra, J.d.A. Thermal Treatment and High-Intensity Ultrasound Processing to Evaluate the Chemical Profile and Antioxidant Activity of Amazon Fig Juices. Processes 2023, 11, 408. [Google Scholar] [CrossRef]
- Bursać Kovačević, D.; Bilobrk, J.; Buntić, B.; Bosiljkov, T.; Karlović, S.; Rocchetti, G.; Lucini, L.; Barba, F.J.; Lorenzo, J.M.; Putnik, P. High-power ultrasound altered the polyphenolic content and antioxidant capacity in cloudy apple juice during storage. J. Food Process. Preserv. 2019, 43, e14023. [Google Scholar] [CrossRef]
- Atalar, I.; Gul, O.; Saricaoglu, F.T.; Besir, A.; Gul, L.B.; Yazici, F. Influence of thermosonication (TS) process on the quality parameters of high pressure homogenized hazelnut milk from hazelnut oil by-products. J. Food Sci. Technol. 2019, 56, 1405–1415. [Google Scholar] [CrossRef]
- Lynch, J.M.; Barbano, D.M. Kjeldahl nitrogen analysis as a reference method for protein determination in dairy prod-ucts. J. AOAC Int. 1999, 82, 1389–1398. [Google Scholar] [CrossRef]
- Nayak, P.K.; Chandrasekar, C.M.; Kesavan, R.K. Effect of thermosonication on the quality attributes of star fruit juice. J. Food Process Eng. 2018, 41, e12857. [Google Scholar] [CrossRef]
- Cervantes-Elizarrarás, A.; Piloni-Martini, J.; Ramírez-Moreno, E.; Alanís-García, E.; Güemes-Vera, N.; Gómez-Aldapa, C.A.; del Socorro Cruz-Cansino, N. Enzymatic inactivation and antioxidant properties of blackberry juice after ther-moultrasound: Optimization using response surface methodology. Ultrason. Sonochem. 2017, 34, 371–379. [Google Scholar] [CrossRef] [PubMed]
- GBehera; Rayaguru, K.; Nayak, P.K. Effect of microwave blanching on lice thickness and quality analysis of star fruit. Curr. Res. Nutr. Food Sci. J. 2017, 5, 274–281. [Google Scholar]
- Alam, A.; Biswas, M.; Ahmed, T.; Zahid, A.; Alam, M.; Hasan, M.K.; Biswas, B.; Zaman, R. Effect of Ultrasound and Thermal Pasteurization on Physicochemical Properties and Antioxidant Activity of Juice Extracted from Ripe and Overripe Pineapple. Food Nutr. Sci. 2023, 14, 300–314. [Google Scholar] [CrossRef]
- Nayak, P.K.; Rayaguru, K.; Krishnan, K.R. Quality comparison of elephant apple juices after high-pressure pro-cessing and thermal treatment. J. Sci. Food Agric. 2017, 97, 1404–1411. [Google Scholar] [CrossRef]
- Tomadoni, B.; Cassani, L.; Ponce, A.; Moreira, M.D.R.; Agüero, M.V. Optimization of ultrasound, vanillin and pomegranate extract treatment for shelf-stable unpasteurized strawberry juice. LWT-Food Sci. Technol. 2016, 72, 475–484. [Google Scholar] [CrossRef]
- Kashyap, P.; Riar, C.S.; Jindal, N. Optimization of ultrasound assisted extraction of polyphenols from Meghalayan cherry fruit (Prunus nepalensis) using response surface methodology (RSM) and artificial neural network (ANN) approach. J. Food Meas. Charact. 2021, 15, 119–133. [Google Scholar] [CrossRef]
