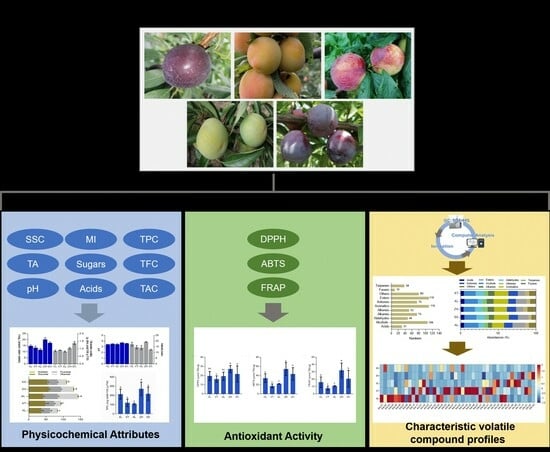
The Physicochemical Attributes, Volatile Compounds, and Antioxidant Activities of Five Plum Cultivars in Sichuan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Materials
2.2. Reagents
2.3. Soluble Solids Content (SSC), Titratable Acidity (TA), pH, and Maturity Index (MI)
2.4. Sugars and Organic Acids
2.4.1. Extraction of Sugars and Organic Acids
2.4.2. HPLC System
2.5. Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Total Anthocyanin Content (TAC)
2.6. Antioxidant Activity
2.7. Volatile Compounds
2.8. Statistical Analysis
3. Results and Discussion
3.1. SSC, TA, pH, and MI of the Five Plum Cultivars
3.2. Individual and Total Sugar Content in Five Plum Cultivars
3.3. Individual and Total Organic Acid Content in the Five Plum Cultivars
3.4. TPC, TFC, and TAC in the Five Plum Cultivars
3.5. DPPH, ABTS, and FRAP in the Five Plum Cultivars
3.6. Volatile Profiles of the Five Plum Cultivars
3.6.1. Analysis of Volatile Metabolites
3.6.2. Identification and Analysis of Differential Metabolites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Feng, C.; Peng, W.; Hao, J.; Wang, J.; Pan, J.; He, Y. Chromosome-level draft genome of a diploid plum (Prunus salicina). GigaScience 2020, 9, giaa130. [Google Scholar] [CrossRef] [PubMed]
- Vlaic, R.; Muresan, V.; Muresan, A.; Muresan, C.; Paucean, A.; Mitre, V.; Chis, S.; Muste, S. The changes of polyphenols, flavonoids, anthocyanins and chlorophyll content in plum peels during growth phases: From fructification to ripening. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 46, 148–155. [Google Scholar] [CrossRef]
- Li, P.; Wu, W.; Chen, F.; Liu, X.; Lin, Y.; Chen, J. Prunus salicina ‘Crown’, a yellow-fruited Chinese plum. Hortscience 2015, 50, 1822–1824. [Google Scholar] [CrossRef]
- Walkowiak-Tomczak, D.; Reguła, J.; Łysiak, G. Physico-chemical properties and antioxidant activity of selected plum cultivars fruit. Acta Sci. Pol. Technol. Aliment. 2008, 7, 15–22. [Google Scholar]
- Sun, P.; Xu, B.; Wang, Y.; Lin, X.; Chen, C.; Zhu, J.; Jia, H.; Wang, X.; Shen, J.; Feng, T. Characterization of volatile constituents and odorous compounds in peach (Prunus persica L.) fruits of different varieties by gas chromatography–ion mobility spectrometry, gas chromatography–mass spectrometry, and relative odor activity value. Front. Nutr. 2022, 9, 965796. [Google Scholar] [CrossRef]
- Liu, Y.; Sang, Y.; Guo, J.; Zhang, W.; Zhang, T.; Wang, H.; Cheng, S.; Chen, G. Analysis of volatility characteristics of five jujube varieties in Xinjiang Province, China, by HS-SPME-GC/MS and E-nose. Food Sci. Nutr. 2021, 9, 6617–6626. [Google Scholar] [CrossRef]
- Pino, J.; Quijano, C. Study of the volatile compounds from plum (Prunus domestica L. cv. Horvin) and estimation of their contribution to the fruit aroma. Food Sci. Technol. 2012, 32, 76–83. [Google Scholar] [CrossRef]
- Lubinska-Szczygeł, M.; Polkowska, A.; Dymerski, T.; Gorinstein, S. Comparison of the physical and sensory properties of hybrid citrus fruit Jaffa® sweetie in relation to the parent fruits. Molecules 2020, 25, 2748. [Google Scholar] [CrossRef]
