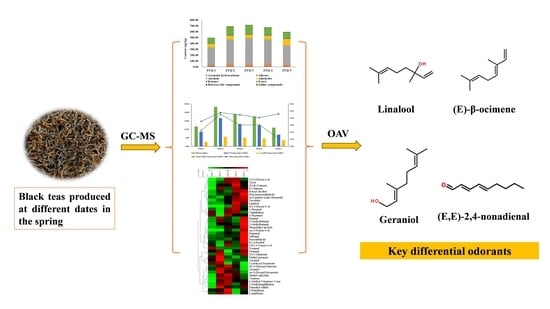
Difference in Aroma Components of Black Teas Processed on Different Dates in the Spring Season
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Black Tea Samples
2.3. Sensory Evaluation of Black Tea Samples
2.4. Extraction and Determination of Volatile Compounds
2.5. Calculation of OAVs
2.6. Data Processing
3. Results and Discussion
3.1. Effect of Production Date on Black Tea Aroma Quality
3.2. Identification and Integral Comparison of Volatile Compounds
3.3. Effect of Production Date on the Individual Aroma Components of Black Teas
3.4. Screening of the Key Differential Odorants Based on OAV
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food. Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef]
- Li, J.; Yao, Y.; Wang, J.; Hua, J.; Wang, J.; Yang, Y.; Dong, C.; Zhou, Q.; Jiang, Y.; Deng, Y.; et al. Rutin, gamma-aminobutyric acid, gallic acid, and caffeine negatively affect the sweet-mellow taste of congou black tea infusions. Molecules 2019, 24, 4221. [Google Scholar] [CrossRef]
- Wang, H.; Shen, S.; Wang, J.; Jiang, Y.; Li, J.; Yang, Y.; Hua, J.; Yuan, H. Novel insight into the effect of fermentation time on quality of Yunnan Congou black tea. LWT-Food Sci. Technol. 2022, 155, 112939. [Google Scholar] [CrossRef]
- Wu, S.; Yu, Q.; Shen, S.; Shan, X.; Hua, J.; Zhu, J.; Qiu, J.; Deng, Y.; Zhou, Q.; Jiang, Y.; et al. Non-targeted metabolomics and electronic tongue analysis reveal the effect of rolling time on the sensory quality and nonvolatile metabolites of congou black tea. LWT-Food Sci. Technol. 2022, 169, 113971. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, Y.; Liu, Y.; Liu, Y.; Dong, C.; Lin, Z.; Teng, J. Black tea aroma formation during the fermentation period. Food Chem. 2022, 374, 131640. [Google Scholar] [CrossRef]
- Zheng, F.; Gan, S.; Zhao, X.; Chen, Y.; Zhang, Y.; Qiu, T.; Zheng, P.; Zhai, X.; Dai, Q. Unraveling the chemosensory attributes of Chinese black teas from different regions using GC-IMS combined with sensory analysis. Food Sci. Technol. 2023, 184, 114988. [Google Scholar] [CrossRef]
- Ho, C.T.; Zheng, X.; Li, S. Tea aroma formation. Food Sci. Human Wellness 2015, 4, 9–27. [Google Scholar] [CrossRef]
- Chen, X.; Sun, H.; Qu, D.; Yan, F.; Jin, W.; Jiang, H.; Chen, C.; Zhang, Y.; Li, C.; Xu, Z. Identification and characterization of key aroma compounds in Chinese high altitude and northernmost black tea (Camellia sinensis) using distillation extraction and sensory analysis methods. Flavour Frag. J. 2020, 35, 666–673. [Google Scholar] [CrossRef]