- Rakshit, M.; Srivastav, P.P. Optimization of pulsed ultrasonic-assisted extraction of punicalagin from pomegranate (Punica granatum) peel: A comparison between response surface methodology and artificial neural network-multiobjective genetic algorithm. J. Food Process. Preserv. 2021, 45, e15078. [Google Scholar] [CrossRef]
- Nadeem, M.; Ubaid, N.; Qureshi, T.M.; Munir, M.; Mehmood, A. Effect of ultrasound and chemical treatment on total phenol, flavonoids and antioxidant properties on carrot-grape juice blend during storage. Ultrason. Sonochem. 2018, 45, 1–6. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Sonication enhances polyphenolic compounds, sugars, carotenoids and mineral elements of apple juice. Ultrason. Sonochem. 2014, 21, 93–97. [Google Scholar] [CrossRef]
- Lan, T.; Bao, S.; Wang, J.; Ge, Q.; Zhang, H.; Yang, W.; Ma, T. Shelf life of non-industrial fresh mango juice: Microbial safety, nutritional and sensory characteristics. Food Biosci. 2021, 42, 101060. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ali, M.; Aadil, R.M.; Ali, A.; Goksen, G.; Li, J.; Zeng, X.-A.; Proestos, C. Sustainable emerging sonication processing: Impact on fungicide reduction and the overall quality characteristics of tomato juice. Ultrason. Sonochem. 2023, 94, 106313. [Google Scholar] [CrossRef] [PubMed]
- Oladunjoye, A.O.; Adeboyejo, F.O.; Okekunbi, T.A.; Aderibigbe, O.R. Effect of thermosonication on quality attributes of hog plum (Spondias mombin L.) juice. Ultrason. Sonochem. 2021, 70, 105316. [Google Scholar] [CrossRef]
- Boghossian, M.; Brassesco, M.E.; Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. Thermosonication Applied to Kiwi Peel: Impact on Nutritional and Microbiological Indicators. Foods 2023, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, K.; Garvín, A.; Ibarz, A.; Augusto, P.E.D. Ascorbic acid stability in fruit juices during thermosonication. Ul-Trasonics Sonochem. 2017, 37, 375–381. [Google Scholar] [CrossRef]
- Putsakum, G.; Tzima, K.; Tiwari, B.K.; O’Donnell, C.P.; Rai, D.K. Effects of thermosonication on ascorbic acid, polyphenols and antioxidant activity in blackberry juice. Int. J. Food Sci. Technol. 2023, 58, 2304–2311. [Google Scholar] [CrossRef]
- Park, J.J.; Olawuyi, I.F.; Lee, W.Y. Influence of thermo-sonication and ascorbic acid treatment on microbial inactivation and shelf-life extension of soft persimmon (Diospyros kaki T.) juice. Food Bioprocess Technol. 2021, 14, 429–440. [Google Scholar] [CrossRef]
- Wang, C.; Xie, Y.; Wang, H.; Bai, Y.; Dai, C.; Li, C.; Xu, X.; Zhou, G. Phenolic compounds in beer inhibit formation of polycyclic aromatic hydrocarbons from charcoal-grilled chicken wings. Food Chem. 2019, 294, 578–586. [Google Scholar] [CrossRef]
