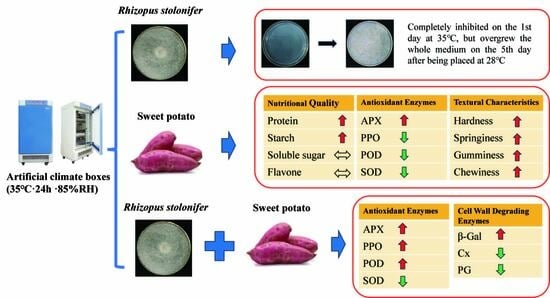
Effect of Heat Treatment on the Quality and Soft Rot Resistance of Sweet Potato during Long-Term Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials and Treatments
2.2. Sample Preparation
2.3. Antifungal Activity of Heat Treatment on R. stolonifer In Vitro
- (1)
- Control A (CK): PDA plates were incubated at 28 °C for 5 days.
- (2)
- Treatment B: PDA plates were incubated at 35 °C for 1 day, followed by incubation at 28 °C for 4 more days.
- (3)
- Treatment C: PDA plates were first incubated at 28 °C for 1 day, then at 35 °C for 1 day, and finally at 28 °C for 3 more days. The colony diameter was measured and averaged after 1, 2, 3, 4 and 5 days by considering two perpendicular diameters for each plate, thus assessing the inhibitory effect of each treatment on mycelium growth.
2.4. Resistance to Soft Rot of Sweet Potato
2.5. Nutritional Quality Indexes
2.5.1. Flavone Determination
2.5.2. Starch and Protein Determination
2.5.3. Soluble Sugar Determination
2.6. Sweet Potato Tuberous Root Texture
2.7. Antioxidant Enzyme Activity
2.8. Cell Wall Degrading Enzyme Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effects of Heat Treatment on the Growth of R. stolonifer in PDA
3.2. Effects of Heat Treatment on Quality Characteristics of Sweet Potato
3.2.1. Nutritional Quality
3.2.2. Antioxidant Enzyme Activity
3.2.3. DPPH Radical Scavenging Rate
3.2.4. Textural Characteristics
3.3. Soft Rot Resistance of Swee Potato and Physiological Changes after Heat Treatment
3.3.1. Sweet Potato Soft Rot Resistance
3.3.2. Antioxidant Enzyme Activity
3.3.3. Cell Wall Degrading Enzyme Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Scruggs, A.C.; Quesada-Ocampo, L.M. Cultural, chemical, and alternative control strategies for rhizopus soft rot of sweet potato. Plant Dis. 2016, 100, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, B.A.; Holmes, G.J. Evaluation of alternative decay control products for control of postharvest rhizopus soft rot of sweet potatoes. Plant Health Prog. 2009, 10, 26. [Google Scholar] [CrossRef]
- Xing, K.; Li, T.J.; Liu, Y.F.; Zhang, J.; Zhang, Y.; Shen, X.Q.; Li, X.Y.; Miao, X.M.; Feng, Z.Z.; Peng, X.; et al. Antifungal and eliciting properties of chitosan against Ceratocystis fimbriata in sweet potato. Food Chem. 2018, 268, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef] [PubMed]
- Abegunde, O.K.; Mu, T.H.; Chen, J.W.; Deng, F.M. Physicochemical characterization of sweet potato starches popularly used in Chinese starch industry. Food Hydrocoll. 2013, 33, 169–177. [Google Scholar] [CrossRef]
- Donado-Pestana, C.M.; Salgado, J.M.; Rios, A.D.O.; Santos, P.R.D.; Jablonski, A. Stability of Carotenoids, Total Phenolics and In Vitro Antioxidant Capacity in the Thermal Processing of Orange-Fleshed Sweet Potato (Ipomoea batatas Lam.) Cultivars Grown in Brazil. Plant Foods Hum. Nutr. 2012, 67, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Karaoglan, M.; Erden, F.; Tetik, N.; Topuz, A.; Ozdemir, F. Effects of Baking and Boiling on the Nutritional and Antioxidant Properties of Sweet Potato [Ipomoea batatas (L.) Lam.] Cultivars. Plant Foods Hum. Nutr. 2011, 66, 341–347. [Google Scholar] [CrossRef]