- Kumazawa, K.; Wada, Y.; Masuda, H. Characterization of epoxydecenal isomers as potent odorants in black tea (Dimbula) infusion. J. Agric. Food. Chem. 2006, 54, 4795–4801. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Yan, H.; Zhu, Y.; Liu, X.; Lv, H.; Zhang, Y.; Dai, W.; Guo, L.; Tan, J.; Peng, Q.; et al. Identification and quantification of key odorants in the world’s four most famous black teas. Food Res. Int. 2019, 121, 73–83. [Google Scholar] [CrossRef]
- Wang, B.; Chen, H.; Qu, F.; Song, Y.; Di, T.; Wang, P.; Zhang, X. Identification of aroma-active components in black teas produced by six Chinese tea cultivars in high-latitude region by GC–MS and GC–O analysis. Eur. Food Res. Technol. 2022, 248, 647–657. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, H.; Niu, Y.; Qiang, L.; Zhu, J.; Chen, H.; Ning, M. Characterization of aroma compositions in different Chinese congou black teas using GC-MS and GC-O combined with partial least squares regression. Flavour Frag. J. 2017, 32, 265–276. [Google Scholar] [CrossRef]
- Joshi, R.; Gulati, A. Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. Food Chem. 2015, 167, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Tontul, I.; Torun, M.; Dincer, C.; Sahin-Nadeem, H.; Topuz, A.; Turna, T.; Ozdemir, F. Comparative study on volatile compounds in Turkish green tea powder: Impact of tea clone, shading level and shooting period. Food Res. Int. 2013, 53, 744–750. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Wu, J.; Wen, J.; Yu, Y.; An, K.; Zou, B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Res. Int. 2021, 150, 110784. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Wu, Y.; Qu, F.; Liu, L.; Wang, B.; Wang, P.; Zhang, X. HS−SPME/GC−MS reveals the season effects on volatile compounds of green tea in high−latitude region. Foods 2022, 11, 3016. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Boorboori, M.R.; Xu, Y.; Lin, W. The appearance of volatile aromas in Tieguanyin tea with different elevations. J. Food Sci. 2021, 86, 4405–4416. [Google Scholar] [CrossRef]
- Yang, Z.; Kobayashi, E.; Katsuno, T.; Asanuma, T.; Fujimori, T.; Ishikawa, T.; Tomomura, M.; Mochizuki, K.; Watase, T.; Nakamura, Y. Characterisation of volatile and non-volatile metabolites in etiolated leaves of tea (Camellia sinensis) plants in the dark. Food Chem. 2012, 135, 2268–2276. [Google Scholar] [CrossRef]