- Lafarga, T.; Ruiz-Aguirre, I.; Abadias, M.; Viñas, I.; Bobo, G.; Aguiló-Aguayo, I. Effect of thermosonication on the bioaccessibiity of antioxidant compounds and the microbiological, physicochemical, and nutritional quality of an anthocyaninenriched tomato juice. Food Bioprocess Technol. 2019, 12, 147–157. [Google Scholar] [CrossRef]
- de Lima Alves, L.; dos Santos, R.L.; Bayer, B.L.; Devens, A.L.M.; Cichoski, A.J.; Mendonça, C.R.B. Thermosonication of tangerine juice: Effects on quality characteristics, bioactive compounds, and antioxidant activity. J. Food Process. Preserv. 2020, 44, e14914. [Google Scholar]
- Santhirasegaram, V.; Razali, Z.; Somasundram, C. Effects of thermal treatment and sonication on quality attributes of Chokanan mango (Mangifera indica L.) juice. Ultrason. Sonochem. 2013, 20, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, S.; Abid, M.; Hu, B.; Hashim, M.M.; Lei, S.; Wu, T.; Zeng, X. Exploring the potential of thermosonication in carrot juice processing. J. Food Sci. Technol. 2015, 52, 7002–7013. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Zhang, Z.-H.; Wang, M.-S.; Han, Z.; Jing, H.; Jabbar, S. Thermosonication: A potential technique that influences the quality of grapefruit juice. Int. J. Food Sci. Technol. 2015, 50, 1275–1282. [Google Scholar] [CrossRef]
- Xu, B.; Feng, M.; Chitrakar, B.; Cheng, J.; Wei, B.; Wang, B.; Ma, H. Multi-frequency power thermosonication treatments of clear strawberry juice: Impact on color, bioactive compounds, flavor volatiles, microbial and polyphenol oxidase inactivation. Innov. Food Sci. Emerg. Technol. 2023, 84, 103295. [Google Scholar] [CrossRef]
- Pradhan, D.; Abdullah, S.; Pradhan, R.C. Chironji (Buchanania lanzan) fruit juice extraction using cellulase enzyme: Modelling and optimization of process by artificial neural network and response surface methodology. J. Food Sci. Technol. 2021, 58, 1051–1060. [Google Scholar] [CrossRef] [PubMed]










| Parameter | Raw Juice | Pasteurised Juice |
|---|---|---|
| pH | 3.77 ± 0.00 a | 3.64 ± 0.01 b |
| TSS (°Brix) | 11 ± 0.00 a | 11 ± 0.00 a |
| TA (%) | 3.30 ± 0.01 a | 3.27 ± 0.01 b |
| Cloudiness | 0.796 ± 0.002 a | 0.794 ± 0.003 a |
| Browning index | 0.118 ± 0.001 a | 0.192 ± 0.001 b |
| Ascorbic acid (mg/100 mL) | 61.73 ± 0.02 a | 41.96 ± 1.31 b |
| Antioxidant activity (%) | 49.21 ± 0.04 a | 44.77 ± 0.06 b |
| TPC (mg GAE/mL) | 118.13 ± 0.02 a | 107.18 ± 0.05 b |
| TFC (mg QE/mL) | 59.07 ± 0.01 a | 41.30 ± 0.08 b |
| Total plate count (log cfu/mL) | 2.44 ± 0.03 | ND |
| Yeast and mould count (log cfu/mL) | 2.94 ± 0.04 | ND |
| Parameter | Amplitude (%) | 15 min | 30 min | 45 min | 60 min | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | 30 °C | 40 °C | 50 °C | ||