- Igwe, E.; Charlton, K. A systematic review on the health effects of plums (Prunus domestica and Prunus salicina). Phytother. Res. 2016, 30, 701–731. [Google Scholar] [CrossRef]
- Miedzińska, K.; Członka, S.; Strąkowska, A.; Strzelec, K. Biobased polyurethane composite foams reinforced with plum stones and silanized plum stones. Int. J. Mol. Sci. 2021, 22, 4757. [Google Scholar] [CrossRef]
- Shahidi, S.; Setareye, S.; Mahmoodi, M. Effect of Prunus domestica L. (mirabelle) on learning and memory in mice. Anc. Sci. Life 2013, 32, 139–143. [Google Scholar] [CrossRef]
- Li, Y.; Wu, L.; Weng, M.; Tang, B.; Lai, P.; Chen, J. Effect of different encapsulating agent combinations on physicochemical properties and stability of microcapsules loaded with phenolics of plum (Prunus salicina lindl.). Powder Technol. 2018, 340, 459–464. [Google Scholar]
- Yu, X.; Rizwan, H.; Li, P.; Luo, S.; Sherameti, I.; Wu, W.; Lin, J.; Zheng, S.; Oelmüller, R.; Chen, F. Comparative studies on the physiochemical properties, phenolic compounds and antioxidant activities in 13 Japanese plum cultivars grown in the subtropical region of China. Appl. Ecol. Environ. Res. 2020, 18, 3147–3159. [Google Scholar] [CrossRef]
- Tommonaro, G.; Morelli, C.; Rabuffetti, M.; Nicolaus, B.; Prisco, R.; Iodice, C.; Speranza, G. Determination of flavor-potentiating compounds in different Italian tomato varieties. J. Food Biochem. 2021, 45, e13736. [Google Scholar] [CrossRef]
- Venter, A.; Joubert, E.; de Beer, D. Nutraceutical value of yellow- and red-fleshed South African plums (Prunus salicina Lindl.): Evaluation of total antioxidant capacity and phenolic composition. Molecules 2014, 19, 3084–3109. [Google Scholar] [CrossRef] [PubMed]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N.; et al. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.; Paponov, I. Supplemental light-emitting diode inter-lighting increases tomato fruit growth through enhanced photosynthetic light use efficiency and modulated root activity. Front. Plant Sci. 2019, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Bellachioma, L.; Morresi, C.; Albacete, A.; Martínez-Melgarejo, P.; Ferretti, G.; Giorgini, G.; Galeazzi, R.; Damiani, E.; Bacchetti, T. Insights on the hypoglycemic potential of crocus sativus tepal polyphenols: An in vitro and in silico study. Int. J. Mol. Sci. 2023, 24, 9213. [Google Scholar] [CrossRef]
- Kodagoda, G.; Hong, H.; O’Hare, T.; Sultanbawa, Y.; Topp, B.; Netzel, M. Effect of storage on the nutritional quality of queen garnet plum. Foods 2021, 10, 352. [Google Scholar] [CrossRef]
- Orazem, P.; Stampar, F.; Hudina, M. Fruit quality of redhaven and royal glory peach cultivars on seven different rootstocks. J. Agric. Food Chem. 2011, 59, 9394–9401. [Google Scholar] [CrossRef]