- Owuor, P.O.; Odhiambo, H.O. Response of some black tea quality parameters to nitrogen fertiliser rates and plucking frequencies. J. Sci. Food. Agric. 1994, 66, 555–561. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, Y.; Xu, S.; Wei, Y.; Bao, G.; Dai, Q.; Deng, W.; Ning, J. Effects of dynamic and static withering technology on volatile and nonvolatile components of Keemun black tea using GC-MS and HPLC combined with chemometrics. LWT-Food Sci. Technol. 2020, 130, 109547. [Google Scholar] [CrossRef]
- Wang, M.; Yang, J.; Li, J.; Zhou, X.; Xiao, Y.; Liao, Y.; Tang, J.; Dong, F.; Zeng, L. Effects of temperature and light on quality-related metabolites in tea [Camellia sinensis (L.) Kuntze] leaves. Food Res. Int. 2022, 161, 111882. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, Y.; Ma, L.; Yi, X.; Ruan, J. Metabolomic analysis using ultra-performance liquid chromatography-quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS) uncovers the effects of light intensity and temperature under shading treatments on the metabolites in tea. PLoS ONE 2014, 9, e112572. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Ma, Q.; Wang, Y. The differences between two tea varieties in their response to natural cold conditions. J. Hortic. Sci. Biotechnol. 2016, 91, 506–513. [Google Scholar] [CrossRef]
- Li, Y.; Jeyaraj, A.; Yu, H.; Wang, Y.; Ma, Q.; Chen, X.; Sun, H.; Zhang, H.; Ding, Z.; Li, X. Metabolic regulation profiling of carbon and nitrogen in tea plants [Camellia sinensis (L.) O. Kuntze] in response to shading. J. Agric. Food. Chem. 2020, 68, 961–974. [Google Scholar] [CrossRef]
- Du, Y.; Shin, S.; Wang, K.; Lu, J.; Liang, Y. Effect of temperature on the expression of genes related to the accumulation of chlorophylls and carotenoids in albino tea. J. Hortic. Sci. Biotechnol. 2009, 84, 365–369. (In Chinese) [Google Scholar] [CrossRef]
- Yin, P.; Liu, P.; Liu, W.; Guo, G.; Cao, L. Effects of different production dates during spring on aroma components in Xinyangmaojian tea. J. South. Agric. 2017, 48, 1671–1675. (In Chinese) [Google Scholar]
- Yin, P.; Liu, W.; Wang, Z.; Liu, P.; Guo, G. Analysis and comparison of the aroma constituents among Anjibaicha processed in different periods during the spring season. Food Res. Dev. 2018, 39, 132–136. (In Chinese) [Google Scholar]
- Zhang, C.; Zhou, C.; Xu, K.; Tian, C.; Zhang, M.; Lu, L.; Zhu, C.; Lai, Z.; Guo, Y. A comprehensive investigation of macro-composition and volatile compounds in spring-picked and autumn-picked white tea. Foods 2022, 11, 3628. [Google Scholar] [CrossRef]
- Huang, H.; Yu, P.; Zhao, X.; Zhong, N.; Zheng, H. HS-SPME-GC-MS analysis of volatile components of Congou black tea processed from Baojing Huangjincha 1 from different harvesting seasons. Food Sci. 2020, 41, 188–196. [Google Scholar]