| pH | 50 | 3.57 ± 0.01 aA | 3.53 ± 0.01 bA | 3.48 ± 0.01 dA | 3.55 ± 0.01 aA | 3.47 ± 0.00 dA | 3.39 ± 0.01 eA | 3.50 ± 0.01 cA | 3.38± 0.00 eA | 3.33 ± 0.01 fgA | 3.47 ± 0.01 dA | 3.35 ± 0.01 fA | 3.32 ± 0.01 gA |
| 75 | 3.55 ± 0.01 aA | 3.48 ± 0.01 bhA | 3.45 ± 0.00 cA | 3.51 ± 0.01 dA | 3.40 ± 0.01 eA | 3.33 ± 0.01 fiA | 3.48 ± 0.01 bA | 3.35 ± 0.00 fA | 3.29 ± 0.00 gA | 3.46 ± 0.01 hcA | 3.33 ± 0.01 iA | 3.30 ± 0.01 gA | |
| 100 | 3.51 ± 0.01aB | 3.43 ± 0.01 bB | 3.39 ± 0.01 cB | 3.47 ± 0.00 dB | 3.38 ± 0.01 cB | 3.27 ± 0.01 eB | 3.44 ± 0.01 bB | 3.31 ± 0.01 fB | 3.22 ± 0.01 gB | 3.40 ± 0.01 cB | 3.27 ± 0.01 eB | 3.21 ± 0.02 gB | |
| TSS (°Brix) | 50 | 10.93 ± 0.06 aA | 11.03 ± 0.06 aA | 11 ± 0.00 aA | 11.03± 0.06 aA | 11 ± 0.10 aA | 11.00 ± 0.10 aA | 10.67 ± 0.58 aA | 11.00± 0.10 aA | 10.93 ± 0.12 aA | 11.13 ± 0.12 aA | 10.70 ± 0.61 aA | 11.03 ± 0.06 aA |
| 75 | 11 ± 0.10 aA | 11.03 ± 0.15 aA | 11.03 ± 0.06 aA | 11.03 ± 0.06 aA | 11 ± 0.00 aA | 11.10 ± 0.10 aA | 11.00 ± 0.10 aA | 11.03 ± 0.06 aA | 11.00 ± 0.00 aA | 10.90 ± 0.10 aA | 11.00 ± 0.00 aA | 11.03 ± 0.06 aA | |
| 100 | 11.03 ± 0.06 aA | 11 ± 0.10 aA | 11.03 ± 0.06 aA | 10.97 ± 0.12 aA | 10.93 ± 0.12 aA | 10.97 ± 0.06 aA | 11.00 ± 0.00 aA | 10.93 ± 0.06 aA | 10.97 ± 0.06 aA | 10.93 ± 0.12 aA | 11.00 ± 0.00 aA | 10.93 ± 0.12 aA | |
| TA (%) | 50 | 3.25 ± 0.01 aA | 3.17 ± 0.01 cA | 3.12 ± 0.01 eA | 3.21 ± 0.01 bA | 3.14 ± 0.02 deA | 3.09 ± 0.01 fA | 3.16 ± 0.01 cdA | 3.12 ± 0.01 eA | 3.03 ± 0.01 gA | 3.15 ± 0.01 cdA | 3.08 ± 0.01 fA | 3.01 ± 0.01 gA |
| 75 | 3.19 ± 0.02 aA | 3.16 ± 0.01 abA | 3.08 ± 0.01 efA | 3.17 ± 0.01 abA | 3.10 ± 0.02 deA | 3.03 ± 0.01 ghA | 3.14 ± 0.01 bcA | 3.09 ± 0.00 eA | 3.02 ± 0.01 ghA | 3.13 ± 0.02 cdA | 3.05 ± 0.01 fgA | 3.01 ± 0.01 hA | |
| 100 | 3.14 ± 0.02 aB | 3.12 ± 0.01 aB | 3.03 ± 0.01 cB | 3.12 ± 0.01 aB | 3.07 ± 0.01 bB | 2.94 ± 0.01eB | 3.08 ± 0.01 bB | 3.00 ± 0.02 dB | 2.90 ± 0.01 fB | 3.05 ± 0.01 bcB | 2.97 ± 0.01 eB | 2.88 ± 0.01 fB | |
| CI | 50 | 0.89 ± 0.00 aA | 1.10 ± 0.01 bA | 1.29 ± 0.00 cA | 1.02 ± 0.00 dA | 1.26 ± 0.02 eA | 1.38 ± 0.00 fA | 1.17 ± 0.00 gA | 1.37 ± 0.00 fA | 1.49 ± 0.00 hA | 1.26 ± 0.00 eA | 1.40 ± 0.00 iA | 1.58 ± 0.01 jA |
| 75 | 1.20 ± 0.00 aB | 1.40 ± 0.01 bB | 1.60 ± 0.00 cB | 1.29 ± 0.00 dB | 1.47 ± 0.00 eB | 1.62 ± 0.00 fB | 1.37 ± 0.00 gB | 1.52 ± 0.00 hB | 1.65 ± 0.00 iB | 1.43 ± 0.00jB | 1.59 ± 0.00 kB | 1.69 ± 0.01 lB | |
| 100 | 1.20 ± 0.00 aB | 1.41 ± 0.01 bB | 1.63 ± 0.01 cB | 1.32 ± 0.00 dB | 1.50 ± 0.00 eB | 1.67 ± 0.00 fB | 1.40 ± 0.00 gB | 1.58 ± 0.00 hB | 1.70 ± 0.00 iB | 1.49 ± 0.00 jB | 1.61 ± 0.00 kB | 1.75 ± 0.01 lB | |