- Wu, H.; Xu, Y.; Wang, H.; Miao, Y.; Li, C.; Zhao, R.; Shi, X.; Wang, B. Physicochemical characteristics, antioxidant activities, and aroma compound analysis of seven peach cultivars (Prunus persica L. Batsch) in Shihezi, Xinjiang. Foods 2022, 11, 2944. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciene, D.; Bobinaite, R.; Viskelis, P.; Bobinas, C.; Petruskevicius, A.; Klavins, L.; Viskelis, J. Geographic variability of biologically active compounds, antioxidant activity and physico-chemical properties in wild bilberries (Vaccinium myrtillus L.). Antioxidants 2022, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, A.; Wu, X.; Zhu, Z.; Yang, Z.; Zhu, Y.; Zha, D. Transcriptome analysis revealed expression of genes related to anthocyanin biosynthesis in eggplant (Solanum melongena L.) under high-temperature stress. BMC Plant Biol. 2019, 19, 387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fang, W.; Zhu, R.; Wang, N.; Yao, Q.; Liu, H.; Wan, J.; Chen, Y.; Wang, Q.; Zhang, D. Comparative study on growth index and nutritional quality of female Chinese mitten crab Eriocheir sinensis selected at different growth periods in rice-crab culture systems. Aquac. Nutr. 2023, 2023, 4805919. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, N.; Pashazadeh, H.; Zannou, O.; Koca, I. Phytochemical content, and antioxidant activity, and volatile compounds associated with the aromatic property, of the vinegar produced from rosehip fruit (Rosa canina L.). LWT-Food Sci. Technol. 2021, 154, 112716. [Google Scholar] [CrossRef]
- Abidi, W.; Jiménez, S.; Moreno, M.; Gogorcena, Y. Evaluation of antioxidant compounds and total sugar content in a nectarine [Prunus persica (L.) Batsch] progeny. Int. J. Mol. Sci. 2011, 12, 6919–6935. [Google Scholar] [CrossRef] [PubMed]
- Petruccelli, R.; Bonetti, A.; Ciaccheri, L.; Ieri, F.; Ganino, T.; Faraloni, C. Evaluation of the fruit quality and phytochemical compounds in peach and nectarine cultivars. Plants 2023, 12, 1618. [Google Scholar] [CrossRef]
- Sifat, S.; Trisha, A.; Huda, N.; Zzaman, W.; Julmohammad, N. Response surface approach to optimize the conditions of foam mat drying of plum in relation to the physical-chemical and antioxidant properties of plum powder. Int. J. Food Sci. 2021, 2021, 3681807. [Google Scholar] [CrossRef]
- Drogoudi, P.; Pantelidis, G. Phenotypic variation and peel contribution to fruit antioxidant contents in European and Japanese plums. Plants 2022, 11, 1338. [Google Scholar] [CrossRef]
- Crisosto, C.; Crisosto, G.; Echeverria, G.; Puy, J. Segregation of plum and pluot cultivars according to their organoleptic characteristics. Postharvest Biol. Technol. 2007, 44, 271–276. [Google Scholar] [CrossRef]
- Win, N.; Yoo, J.; Kwon, S.; Watkins, C.; Kang, I. Characterization of fruit quality attributes and cell wall metabolism in 1-methylcyclopropene (1-MCP)-treated ‘Summer King’ and ‘Green Ball’ apples during cold storage. Front. Plant Sci. 2019, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
- Gorzelany, J.; Basara, O.; Kapusta, I.; Paweł, K.; Belcar, J. Evaluation of the chemical composition of selected varieties of L. caerulea var. kamtschatica and L. caerulea var. emphyllocalyx. Molecules 2023, 28, 2525. [Google Scholar] [PubMed]
- Zacarías-García, J.; Pérez-Través, L.; Gil, J.; Rodrigo, M.; Zacarías, L. Bioactive compounds, nutritional quality and antioxidant capacity of the red-fleshed kirkwood navel and ruby valencia oranges. Antioxidants 2022, 11, 905. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Fang, Z.; Zhou, D.; Pan, S.; Ye, X. Changes in secondary metabolites, organic acids and soluble sugars during the development of plum fruit cv. ‘Furongli’ (Prunus salicina Lindl). J. Sci. Food Agric. 2019, 99, 1010–1019. [Google Scholar] [CrossRef]