- Zhao, L.; Ye, X.; Dong, W.; Shi, J.; Luo, L.; Lu, G. Changes of nutritional quality and starch properties of different types of sweet potato roots during storage. Acta Agric. Zhejiangensis 2021, 33, 2224–2233. [Google Scholar]
- Ojong, P.B.; Njiti, V.; Guo, Z.; Gao, M.; Besong, S.; Barnes, S.L. Variation of Flavonoid Content Among Sweet potato Accessions. J. Am. Soc. Hortic. Sci. J. Amer. Soc. Hort. Sci. 2008, 133, 819–824. [Google Scholar] [CrossRef]
- Wang, A.; Li, R.; Ren, L.; Gao, X.; Zhang, Y.; Ma, Z.; Ma, D.; Luo, Y. A comparative metabolomics study of flavonoids in sweet potato with different flesh colors (Ipomoea batatas (L.) Lam). Food Chem. 2018, 260, 124–134. [Google Scholar] [CrossRef]
- Kusajima, M.; Fujita, M.; Nishiuchi, T.; Nakashita, H.; Asami, T. Induction of tocopherol biosynthesis through heat shock treatment in Arabidopsis. Biosci. Biotechnol. Biochem. 2021, 85, 502–509. [Google Scholar] [CrossRef]
- Fallik, E.; Grinberg, S.; Alkalai, S.; Lurie, S. The effectiveness of postharvest hot water dipping on the control of grey and black moulds in sweet red pepper (Capsicum annuum). Plant Pathol. 1996, 45, 644–649. [Google Scholar] [CrossRef]
- Inkha, S.; Boonyakiat, D. Induction of resistance to penicillium digitatum in tangerine fruit cv. sai num phung flavedo by hot water treatment. Songklanakarin J. Sci. Technol. 2010, 32, 445–451. [Google Scholar]
- Kusajima, M.; Kwon, S.; Nakajima, M.; Sato, T.; Yamakawa, T.; Akutsu, K.; Nakashita, H. Induction of systemic acquired resistance by heat shock treatment in arabidopsis. Biosci. Biotechnol. Biochem. 2012, 76, 2301–2306. [Google Scholar] [CrossRef]
- Lurie, S. Postharvest heat treatments. Postharvest Biol. Technol. 1998, 14, 257–269. [Google Scholar] [CrossRef]
- Schirra, M.; D’Hallewin, G.; Ben-Yehoshua, S.; Fallik, E. Host–pathogen interactions modulated by heat treatment. Postharvest Biol. Technol. 2000, 21, 71–85. [Google Scholar] [CrossRef]
- D’Aquino, S.; Suming, D.; Deng, Z.; Gentile, A.; Angioni, A.; De Pau, L.; Palma, A. A sequential treatment with sodium hypochlorite and a reduced dose of imazalil heated at 50 °C effectively control decay of individually film-wrapped lemons stored at 20 °C. Postharvest Biol. Technol. 2017, 124, 75–84. [Google Scholar] [CrossRef]
- Sivakumar, D.; Fallik, E. Influence of heat treatments on quality retention of fresh and fresh-cut produce. Food Rev. Int. 2013, 29, 294–320. [Google Scholar] [CrossRef]
- Martínez, G.A.; Civello, P.M. Effect of heat treatments on gene expression and enzyme activities associated to cell wall degradation in strawberry fruit. Postharvest Biol. Technol. 2008, 49, 38–45. [Google Scholar] [CrossRef]