| BI | 50 | 0.20 ± 0.00 aA | 0.18 ± 0.01 aA | 0.20 ± 0.01 bA | 0.18 ± 0.00 caA | 0.19 ± 0.00 dA | 0.22 ± 0.00 eA | 0.19± 0.00 daA | 0.20 ± 0.00 bA | 0.23 ± 0.001 fA | 0.19 ± 0.00 bA | 0.21 ± 0.00 gA | 0.26 ± 0.01 hA |
| 75 | 0.20 ± 0.00 aB | 0.22 ± 0.01 bB | 0.27 ± 0.01 cB | 0.21 ± 0.00 dB | 0.24 ± 0.00 eB | 0.28 ± 0.00 fB | 0.22 ± 0.00 bB | 0.25 ± 0.00 gB | 0.28 ± 0.00 fjB | 0.23 ± 0.00 hB | 0.26 ± 0.00 iB | 0.29 ± 0.01 jB | |
| 100 | 0.21 ± 0.00 aC | 0.24 ± 0.01 bC | 0.28 ± 0.01 cC | 0.23 ± 0.00 dC | 0.26 ± 0.00 eC | 0.29 ± 0.00 fjC | 0.24 ± 0.00 bC | 0.27 ± 0.00 gC | 0.30 ± 0.00 hC | 0.25 ± 0.00 iC | 0.29 ± 0.00 jC | 0.30 ± 0.01 fhC | |
| Treatment | pH | TSS | TA | CI | BI | AA | AOA | TPC | TFC | YM |
|---|---|---|---|---|---|---|---|---|---|---|
| TSLJ-50-30-15 | 3.57 | 10.93 | 3.25 | 0.897 | 0.184 | 58.31 | 50.37 | 125.22 | 64.20 | 2.82 |
| TSLJ-50-30-30 | 3.55 | 11.03 | 3.21 | 1.023 | 0.187 | 59.18 | 51.76 | 130.05 | 71.86 | 2.77 |
| TSLJ-50-30-45 | 3.50 | 10.67 | 3.16 | 1.174 | 0.190 | 59.85 | 54.28 | 136.15 | 80.27 | 2.64 |
| TSLJ-50-30-60 | 3.47 | 11.13 | 3.15 | 1.268 | 0.197 | 60.00 | 57.54 | 144.05 | 76.07 | 2.56 |
| TSLJ-50-40-15 | 3.53 | 11.03 | 3.17 | 1.091 | 0.185 | 60.17 | 53.18 | 134.23 | 79.21 | 2.65 |
| TSLJ-50-40-30 | 3.47 | 11.00 | 3.14 | 1.263 | 0.193 | 63.67 | 57.45 | 146.24 | 84.17 | 2.56 |
| TSLJ-50-40-45 | 3.38 | 11.00 | 3.12 | 1.373 | 0.200 | 69.03 | 60.02 | 151.45 | 90.17 | 2.52 |
| TSLJ-50-40-60 | 3.35 | 10.70 | 3.08 | 1.408 | 0.211 | 66.34 | 63.42 | 158.76 | 98.36 | 2.45 |
| TSLJ-50-50-15 | 3.48 | 11.00 | 3.12 | 1.295 | 0.199 | 64.33 | 61.25 | 156.27 | 94.53 | 2.46 |
| TSLJ-50-50-30 | 3.39 | 11.00 | 3.09 | 1.384 | 0.222 | 61.72 | 64.73 | 162.76 | 107.32 | 2.41 |
| TSLJ-50-50-45 | 3.33 | 10.93 | 3.03 | 1.494 | 0.233 | 62.18 | 67.63 | 169.30 | 116.86 | 2.34 |
| TSLJ-50-50-60 | 3.32 | 11.03 | 3.01 | 1.583 | 0.258 | 60.53 | 68.08 | 171.43 | 119.30 | 2.28 |
| TSLJ-75-30-15 | 3.55 | 11.00 | 3.19 | 1.157 | 0.198 | 59.13 | 53.18 | 147.85 | 77.16 | 2.76 |
| TSLJ-75-30-30 | 3.51 | 11.03 | 3.17 | 1.291 | 0.212 | 59.98 | 55.47 | 153.86 | 85.48 | 2.66 |
| TSLJ-75-30-45 | 3.48 | 11.00 | 3.14 | 1.375 | 0.225 | 60.39 | 58.21 | 162.49 | 93.05 | 2.52 |
| TSLJ-75-30-60 | 3.46 | 10.90 | 3.13 | 1.436 | 0.236 | 61.16 | 60.07 | 168.73 | 88.81 | 2.43 |
| TSLJ-75-40-15 | 3.48 | 11.03 | 3.16 | 1.383 | 0.228 | 61.28 | 56.27 | 160.13 | 94.57 | 2.53 |
| TSLJ-75-40-30 | 3.40 | 11.00 | 3.10 | 1.472 | 0.244 | 64.81 | 61.34 | 166.89 | 103.25 | 2.44 |
| TSLJ-75-40-45 | 3.35 | 11.03 | 3.09 | 1.524 | 0.252 | 70.09 | 64.26 | 173.24 | 111.23 | 2.40 |
| TSLJ-75-40-60 | 3.33 | 11.00 | 3.05 | 1.596 | 0.261 | 66.26 | 67.46 | 179.63 | 117.25 | 2.35 |
| TSLJ-75-50-15 | 3.45 | 11.03 | 3.08 | 1.603 | 0.277 | 63.11 | 66.35 | 175.29 | 110.22 | 2.37 |
| TSLJ-75-50-30 | 3.33 | 11.10 | 3.03 | 1.626 | 0.283 | 62.05 | 71.42 | 179.28 | 114.13 | 2.26 |