- García-Mariño, N.; De, l.; Matilla, A. Organic acids and soluble sugars in edible and nonedible parts of damson plum (Prunus domestica L. subsp. insititia cv. Syriaca) fruits during development and ripening. Food Sci. Technol. Int. 2008, 14, 187–193. [Google Scholar]
- Li, C.; Xu, Y.; Wu, H.; Zhao, R.; Wang, X.; Wang, F.; Fu, Q.; Tang, T.; Shi, X.; Wang, B. Flavor characterization of native Xinjiang flat peaches based on constructing aroma fingerprinting and stoichiometry analysis. Foods 2023, 12, 2554. [Google Scholar] [CrossRef]
- Shan, N.; Gan, Z.; Nie, J.; Liu, H.; Wang, Z.; Sui, X. Comprehensive characterization of fruit volatiles and nutritional quality of three Cucumber (Cucumis sativus L.) genotypes from different geographic groups after bagging treatment. Foods 2020, 9, 294. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red fruits composition and their health benefits—A review. Foods 2022, 11, 644. [Google Scholar] [CrossRef]
- Kim, D.; Chun, O.; Kim, Y.; Moon, H.; Lee, C. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.; Suleria, H. LC-ESI-QTOF/MS characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Maietti, A.; Tedeschi, P.; Stagno, C.; Bordiga, M.; Travaglia, F.; Locatelli, M.; Arlorio, M.; Brandolini, V. Analytical traceability of melon (Cucumis melo var reticulatus): Proximate composition, bioactive compounds, and antioxidant capacity in relation to cultivar, plant physiology state, and seasonal variability. J. Food Sci. 2012, 77, C646–C652. [Google Scholar] [CrossRef] [PubMed]
- Phommalath, S.; Teraishi, M.; Yoshikawa, T.; Saito, H.; Tsukiyama, T.; Nakazaki, T.; Tanisaka, T.; Okumoto, Y. Wide genetic variation in phenolic compound content of seed coats among black soybean cultivars. Breed. Sci. 2014, 64, 409–415. [Google Scholar] [CrossRef]
- Gu, C.; Liao, L.; Zhou, H.; Wang, L.; Deng, X.; Han, Y. Constitutive activation of an anthocyanin regulatory gene PcMYB10.6 is related to red coloration in purple-foliage plum. PLoS ONE 2015, 10, e0135159. [Google Scholar] [CrossRef]
- Liao, L.; Li, Y.; Lan, X.; Yang, Y.; Wei, W.; Ai, J.; Feng, X.; Chen, H.; Tang, Y.; Xi, L.; et al. Integrative analysis of fruit quality and anthocyanin accumulation of plum cv. ‘Cuihongli’ (Prunus salicina Lindl.) and its bud mutation. Plants 2023, 12, 1357. [Google Scholar] [CrossRef] [PubMed]
- Nour, V. Effect of sour cherry or plum juice marinades on quality characteristics and oxidative stability of pork loin. Foods 2022, 11, 1088. [Google Scholar] [CrossRef]
- Hameed, A.; Liu, Z.; Wu, H.; Zhong, B.; Ciborowski, M.; Suleria, H. A comparative and comprehensive characterization of polyphenols of selected fruits from the Rosaceae family. Metabolites 2022, 12, 271. [Google Scholar] [CrossRef]
- Goldner, K.; Michaelis, S.; Stammler, J.; Neumüller, M.; Treutter, D. Different phenotypes of plum cultivars due to specific phenolic profiles. Acta. Hortic. 2019, 2019, 137–144. [Google Scholar] [CrossRef]