- Saltveit, M.E. Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol. Technol. 2000, 21, 61–69. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Ben-Yehoshua, S.; Mitcham, E.J.; Mcdonald, R.E.; Lurie, S. Postharvest heat treatments: Introduction and workshop summary. Postharvest Biol. Technol. 2000, 21, 1–6. [Google Scholar] [CrossRef]
- Giri, S.K.; Singh, R.; Tripathi, M.K.; More, S.N. Post-harvest heat treatment of bananas—Effect on shelf life and quality. J. Food Saf. Food Qual. Arch. Leb. 2016, 67, 132–138. [Google Scholar]
- Spadoni, A.; Guidarelli, M.; Sanzani, S.M.; Ippolito, A.; Mari, M. Influence of hot water treatment on brown rot of peach and rapid fruit response to heat stress. Postharvest Biol. Technol. 2014, 94, 66–73. [Google Scholar] [CrossRef]
- Wang, K.; Shao, X.F.; Gong, Y.F.; Xu, F.; Wang, H.F. Effects of postharvest hot air treatment on gene expression associated with ascorbic acid metabolism in peach fruit. Plant Mol. Biol. Rep. 2014, 32, 881–887. [Google Scholar] [CrossRef]
- Zhou, H.J.; Ye, Z.W.; Su, M.S.; Du, J.H.; Li, X.W. Effect of heat treatment on protein content and the quality of ‘Hujingmilu’ peach Prunus persica (L.) batsch. HortScience 2015, 50, 1531–1536. [Google Scholar] [CrossRef]
- Kang, H.M.; Park, K.-W.; Stop, K.I. Effects of postharvest heat treatment on alleviation chilling injury and improvement storability of oriental melon. J. Bio Environ. Control 2005, 14, 137–143. [Google Scholar]
- Widiastuti, A.; Yoshino, M.; Hasegawa, M.; Nitta, Y.; Sato, T. Heat shock-induced resistance increases chitinase-1 gene expression and stimulates salicylic acid production in melon (Cucumis melo L.). Physiol. Mol. Plant Pathol. 2013, 82, 51–55. [Google Scholar] [CrossRef]
- Li, L.; Li, X.H.; Wang, A.L.; Jiang, Y.Q.; Ban, Z.J. Effect of heat treatment on physiochemical, colour, antioxidant and microstructural characteristics of apples during storage. Int. J. Food Sci. Technol. 2013, 48, 727–734. [Google Scholar] [CrossRef]
- Rodriguez-Arzuaga, M.; Rios, G.; Piagentini, A.M. Mild heat treatments before minimal processing reduce browning susceptibility and increase total phenolic content of low-chill apple cultivars. J. Food Process. Preserv. 2019, 43, e14209. [Google Scholar] [CrossRef]
- Yi, M.J.; Kong, J.; Yu, Z.F. Effect of heat treatment on the quality and energy metabolism in “Golden Delicious” apple fruit. J. Food Biochem. 2021, 45, e13759. [Google Scholar] [CrossRef]
- Luo, K.; Oh, D.H. Inactivation kinetics of Listeria monocytogenes and Salmonella enterica serovar Typhimurium on fresh-cut bell pepper treated with slightly acidic electrolyzed water combined with ultrasound and mild heat. Food Microbiol. 2016, 53, 165–171. [Google Scholar] [CrossRef]
- Luengwilai, K.; Saltveit, M.; Beckles, D.M. Metabolite content of harvested Micro-Tom tomato (Solanum lycopersicum L.) fruit is altered by chilling and protective heat-shock treatments as shown by GC-MS metabolic profiling. Postharvest Biol. Technol. 2012, 63, 116–122. [Google Scholar] [CrossRef]