| TSLJ-75-50-45 | 3.29 | 11.00 | 3.02 | 1.657 | 0.287 | 60.47 | 71.57 | 182.79 | 121.19 | 2.22 |
| TSLJ-75-50-60 | 3.30 | 11.03 | 3.01 | 1.696 | 0.289 | 58.13 | 71.23 | 180.70 | 124.20 | 2.18 |
| TSLJ-100-30-15 | 3.51 | 11.03 | 3.14 | 1.196 | 0.216 | 60.15 | 57.25 | 163.18 | 87.31 | 2.69 |
| TSLJ-100-30-30 | 3.47 | 10.97 | 3.12 | 1.322 | 0.232 | 61.33 | 60.16 | 169.94 | 93.84 | 2.52 |
| TSLJ-100-30-45 | 3.44 | 11.00 | 3.08 | 1.401 | 0.242 | 61.95 | 63.31 | 174.04 | 111.60 | 2.42 |
| TSLJ-100-30-60 | 3.40 | 10.93 | 3.05 | 1.494 | 0.254 | 62.44 | 67.17 | 178.23 | 97.07 | 2.33 |
| TSLJ-100-40-15 | 3.43 | 11.00 | 3.12 | 1.411 | 0.244 | 63.93 | 62.20 | 172.63 | 113.25 | 2.43 |
| TSLJ-100-40-30 | 3.38 | 10.93 | 3.07 | 1.502 | 0.262 | 66.88 | 68.28 | 183.31 | 119.05 | 2.37 |
| TSLJ-100-40-45 | 3.31 | 10.93 | 3.00 | 1.589 | 0.277 | 71.80 | 69.99 | 185.40 | 127.27 | 2.29 |
| TSLJ-100-40-60 | 3.27 | 11.00 | 2.97 | 1.613 | 0.295 | 69.06 | 74.60 | 187.33 | 125.16 | 2.26 |
| TSLJ-100-50-15 | 3.39 | 11.03 | 3.03 | 1.636 | 0.282 | 62.95 | 69.66 | 182.54 | 122.26 | 2.21 |
| TSLJ-100-50-30 | 3.27 | 10.97 | 2.94 | 1.675 | 0.297 | 61.57 | 73.20 | 185.59 | 124.93 | 2.14 |
| TSLJ-100-50-45 | 3.22 | 10.97 | 2.90 | 1.707 | 0.302 | 59.13 | 72.63 | 184.20 | 123.94 | 2.09 |
| TSLJ-100-50-60 | 3.21 | 10.93 | 2.88 | 1.757 | 0.301 | 57.59 | 71.98 | 184.00 | 125.90 | 2.02 |
| Treatment | pH | TSS | TA | CI | BI | AA | AOA | TPC | TFC | YM |
|---|---|---|---|---|---|---|---|---|---|---|
| TSLJ-50-30-15 | 3.55 | 10.97 | 3.22 | 0.958 | 0.203 | 58.31 | 51.63 | 129.22 | 68.23 | 2.82 |
| TSLJ-50-30-30 | 3.54 | 11.00 | 3.21 | 1.029 | 0.204 | 59.23 | 52.51 | 132.12 | 71.99 | 2.77 |
| TSLJ-50-30-45 | 3.52 | 11.00 | 3.14 | 1.259 | 0.204 | 59.91 | 52.49 | 136.69 | 82.69 | 2.64 |
| TSLJ-50-30-60 | 3.39 | 10.97 | 3.02 | 1.382 | 0.200 | 59.43 | 56.26 | 148.36 | 75.54 | 2.56 |
| TSLJ-50-40-15 | 3.54 | 11.01 | 3.20 | 1.052 | 0.233 | 63.61 | 53.74 | 134.11 | 75.57 | 2.66 |
| TSLJ-50-40-30 | 3.47 | 11.03 | 3.13 | 1.262 | 0.226 | 64.98 | 56.69 | 143.83 | 84.04 | 2.56 |
| TSLJ-50-40-45 | 3.35 | 10.99 | 3.04 | 1.456 | 0.213 | 67.83 | 56.03 | 149.34 | 100.89 | 2.52 |
| TSLJ-50-40-60 | 3.35 | 10.98 | 3.03 | 1.493 | 0.221 | 65.89 | 60.98 | 158.93 | 96.69 | 2.51 |
| TSLJ-50-50-15 | 3.45 | 11.02 | 3.11 | 1.265 | 0.210 | 64.74 | 60.68 | 148.93 | 88.29 | 2.46 |
| TSLJ-50-50-30 | 3.36 | 11.00 | 3.06 | 1.385 | 0.230 | 61.76 | 66.50 | 162.30 | 99.41 | 2.40 |
| TSLJ-50-50-45 | 3.32 | 10.98 | 3.03 | 1.407 | 0.264 | 61.89 | 67.07 | 168.72 | 116.72 | 2.35 |
| TSLJ-50-50-60 | 3.32 | 10.98 | 3.02 | 1.451 | 0.266 | 60.61 | 69.60 | 172.78 | 117.94 | 2.28 |
| TSLJ-75-30-15 | 3.55 | 11.02 | 3.20 | 0.964 | 0.244 | 59.48 | 54.06 | 144.90 | 76.51 | 2.76 |