- Tian, X.; Schaich, K. Effects of molecular structure on kinetics and dynamics of the trolox equivalent antioxidant capacity assay with ABTS+•. J. Agric. Food Chem. 2013, 61, 5511–5519. [Google Scholar] [CrossRef]
- Ma, Y.; Meng, A.; Liu, P.; Chen, Y.; Yuan, A.; Dai, Y.; Ye, K.; Yang, Y.; Wang, Y.; Li, Z. Reflux extraction optimization and antioxidant activity of phenolic compounds from Pleioblastus amarus (Keng) shell. Molecules 2022, 27, 362. [Google Scholar] [CrossRef]
- Lim, V.; Gorji, S.; Daygon, V.; Fitzgerald, M. Untargeted and targeted metabolomic profiling of Australian indigenous fruits. Metabolites 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, F.; Moreno-Rojas, J.; Arroyo, F.; Daza, A.; Ruiz-Moreno, M. Effect of management (organic vs conventional) on volatile profiles of six plum cultivars (Prunus salicina Lindl.). A chemometric approach for varietal classification and determination of potential markers. Food Chem. 2016, 199, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Song, Y.; Lu, A.; Qin, L.; Tan, D.; Zhang, Q.; He, Y.; Lu, Y. Metabolomics-based analysis of the effects of different cultivation strategies on metabolites of Dendrobium officinale Kimura et Migo. Metabolites 2023, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Joulain, D.; Casazza, A.; Laurent, R.; Portier, D.; Guillamon, N.; Pandya, R.; Le, M.; Viljoen, A. Volatile flavor constituents of fruits from Southern Africa: Mobola plum (Parinari curatellifolia). J. Agric. Food Chem. 2004, 52, 2322–2325. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Lopez, R. The actual and potential aroma of winemaking grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef]
- Chai, Q.; Wu, B.; Liu, W.; Wang, L.; Yang, C.; Wang, Y.; Fang, J.; Liu, Y.; Li, S. Volatiles of plums evaluated by HS-SPME with GC–MS at the germplasm level. Food Chem. 2012, 130, 432–440. [Google Scholar] [CrossRef]
- Ismail, H.; Brown, G.; Tucknott, O.; Holloway, P.; Williams, A. Nonanal in epicuticular wax of golden egg plums (Prunus domestica). Phytochemistry 1977, 16, 769–770. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Li, B.; Liao, M.; Liu, J.; Zhou, Y.; Liang, Y.; Yuan, H.; Li, K.; Li, H. The Physicochemical Attributes, Volatile Compounds, and Antioxidant Activities of Five Plum Cultivars in Sichuan. Foods 2023, 12, 3801. https://doi.org/10.3390/foods12203801
Lin Z, Li B, Liao M, Liu J, Zhou Y, Liang Y, Yuan H, Li K, Li H. The Physicochemical Attributes, Volatile Compounds, and Antioxidant Activities of Five Plum Cultivars in Sichuan. Foods. 2023; 12(20):3801. https://doi.org/10.3390/foods12203801
Chicago/Turabian StyleLin, Zixi, Binbin Li, Maowen Liao, Jia Liu, Yan Zhou, Yumei Liang, Huaiyu Yuan, Ke Li, and Huajia Li. 2023. "The Physicochemical Attributes, Volatile Compounds, and Antioxidant Activities of Five Plum Cultivars in Sichuan" Foods 12, no. 20: 3801. https://doi.org/10.3390/foods12203801
APA StyleLin, Z., Li, B., Liao, M., Liu, J., Zhou, Y., Liang, Y., Yuan, H., Li, K., & Li, H. (2023). The Physicochemical Attributes, Volatile Compounds, and Antioxidant Activities of Five Plum Cultivars in Sichuan. Foods, 12(20), 3801. https://doi.org/10.3390/foods12203801