- Polenta, G.; Budde, C.; Sivakumar, D.; Nanni, M.; Guidi, S. Evaluation of biochemical and quality attributes to monitor the application of heat and cold treatments in tomato fruit (Lycopersicon EsculentumMill.). J. Food Qual. 2015, 38, 153–163. [Google Scholar] [CrossRef]
- Wang, L.B.; Baldwin, E.A.; Zhao, W.; Plotto, A.; Sun, X.X.; Wang, Z.; Brecht, J.K.; Bai, J.H.; Yu, Z.F. Suppression of volatile production in tomato fruit exposed to chilling temperature and alleviation of chilling injury by a pre-chilling heat treatment. Lwt Food Sci. Technol. 2015, 62, 115–121. [Google Scholar] [CrossRef]
- Su, H.N.; Chen, G.; Yang, L.M.; Zhang, Y.Y.; Wang, Y.; Fang, Z.Y.; Lv, H.H. Proteomic variations after short-term heat shock treatment reveal differentially expressed proteins involved in early microspore embryogenesis in cabbage (Brassica oleracea). Peerj 2020, 8, e8897. [Google Scholar] [CrossRef]
- Yarra, R.; Xue, Y.B. Ectopic expression of nucleolar DEAD-Box RNA helicase OsTOGR1 confers improved heat stress tolerance in transgenic Chinese cabbage. Plant Cell Rep. 2020, 39, 1803–1814. [Google Scholar] [CrossRef]
- Gou, M.; Wu, H.; Saleh, A.S.M.; Jing, L.Z.; Liu, Y.; Zhao, K.; Su, C.Y.; Zhang, B.; Jiang, H.; Li, W.H. Effects of repeated and continuous dry heat treatments on properties of sweet potato starch. Int. J. Biol. Macromol. 2019, 129, 869–877. [Google Scholar] [CrossRef]
- Musilova, J.; Lidikova, J.; Vollmannova, A.; Frankova, H.; Urminska, D.; Bojnanska, T.; Toth, T. Influence of heat treatments on the content of bioactive substances and antioxidant properties of sweet potato (Ipomoea batatas L.) tubers. J. Food Qual. 2020, 2020, 8856206. [Google Scholar] [CrossRef]
- Morgado, C.M.A.; Sallanon, H.; Mattiuz, B.H.; Nilprapruck, P.; Charles, F. Heat treatment and active packaging to improve the storage of fresh-cut melons (Cucumis melo L.). Fruits 2016, 71, 9–15. [Google Scholar] [CrossRef]
- Rodoni, L.M.; Hasperue, J.H.; Ortiz, C.M.; Lemoine, M.L.; Concellon, A.; Vicente, A.R. Combined use of mild heat treatment and refrigeration to extend the postharvest life of organic pepper sticks, as affected by fruit maturity stage. Postharvest Biol. Technol. 2016, 117, 168–176. [Google Scholar] [CrossRef]
- Kim, M.Y.; Lee, B.W.; Lee, H.U.; Lee, Y.Y.; Kim, M.H.; Lee, J.Y.; Lee, B.K.; Woo, K.S.; Kim, H.J. Phenolic compounds and antioxidant activity in sweet potato after heat treatment. J. Sci. Food Agric. 2019, 99, 6833–6840. [Google Scholar] [CrossRef]
- Arrebola, E.; Sivakumar, D.; Bacigalupo, R.; Korsten, L. Combined application of antagonist bacillus amyloliquefaciens and essential oils for the control of peach postharvest diseases. Crop Prot. 2010, 29, 369–377. [Google Scholar] [CrossRef]