| TSLJ-75-30-30 | 3.50 | 11.00 | 3.16 | 1.124 | 0.235 | 60.28 | 57.32 | 157.25 | 86.04 | 2.66 |
| TSLJ-75-30-45 | 3.48 | 11.00 | 3.15 | 1.367 | 0.231 | 60.90 | 57.80 | 161.98 | 93.16 | 2.57 |
| TSLJ-75-30-60 | 3.41 | 11.00 | 3.07 | 1.551 | 0.206 | 60.18 | 59.45 | 165.06 | 84.34 | 2.43 |
| TSLJ-75-40-15 | 3.50 | 11.05 | 3.18 | 1.187 | 0.225 | 62.80 | 58.41 | 159.43 | 92.58 | 2.53 |
| TSLJ-75-40-30 | 3.42 | 11.03 | 3.13 | 1.537 | 0.203 | 65.84 | 61.90 | 167.54 | 99.56 | 2.55 |
| TSLJ-75-40-45 | 3.34 | 11.00 | 3.05 | 1.670 | 0.190 | 69.85 | 63.45 | 172.89 | 110.06 | 2.40 |
| TSLJ-75-40-60 | 3.30 | 10.98 | 2.97 | 1.689 | 0.188 | 66.53 | 65.99 | 177.42 | 113.05 | 2.33 |
| TSLJ-75-50-15 | 3.37 | 11.05 | 3.07 | 1.470 | 0.193 | 63.98 | 66.16 | 175.13 | 108.38 | 2.37 |
| TSLJ-75-50-30 | 3.35 | 11.04 | 3.04 | 1.575 | 0.190 | 61.67 | 70.50 | 180.54 | 115.91 | 2.26 |
| TSLJ-75-50-45 | 3.32 | 11.00 | 3.01 | 1.558 | 0.201 | 60.20 | 70.96 | 180.89 | 120.18 | 2.22 |
| TSLJ-75-50-60 | 3.27 | 10.93 | 2.95 | 1.517 | 0.233 | 58.38 | 69.85 | 178.88 | 121.57 | 2.14 |
| TSLJ-100-30-15 | 3.49 | 10.99 | 3.15 | 0.991 | 0.253 | 60.94 | 57.72 | 164.01 | 87.20 | 2.69 |
| TSLJ-100-30-30 | 3.46 | 10.98 | 3.13 | 1.233 | 0.261 | 62.26 | 60.47 | 169.09 | 95.38 | 2.52 |
| TSLJ-100-30-45 | 3.44 | 10.99 | 3.10 | 1.422 | 0.256 | 63.62 | 63.35 | 173.72 | 98.28 | 2.42 |
| TSLJ-100-30-60 | 3.43 | 11.00 | 3.08 | 1.565 | 0.230 | 63.66 | 64.88 | 178.03 | 99.63 | 2.33 |
| TSLJ-100-40-15 | 3.40 | 10.96 | 3.07 | 1.295 | 0.273 | 64.58 | 63.05 | 174.28 | 112.16 | 2.43 |
| TSLJ-100-40-30 | 3.37 | 10.95 | 3.04 | 1.563 | 0.250 | 71.29 | 68.20 | 181.98 | 120.78 | 2.42 |
| TSLJ-100-40-45 | 3.33 | 10.96 | 3.00 | 1.626 | 0.226 | 71.47 | 71.01 | 184.89 | 124.04 | 2.34 |
| TSLJ-100-40-60 | 3.30 | 10.99 | 2.95 | 1.635 | 0.217 | 69.29 | 70.42 | 185.21 | 124.91 | 2.26 |
| TSLJ-100-50-15 | 3.36 | 11.00 | 3.02 | 1.602 | 0.197 | 62.70 | 69.97 | 182.86 | 122.66 | 2.21 |
| TSLJ-100-50-30 | 3.29 | 10.98 | 2.96 | 1.627 | 0.193 | 61.03 | 71.70 | 184.04 | 124.04 | 2.14 |
| TSLJ-100-50-45 | 3.27 | 10.96 | 2.94 | 1.635 | 0.191 | 58.87 | 72.38 | 184.32 | 124.61 | 2.09 |
| TSLJ-100-50-60 | 3.25 | 10.95 | 2.92 | 1.633 | 0.190 | 57.79 | 71.12 | 183.31 | 125.48 | 2.02 |
| R² | 0.978 | 0.912 | 0.996 | 0.999 | 0.996 | 0.999 | 0.977 | 0.999 | 0.990 | 0.998 |
| MSE | 0.426 | 0.173 | 0.775 | 0.478 | 0.825 | 0.101 | 0.214 | 0.253 | 0.961 | 0.136 |
| MAE | 0.006 | −0.013 | 0.012 | 0.000 | 0.020 | −0.412 | 0.352 | 0.394 | 0.998 | −0.007 |
| Treatment | pH | TSS | TA | CI | BI | AA | AO | TPC | TFC | YM |
|---|---|---|---|---|---|---|---|---|---|---|
| RLJ | 3.77 ± 0.00 a | 11 ± 0.00 a | 3.29 ± 0.01 a | 0.79 ± 0.00 a | 0.11 ± 0.00 a | 61.73 ± 0.02 a | 49.21 ± 0.04 a | 118.13 ± 0.02 a | 59.07± 0.01 a | 2.94 ± 0.04 a |