- Wang, M.Q.; Ma, W.J.; Shi, J.; Zhu, Y.; Lin, Z.; Lv, H.P. Characterization of the key aroma compounds in Longjing tea using stir bar sorptive extraction (SBSE) combined with gas chromatography-mass spectrometry (GC-MS), gas chromatography-olfactometry (GC-O), odor activity value (OAV), and aroma recombination. Food Res. Int. 2020, 130, 108908. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Yu, D.; Shu, C.; Chen, H.; Wang, H.; Xiao, Z. Comparison of aroma-active volatiles in oolong tea infusions using GC-olfactometry, GC-FPD, and GC-MS. J. Agric. Food. Chem. 2015, 63, 7499–7510. [Google Scholar] [CrossRef]
- Cui, J.; Zhai, X.; Guo, D.; Du, W.; Gao, T.; Zhou, J.; Schwab, W.G.; Song, C. Characterization of Key Odorants in Xinyang Maojian Green Tea and Their Changes During the Manufacturing Process. J. Agric. Food. Chem. 2022, 70, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Schwab, W.; Ho, C.T.; Song, C.; Wan, X. Characterization of the aroma profiles of oolong tea made from three tea cultivars by both GC-MS and GC-IMS. Food Chem. 2022, 376, 131933. [Google Scholar] [CrossRef]
- Guo, X.; Ho, C.; Schwab, W.; Wan, X. Effect of the roasting degree on flavor quality of large-leaf yellow tea. Food Chem. 2021, 347, 129016. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhu, Y.; Dai, W.; Lv, H.; Mu, B.; Li, P.; Tan, J.; Ni, D.; Lin, Z. Aroma formation and dynamic changes during white tea processing. Food Chem. 2019, 274, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Parthiban, R. The impact of processing techniques on tea volatiles. Food Chem. 1998, 62, 347–353. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, P.; Li, Z.; Wang, Y.; Liu, Y.; Zhu, Y.; Fu, H. Re-rolling treatment in the fermentation process improves the aroma quality of black tea. Foods 2023, 12, 3702. [Google Scholar] [CrossRef]
- Mao, S.; Lu, C.; Li, M.; Ye, Y.; Wei, X.; Tong, H. Identification of key aromatic compounds in Congou black tea by partial least-square regression with variable importance of projection scores and gas chromatography-mass spectrometry/gas chromatography-olfactometry. J. Sci. Food. Agric. 2018, 98, 5278–5286. [Google Scholar] [CrossRef]
- Huang, W.; Fang, S.; Wang, J.; Zhuo, C.; Luo, Y.; Yu, Y.; Li, L.; Wang, Y.; Deng, W.; Ning, J. Sensomics analysis of the effect of the withering method on the aroma components of Keemun black tea. Food Chem. 2022, 395, 133549. [Google Scholar] [CrossRef]