- Yang, D.J.; Zhen, X.U.; Zhao, Y.Q.; Zhang, C.L.; Sun, H.J.; Xie, Y.P. The study of identification method of sweet potato soft rot resistance and the evaluation of their disease resistance. Acta Agric. Boreali Sin. 2014, 29, 54–56. [Google Scholar]
- Mohammed, E.A.; Abdalla, I.G.; Alfawaz, M.A.; Mohammed, M.A.; Al Maiman, S.A.; Osman, M.A.; Yagoub, A.A.; Hassan, A.B. Effects of extraction solvents on the total phenolic content, total flavonoid content, and antioxidant activity in the aerial part of root vegetables. Agriculture 2022, 12, 1820. [Google Scholar] [CrossRef]
- Takac, V.; Toth, V.; Rakszegi, M.; Miko, P.; Mikic, S.; Mirosavljevic, M. The influence of farming systems, genotype and their interaction on bioactive compound, protein and starch content of bread and spelt wheat. Foods 2022, 11, 4028. [Google Scholar] [CrossRef]
- DONG, S.; Li, H.; Li, D.; ZHANG, S. Comparative analysis of two assay methods for protein content in different wheat cultivars. J. Henan Inst. Sci. Technol. Nat. Sci. Ed. 2020, 48, 1–5+9. [Google Scholar]
- Aung, L.H.; Fouse, D.C.; Brandl, D.G.; Harris, C.M. Effects of imbibition and modified atmospheres on the soluble sugar content of supersweet sweet corn embryo and endosperm. J. Hortic. Sci. 1993, 68, 37–43. [Google Scholar] [CrossRef]
- Aiessandrini, L.; Balestra, F.; Romani, S.; Rocculi, P.; Rosa, M.D. Physicochemical and sensory properties of fresh potato-based pasta (Gnocchi). J. Food Sci. 2010, 75, S542–S547. [Google Scholar]
- Matvieieva, N.; Shakhovsky, A.; Tashyreva, H.; Ratushnyak, Y.; Duplij, V.; Bohdanovych, T.; Kuchuk, M. Study of superoxide dismutase activity in long-term cultivated artemisia and althaea “hairy” roots. Curr. Microbiol. 2021, 79, 14. [Google Scholar] [CrossRef]
- Apiamu, A.; Asagba, S.O. Zinc-cadmium interactions instigated antagonistic alterations in lipid peroxidation, ascorbate peroxidase activity and chlorophyll synthesis in Phaseolus vulgaris leaves. Sci. Afr. 2021, 11, e00688. [Google Scholar] [CrossRef]
- Yang, J.W.; Park, S.U.; Lee, H.U.; Nam, K.J.; Lee, K.L.; Lee, J.J.; Kim, J.H.; Kwak, S.S.; Kim, H.S.; Kim, Y.H. Differential Responses of Antioxidant Enzymes and Lignin Metabolism in Susceptible and Resistant Sweet potato Cultivars during Root-Knot Nematode Infection. Antioxidants 2023, 12, 1164. [Google Scholar] [CrossRef]
- Torres, A.; Aguilar-Osorio, G.; Camacho, M.; Basurto, F.; Navarro-Ocana, A. Characterization of polyphenol oxidase from purple sweet potato (Ipomoea batatas L. Lam) and its affinity towards acylated anthocyanins and caffeoylquinic acid derivatives. Food Chem. 2021, 356, 129709. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Cai, Y.L.; Li, X. Free radical scavenging activity and reaction conditions optimization of Maillard reaction products derived from L-lysine-D-arabinose. J. Light Ind. 2017, 32, 1–7. [Google Scholar]