| PSLJ | 3.64 ± 0.01 b | 11 ± 0.00 a | 3.26 ± 0.01 b | 0.79 ± 0.00 a | 0.19 ± 0.00 b | 41.96 ± 1.31 b | 44.77 ± 0.06 b | 107.18 ± 0.05 b | 41.30 ± 0.08 b | ND |
| TSLJ-50-50-60 | 3.32 ± 0.01 c | 11.02 ± 0.06 a | 3.01 ± 0.01 cd | 1.58 ± 0.00 b | 0.25 ± 0.00 c | 60.53 ± 0.21 c | 68.08 ± 0.07 c | 171.43 ± 0.05 c | 119.30 ± 0.03 c | 2.28 ± 0.01 b |
| TSLJ-75-50-45 | 3.29 ± 0.0 d | 11 ± 0.00 a | 3.02 ± 0.01 d | 1.65 ± 0.00 c | 0.28 ± 0.00 d | 60.47 ± 0.03 c | 71.57 ± 0.06 d | 182.79 ± 0.12 d | 121.19 ± 0.06 d | 2.22 ± 0.03 ce |
| TSLJ-75-40-45 | 3.35 ± 0.0 e | 11.03 ± 0.05 a | 3.09 ± 0.00 e | 1.52 ± 0.00 d | 0.25 ± 0.00 e | 70.09 ± 0.02 d | 64.26 ± 0.03 e | 173.24 ± 0.01 e | 111.23 ± 0.04 e | 2.40 ± 0.03 d |
| TSLJ-75-50-60 | 3.29 ± 0.01 d | 11.03 ± 0.05 a | 3.01 ± 0.00 cd | 1.69 ± 0.00 e | 0.28 ± 0.00 f | 58.13 ± 0.03 e | 71.23 ± 0.17 f | 180.70 ± 0.07 f | 124.20 ± 0.03 f | 2.18 ± 0.03 c |
| TSLJ-100-40-45 | 3.31 ± 0.01 c | 10.93 ± 0.05 a | 3.00 ± 0.01 c | 1.58 ± 0.00 f | 0.27 ± 0.00 g | 71.80 ± 0.06 f | 69.99 ± 0.07 g | 185.40 ± 0.03 g | 127.27 ± 0.05 g | 2.29 ± 0.02 b |
| TSLJ-100-40-60 | 3.26 ± 0.01 f | 11.00 ± 0.00 a | 2.96 ± 0.01 f | 1.61 ± 0.00 g | 0.29 ± 0.00 h | 69.06 ± 0.04 g | 74.60 ± 0.28 h | 187.33 ± 0.03 h | 125.16 ± 0.04 h | 2.26 ± 0.03 e |
| TSLJ-100-50-60 | 3.20 ± 0.01 g | 10.93 ± 0.11 a | 2.87 ± 0.01 g | 1.75 ± 0.00 h | 0.30 ± 0.00 i | 57.59 ± 0.10 e | 71.98 ± 0.18 i | 184.00 ± 0.04 i | 125.90 ± 0.07 i | 2.02 ± 0.02 f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, P.; Nayak, P.K.; Stephen Inbaraj, B.; Sharma, M.; Kesavan, R.k.; Sridhar, K. Effect of Thermosonication on the Nutritional Quality of Lapsi (Choerospondias axillaris) Fruit Juice: Application of Advanced Artificial Neural Networks. Foods 2023, 12, 3723. https://doi.org/10.3390/foods12203723
Das P, Nayak PK, Stephen Inbaraj B, Sharma M, Kesavan Rk, Sridhar K. Effect of Thermosonication on the Nutritional Quality of Lapsi (Choerospondias axillaris) Fruit Juice: Application of Advanced Artificial Neural Networks. Foods. 2023; 12(20):3723. https://doi.org/10.3390/foods12203723
Chicago/Turabian StyleDas, Puja, Prakash Kumar Nayak, Baskaran Stephen Inbaraj, Minaxi Sharma, Radha krishnan Kesavan, and Kandi Sridhar. 2023. "Effect of Thermosonication on the Nutritional Quality of Lapsi (Choerospondias axillaris) Fruit Juice: Application of Advanced Artificial Neural Networks" Foods 12, no. 20: 3723. https://doi.org/10.3390/foods12203723
APA StyleDas, P., Nayak, P. K., Stephen Inbaraj, B., Sharma, M., Kesavan, R. k., & Sridhar, K. (2023). Effect of Thermosonication on the Nutritional Quality of Lapsi (Choerospondias axillaris) Fruit Juice: Application of Advanced Artificial Neural Networks. Foods, 12(20), 3723. https://doi.org/10.3390/foods12203723