- Plutowska, B.; Wardencki, W. Application of gas chromatography-olfactometry (GC-O) in analysis and quality assessment of alcoholic beverages—A review. Food Chem. 2008, 107, 449–463. [Google Scholar] [CrossRef]
- Ma, L.; Gao, M.; Zhang, L.; Qiao, Y.; Li, J.; Du, L.; Zhang, H.; Wang, H. Characterization of the key aroma-active compounds in high-grade Dianhong tea using GC-MS and GC-O combined with sensory-directed flavor analysis. Food Chem. 2022, 378, 132058. [Google Scholar] [CrossRef] [PubMed]




| ZYQ-1 | ZYQ-2 | ZYQ-3 | ZYQ-4 | ZYQ-5 | |
|---|---|---|---|---|---|
| Aroma description | Sweet, approaching strong | Sweet, strong and lasting | Sweet, strong and lasting | Sweet, nearly strong, with slight floral scent | Sweet, strong and lasting |
| Score | 92.2 | 93.2 | 93.5 | 93.0 | 93.2 |
| No. | Class | OT (μg/L) | ZYQ-1 | ZYQ-2 | ZYQ-3 | ZYQ-4 | ZYQ-5 | p-Value | VIP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Aromatic hydrocarbons | 11.17 ± 1.67 | 4.87 ± 0.85 | 6.78 ± 0.57 | 5.86 ± 0.78 | 5.54 ± 0.52 | |||||
| 1 | Methylbenzene | 73 [31] | nd | 0.47 ± 0.09 | 0.48 ± 0.05 | 0.40 ± 0.03 | 0.31 ± 0.06 | 0.034 | 0.91 | |
| 2 | Styrene | 1.53 ± 0.13 | 0.50 ± 0.07 | 0.76 ± 0.11 | 0.55 ± 0.14 | 0.72 ± 0.10 | 0.000 | 0.89 | ||
| 3 | Naphthalene | 0.44 [32] | 2.73 ± 0.64 | 1.19 ± 0.67 | 2.73 ± 0.37 | 3.00 ± 0.63 | 1.79 ± 0.33 | 0.011 | 1.19 | |
| 4 | 1-Methylnaphthalene | 8 [33] | 0.49 ± 0.11 | 1.11 ± 0.16 | 1.20 ± 0.21 | 0.67 ± 0.09 | 0.97 ± 0.15 | 0.001 | 1.27 | |
| 5 | 2-Methylnaphthalene | 1.47 ± 0.53 | 0.56 ± 0.12 | 0.62 ± 0.03 | 0.40 ± 0.12 | 0.48 ± 0.04 | 0.002 | 0.78 | ||
| 6 | 2,6-Di-tert-butylbenzoquinone | 4.94 ± 0.59 | 1.04 ± 0.19 | 0.98 ± 0.11 | 0.84 ± 0.10 | 1.27 ± 0.04 | 0.000 | 0.86 | ||
| Alkenes | 28.41 ± 3.08 | 37.79 ± 2.83 | 32.87 ± 1.48 | 37.3 ± 4.34 | 28.56 ± 1.51 | |||||
| 7 | Cubenene | VT | 4.25 ± 0.62 | 1.71 ± 0.32 | 1.40 ± 0.04 | 2.03 ± 0.29 | 1.55 ± 0.15 | 0.000 | 0.93 | |
| 8 | Myrcene | VT | 15 [3,10] | 9.18 ± 1.24 | 11.75 ± 1.15 | 9.62 ± 0.39 | 10.71 ± 1.17 | 8.22 ± 0.46 | 0.009 | 0.99 |
| 9 | Limonene | VT | 10 [10] | 5.74 ± 0.61 | 2.90 ± 0.38 | 2.55 ± 0.11 | 2.86 ± 0.36 | 2.35 ± 0.40 | 0.000 | 0.87 |
| 10 | (E)-β-Ocimene | VT | 0.02 [34] | 2.31 ± 0.34 | 4.13 ± 0.66 | 4.47 ± 0.67 | 6.24 ± 0.90 | 4.11 ± 0.77 | 0.001 | 1.16 |
| 11 | Ocimene | VT | 6.7 [15] | 2.64 ± 0.68 | 8.65 ± 0.49 | 8.65 ± 0.49 | 6.86 ± 0.54 | 5.01 ± 0.68 | 0.000 | 1.06 |
| 12 | α-Cubebene | VT | 14 [20] | 1.96 ± 0.25 | 2.19 ± 0.39 | 1.58 ± 0.18 | 2.39 ± 0.40 | 1.82 ± 0.36 | 0.088 | 1.19 |