- Zdunek, A.; Kozioł, A.; Pieczywek, P.M.; Cybulska, J. Evaluation of the nanostructure of pectin, hemicellulose and cellulose in the cell walls of pears of different texture and firmness. Food Bioprocess. Technol. 2014, 7, 3525–3535. [Google Scholar] [CrossRef]
- González-Andrada, J.I.; Romero, C.; Morales, F.J.; Jiménez-Pérez, S. An improved method for determination of the activity of β-galactosidase in yoghurt by high-performance liquid chromatography. Chromatographia 1996, 43, 85–88. [Google Scholar] [CrossRef]
- Ng, T.K.; Zeikus, J.G. A continuous spectrophotometric assay for the determination of cellulase solubilizing activity. Anal. Biochem. 1980, 103, 42–50. [Google Scholar] [CrossRef]
- Song, S.Z.; Fang, Z.Y.; Tian, Y.P. Protective effect of total flavone of puerarin against H2O2-induced oxidative damage of cultured PC12 cells. Chin. J. Clin. Rehabil. 2006, 10, 171–173. [Google Scholar]
- Lyu, X.L. Effects of Postharvest Heat Shock Treatment on Callus Formation and Storage Quality of Sweet Potato. Master’s Thesis, Sichuan Agricultural University, Ya’an, China, 2020. [Google Scholar]
- Zhang, S.Z.; Pu, H.M.; Zhang, J.; Yang, W.L.; HU, X.; Ma, F.F.; Yang, F. Effects of culture conditions and drying methods on the quality of tartary buckwheat sprouts. J. Food Saf. Qual. 2020, 11, 2109–2115. [Google Scholar]
- Weng, J.; Hong, Y. The roles of plant heat shock transcription factors in abiotic stress. Mol. Plant Breed. 2006, 1, 88–94. [Google Scholar]
- Pan, Y.F.; Chen, L.; Chen, X.T.; Jia, X.Y.; Zhang, J.M.; Ban, Z.J.; Li, X.H. Postharvest intermittent heat treatment alleviates chilling injury in cold-stored sweet potato roots through the antioxidant metabolism regulation. J. Food Process. Preserv. 2019, 43, e14274. [Google Scholar] [CrossRef]
- Meng, X.L. Photoprotection Mediated by Mitochondrial Alternative Oxidase Pathway in Cucumber Leaves under Different Light Intensities and Different Temperatures. Master’s Thesis, Shandong Agricultural University, Tai’an City, China, 2013. [Google Scholar]
- Yang, G.L.; Fan, Y.Y.; Deng, X.Z. Effects of heat shock treatment on total phenol content and PAL. PPO. POD ativies in Diospyros vaki explants. Non Wood For. Res. 2015, 33, 115–118. [Google Scholar]
- Wang, Q.G.; Liu, J.; Fan, M.N.; Mu, W.L. Effects of heat shock treatment on the storage of ‘Kyoho’ grape. Food Ferment. Ind. 2013, 39, 219–223. [Google Scholar]
- Yao, J.Y.; Ji, R.; Liu, J.K.; He, L. Study on the effects of preservative combined heat shock on storage of ‘Jimei’ cherry. J. Tarim Univ. 2022, 34, 49–58. [Google Scholar]
- Sato, A.; Truong, V.D.; Johanningsmeier, S.D.; Reynolds, R.; Pecota, K.V.; Yencho, G.C. Chemical constituents of sweet potato genotypes in relation to textural characteristics of processed french fries. J. Food Sci. 2018, 83, 60–73. [Google Scholar] [CrossRef]
- Wang, H.D.; Wang, H.Q.; Zheng, C.; Wang, J.; Zheng, Y.H. Induction of disease resistances in loquat fruit by postharvest hot ain treatment. Food Sci. 2014, 35, 227–231. [Google Scholar]
- Huang, T.H.; Li, Y.C.; Luo, J.; Wang, J.; Cai, Z.P.; Shen, Y.G.; Li, Y.X.; Zhang, W.; Chen, J.; Zhu, L. Hydrogen sulfide enhances resistance to Penicillium italicum by activating phenylpropanoid metabolism in postharvest navel orange fruit. Postharvest Biol. Technol. 2023, 198, 112259. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, C.; Lin, C.Y.; Li, Q. Editorial: Rising stars in plant metabolism and chemodiversity 2022—Phenylpropanoid metabolism and regulation. Front Plant Sci. 2023, 14, 1159100. [Google Scholar] [CrossRef]
- Song, L.; Liu, X. Research progress of cellulase. Asian Agric. Res. 2019, 10, 5. [Google Scholar]
- Sezgintürk, M.K.; DincKaya, E. β-Galactosidase monitoring by a biosensor based on Clark electrode: Its optimization, characterization and application. Biosens. Bioelectron. 2008, 23, 1799–1804. [Google Scholar] [CrossRef]
- Dopico, B.; Nicolos, G.; Labrador, E. Cell wall localization of the natural substrate of a β-alactosidase, the main enzyme responsible for the autolytic process of Cicer arietinum epicotyl cell walls. Physiol. Plant 1990, 80, 636–641. [Google Scholar] [CrossRef]
- Chopsri, A.; Sekozawa, Y.; Sugaya, S. Effects of hot air treatment on cell wall-degrading enzymes, pulp softening and ripening in bananas. Int. Food Res. J. 2018, 25, 2195–2203. [Google Scholar]
- Wei, Y.Y.; Zhou, D.D.; Wang, Z.J.; Tu, S.C.; Shao, X.F.; Peng, J.; Pan, L.Q.; Tu, K. Hot air treatment reduces postharvest decay and delays softening of cherry tomato by regulating gene expression and activities of cell wall-degrading enzymes. J. Sci. Food Agric. 2018, 98, 2105–2112. [Google Scholar] [CrossRef]
- Falagan, N.; Artes, F.; Aguayo, E. Heat treatment as postharvest tool for improving quality in extra-early nectarines. J. Sci. Food Agric. 2018, 98, 1469–1475. [Google Scholar] [CrossRef]







| Texture | Treatment | Storage Days | |||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 15 | 30 | 45 | 60 | ||
| Hardness | P32 CK0 | 97.59 ± 0.86 b | 91.93 ± 4.04 c | 93.72 ± 1.03 bc | 102.57 ± 1.43 a | 104.8 ± 2.59 a | 104.55 ± 0.89 a |
| P32 HT0 | 92.05 ± 1.34 c | 95.78 ± 4.17 c | 97.71 ± 3.69 c | 106.96 ± 0.78 b | 116.65 ± 1.7 a | 108.84 ± 0.87 b | |
| Xinxiang CK0 | 115.72 ± 4.05 a | 101.2 ± 8.55 b | 99.23 ± 5 b | 107.3 ± 5.15 ab | 110.12 ± 0.95 ab | 104.2 ± 2.77 ab | |
| Xinxiang HT0 | 110.8 ± 4.05 a | 105.98 ± 6.25 a | 105.76 ± 1.17 a | 113.71 ± 3.06 a | 113.82 ± 1.16 a | 111.27 ± 1.44 a | |
| Adhesiveness | P32 CK0 | 20.28 ± 2.04 ab | 21.72 ± 0.99 ab | 11.25 ± 1.18 c | 12.58 ± 2.61 c | 18.57 ± 1.66 b | 22.94 ± 1.23 a |
| P32 HT0 | 20.91 ± 1.93 bc | 25 ± 1.13 ab | 13.04 ± 2.26 d | 17.43 ± 1.74 c | 21.45 ± 1.7 bc | 27.07 ± 2.32 a | |
| Xinxiang CK0 | 11.91 ± 1.33 bc | 10.31 ± 0.48 bc | 9.68 ± 0.34 bc | 8.16 ± 0.42 c | 13.42 ± 2.6 ab | 16.04 ± 2.72 a | |
| Xinxiang HT0 | 14.87 ± 0.48 ab | 13.58 ± 2 bc | 10.57 ± 2.17 c | 10.81 ± 1.29 c | 16.47 ± 0.89 ab | 17 ± 1.15 a | |