| 13 | Longifolene | VT | 2 [15] | 0.84 ± 0.30 | 1.15 ± 0.23 | 1.35 ± 0.24 | 0.61 ± 0.06 | 0.97 ± 0.21 | 0.020 | 1.38 |
| 14 | δ-Cadinene | VT | 1.5 [32] | 1.50 ± 0.18 | 5.31 ± 0.45 | 3.24 ± 0.15 | 5.61 ± 1.07 | 4.54 ± 0.84 | 0.000 | 1.06 |
| Alcohols | 295.55 ± 19.71 | 428.25 ± 18.14 | 453.85 ± 25.81 | 428.22 ± 18.98 | 335.15 ± 8.38 | |||||
| 15 | cis-2-Penten-1-ol | FADV | 720 [33] | nd | nd | 0.43 ± 0.07 | 0.25 ± 0.06 | 0.84 ± 0.14 | 0.001 | 1.07 |
| 16 | (Z)-3-Hexen-1-ol | FADV | 13 [7] | nd | 1.64 ± 0.08 | 2.46 ± 0.42 | 2.49 ± 0.37 | 1.87 ± 0.29 | 0.025 | 1.04 |
| 17 | (E)-2-Hexen-1-ol | FADV | 100 [33] | nd | nd | 0.54 ± 0.07 | 1.55 ± 0.19 | 0.86 ± 0.06 | 0.000 | 1.28 |
| 18 | 1-Hexanol | FADV | 5.6 [33] | 0.85 ± 0.17 | 0.68 ± 0.06 | 1.15 ± 0.16 | 1.24 ± 0.07 | 1.00 ± 0.19 | 0.005 | 1.15 |
| 19 | 1-Heptanol | FADV | 3 [31] | 0.63 ± 0.05 | 0.24 ± 0.02 | 0.71 ± 0.04 | 0.54 ± 0.07 | 0.50 ± 0.08 | 0.000 | 1.32 |
| 20 | 1-Octen-3-ol | FADV | 1.5 [31] | 0.18 ± 0.01 | 0.57 ± 0.10 | 0.51 ± 0.02 | 0.63 ± 0.10 | 0.46 ± 0.06 | 0.000 | 0.88 |
| 21 | Benzyl alcohol | AADV | 100 [3,31] | 0.71 ± 0.13 | 1.19 ± 0.31 | 0.95 ± 0.29 | 1.82 ± 0.04 | 1.92 ± 0.75 | 0.013 | 1.02 |
| 22 | cis-Linalool oxide (furanoid) | VT | 6 [15] | 5.49 ± 0.33 | 4.10 ± 0.35 | 7.98 ± 0.76 | 10.51 ± 0.94 | 12.06 ± 1.12 | 0.000 | 1.11 |
| 23 | trans-Linalool oxide (furanoid) | VT | 6 [15] | 5.40 ± 0.07 | 10.57 ± 1.03 | 19.66 ± 1.43 | 18.93 ± 1.64 | 23.71 ± 1.59 | 0.000 | 0.96 |
| 24 | Linalool | VT | 0.22 [33] | 62.61 ± 4.66 | 61.25 ± 3.81 | 88.78 ± 5.32 | 129.12 ± 4.66 | 112.35 ± 8.74 | 0.000 | 1.22 |
| 25 | Phenylethyl alcohol | AADV | 60 [31] | 10.63 ± 0.52 | 11.88 ± 0.67 | 20.59 ± 1.73 | 19.15 ± 1.06 | 27.74 ± 2.98 | 0.000 | 1.01 |
| 26 | (E)-Pinocarveol | VT | 1.07 ± 0.29 | 0.78 ± 0.09 | 0.70 ± 0.13 | 0.34 ± 0.07 | 0.51 ± 0.12 | 0.002 | 0.97 | |
| 27 | (Z)-3-Nonen-1-ol | FADV | 1.14 ± 0.19 | 0.67 ± 0.14 | 1.61 ± 0.05 | 1.01 ± 0.04 | 1.67 ± 0.45 | 0.001 | 1.20 | |
| 28 | 1-Nonanol | FADV | 45.5 [33] | nd | nd | 5.51 ± 1.07 | 5.74 ± 0.40 | 4.92 ± 0.83 | 0.487 | 0.52 |
| 29 | Nerol | VT | 49 [33] | 2.49 ± 0.52 | 7.03 ± 0.54 | 13.03 ± 0.11 | 14.74 ± 1.41 | 10.02 ± 0.82 | 0.000 | 1.04 |
| 30 | Geraniol | VT | 3.2 [7] | 202.59 ± 15.1 | 320.11 ± 20.68 | 278.79 ± 21.78 | 204.32 ± 16.56 | 119.36 ± 6.8 | 0.000 | 1.20 |
| 31 | Nerolidol | VT | 10 [20,31] | 1.79 ± 0.54 | 5.11 ± 0.88 | 8.86 ± 0.68 | 13.45 ± 1.01 | 13.92 ± 1.73 | 0.000 | 1.01 |
| 32 | Epicubenol | VT | nd | 2.43 ± 0.65 | 1.58 ± 0.24 | 2.40 ± 0.56 | 1.43 ± 0.26 | 0.055 | 0.97 | |