| Cohesiveness | P32 CK0 | 0.22 ± 0 a | 0.23 ± 0.02 a | 0.24 ± 0.01 a | 0.21 ± 0.01 a | 0.22 ± 0 a | 0.23 ± 0.01 a |
| P32 HT0 | 0.24 ± 0 a | 0.25 ± 0.02 a | 0.24 ± 0.01 a | 0.24 ± 0.01 a | 0.22 ± 0.01 a | 0.23 ± 0.01 a | |
| Xinxiang CK0 | 0.27 ± 0.03 a | 0.2 ± 0.03 b | 0.21 ± 0.02 b | 0.2 ± 0.02 b | 0.21 ± 0.01 b | 0.22 ± 0.02 b | |
| Xinxiang HT0 | 0.22 ± 0.02 a | 0.22 ± 0.01 a | 0.22 ± 0.01 a | 0.21 ± 0.02 a | 0.22 ± 0.01 a | 0.22 ± 0 a | |
| Springiness | P32 CK0 | 6.56 ± 0.12 a | 6.31 ± 0.17 ab | 5.58 ± 0.18 bc | 5.19 ± 0.4 c | 5.99 ± 0.14 ab | 5.95 ± 0.65 abc |
| P32 HT0 | 6.33 ± 0.35 ab | 6.58 ± 0.13 ab | 6.18 ± 0.21 ab | 5.78 ± 0.08 b | 6.91 ± 0.06 a | 6.96 ± 0.74 a | |
| Xinxiang CK0 | 7.62 ± 0.35 a | 5.76 ± 0.28 b | 5.62 ± 0.33 bc | 5.08 ± 0.29 c | 5.91 ± 0.11 b | 5.87 ± 0.09 b | |
| Xinxiang HT0 | 6.37 ± 0.23 a | 6.07 ± 0.24 ab | 6.49 ± 0.18 a | 5.58 ± 0.28 b | 6.39 ± 0.29 a | 6.03 ± 0.14 ab | |
| Gumminess | P32 CK0 | 22.02 ± 0.21 ab | 21.96 ± 2.88 ab | 20.59 ± 0.28 b | 20.24 ± 0.65 b | 24.01 ± 0.46 a | 21.45 ± 0.45 ab |
| P32 HT0 | 21.53 ± 0.85 a | 23.57 ± 2.73 a | 22.06 ± 0.37 a | 23.57 ± 0.68 a | 24.71 ± 1.58 a | 23.81 ± 0.33 a | |
| Xinxiang CK0 | 25.78 ± 3.08 a | 21.06 ± 0.4 b | 20.94 ± 0.8 b | 21.38 ± 1.56 b | 22.66 ± 0.55 ab | 22.92 ± 0.16 ab | |
| Xinxiang HT0 | 23.99 ± 1.36 a | 23.05 ± 0.93 a | 23.67 ± 0.91 a | 24 ± 0.53 a | 25.15 ± 0.78 a | 24.54 ± 0.8 a | |
| Chewiness | P32 CK0 | 144.61 ± 2.07 a | 139.61 ± 8.35 ab | 127.69 ± 6.81 bc | 121.32 ± 8.59 c | 144.03 ± 1.27 a | 150.79 ± 4.25 a |
| P32 HT0 | 136.67 ± 9.7 b | 153.98 ± 22 ab | 135.53 ± 1.91 b | 143.52 ± 5.54 ab | 170.89 ± 12.44 a | 166.89 ± 11.11 a | |
| Xinxiang CK0 | 202.39 ± 24 a | 140.09 ± 13.1 b | 130.72 ± 12.3 b | 114.25 ± 19.01 b | 139.54 ± 2.53 b | 144.29 ± 2.04 b | |
| Xinxiang HT0 | 153.42 ± 13.06 ab | 153.71 ± 4.36 ab | 147.24 ± 11.54 ab | 133.73 ± 11.2 b | 161.18 ± 8.47 a | 155.08 ± 3.4 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Zhang, J.; Ni, W.; Xu, X.; George, M.S.; Lu, G. Effect of Heat Treatment on the Quality and Soft Rot Resistance of Sweet Potato during Long-Term Storage. Foods 2023, 12, 4352. https://doi.org/10.3390/foods12234352
Wu J, Zhang J, Ni W, Xu X, George MS, Lu G. Effect of Heat Treatment on the Quality and Soft Rot Resistance of Sweet Potato during Long-Term Storage. Foods. 2023; 12(23):4352. https://doi.org/10.3390/foods12234352
Chicago/Turabian StyleWu, Jifeng, Jingzhen Zhang, Wenrong Ni, Ximing Xu, Melvin Sidikie George, and Guoquan Lu. 2023. "Effect of Heat Treatment on the Quality and Soft Rot Resistance of Sweet Potato during Long-Term Storage" Foods 12, no. 23: 4352. https://doi.org/10.3390/foods12234352
APA StyleWu, J., Zhang, J., Ni, W., Xu, X., George, M. S., & Lu, G. (2023). Effect of Heat Treatment on the Quality and Soft Rot Resistance of Sweet Potato during Long-Term Storage. Foods, 12(23), 4352. https://doi.org/10.3390/foods12234352