| Aldehydes | 59.2 ± 3.65 | 63.72 ± 1.32 | 57.00 ± 3.72 | 63.33 ± 1.53 | 108.48 ± 0.95 | |||||
| 33 | Butanal | 17 [31] | 0.98 ± 0.12 | 1.94 ± 0.31 | 1.36 ± 0.23 | 1.00 ± 0.18 | 2.16 ± 0.11 | 0.000 | 1.29 | |
| 34 | 3-Methylbutanal | AADV | 0.2 [31] | 0.87 ± 0.24 | 2.62 ± 0.49 | 1.74 ± 0.13 | 1.13 ± 0.14 | 3.54 ± 0.60 | 0.000 | 1.25 |
| 35 | 2-Methylbutanal | AADV | 1 [31] | 0.21 ± 0.08 | 3.70 ± 0.45 | 3.49 ± 0.47 | 2.29 ± 0.27 | 7.91 ± 0.78 | 0.000 | 1.14 |
| 36 | Pentanal | FADV | 12 [31] | 0.28 ± 0.03 | 0.33 ± 0.03 | 0.17 ± 0.02 | 0.27 ± 0.06 | 0.33 ± 0.10 | 0.027 | 1.21 |
| 37 | Hexanal | FADV | 4.5 [10,33] | 8.82 ± 1.45 | 4.41 ± 0.50 | 3.40 ± 0.45 | 2.71 ± 0.23 | 3.14 ± 0.38 | 0.000 | 0.84 |
| 38 | (E)-2-hexenal | FADV | 17 [8,15] | 1.07 ± 0.07 | 3.40 ± 0.36 | 3.42 ± 0.48 | 4.15 ± 0.66 | 5.65 ± 0.34 | 0.000 | 0.93 |
| 39 | Heptanal | FADV | 2.8 [33] | 0.30 ± 0.04 | 0.64 ± 0.08 | 1.16 ± 0.14 | 0.88 ± 0.12 | 2.18 ± 0.11 | 0.000 | 1.07 |
| 40 | (E)-2-Heptenal | FADV | 13 [31] | 0.17 ± 0.03 | 0.24 ± 0.03 | 0.15 ± 0.02 | 0.18 ± 0.03 | 0.24 ± 0.04 | 0.017 | 1.16 |
| 41 | Benzaldehyde | AADV | 350 [33] | 4.10 ± 0.62 | 4.62 ± 0.22 | 3.68 ± 0.24 | 6.26 ± 0.61 | 13.14 ± 1.13 | 0.000 | 1.17 |
| 42 | (E,E)-2,4-Heptadienal | FADV | 56 [31] | 2.55 ± 0.77 | 2.04 ± 0.22 | 1.42 ± 0.06 | 1.60 ± 0.19 | 2.56 ± 0.57 | 0.031 | 0.97 |
| 43 | Benzeneacetaldehyde | AADV | 4 [3,8,31] | 4.17 ± 0.99 | 9.72 ± 0.79 | 9.28 ± 0.99 | 15.71 ± 0.98 | 23.21 ± 2.21 | 0.000 | 1.07 |
| 44 | 2,6-Dimethyl-5-hepten-1-al | 0.68 ± 0.20 | 0.69 ± 0.14 | 0.55 ± 0.06 | nd | nd | 0.441 | 0.62 | ||
| 45 | (E)-2-Octenal | FADV | 3 [10] | 3.37 ± 0.25 | 2.01 ± 0.31 | 1.32 ± 0.11 | 1.61 ± 0.16 | 1.78 ± 0.12 | 0.000 | 0.92 |
| 46 | Nonanal | FADV | 1 [10] | 13.89 ± 1.83 | 6.28 ± 1.05 | 6.87 ± 0.71 | 6.65 ± 0.76 | 15.65 ± 1.77 | 0.000 | 1.09 |
| 47 | (E)-2-Nonenal | FADV | 0.08 [15] | 1.93 ± 0.42 | 1.37 ± 0.11 | 0.88 ± 0.19 | 1.11 ± 0.05 | 1.19 ± 0.30 | 0.005 | 0.88 |
| 48 | Safranal | CDV | 3 [33] | 2.00 ± 0.39 | 2.21 ± 0.09 | 3.18 ± 0.24 | 3.02 ± 0.32 | 8.43 ± 0.70 | 0.000 | 1.12 |
| 49 | Decanal | FADV | 6 [15] | 0.92 ± 0.34 | 2.44 ± 0.10 | 2.71 ± 0.22 | 2.61 ± 0.08 | 2.71 ± 0.21 | 0.000 | 0.86 |
| 50 | (E,E)-2,4-Nonadienal | FADV | 0.02 [8] | 1.92 ± 0.69 | 1.14 ± 0.22 | 1.05 ± 0.13 | 0.80 ± 0.12 | 1.28 ± 0.16 | 0.024 | 0.77 |
| 51 | β-Cyclocitral | CDV | 3 [33] | 2.73 ± 0.14 | 1.81 ± 0.43 | 3.47 ± 0.07 | 2.60 ± 0.33 | 4.68 ± 0.43 | 0.000 | 1.03 |
| 52 | Neral | VT | 53 [33] | 1.02 ± 0.19 | 2.59 ± 0.81 | 1.68 ± 0.07 | 2.35 ± 0.29 | 2.35 ± 0.09 | 0.004 | 0.96 |
| 53 | Geranial | VT | 32 [20] | 7.21 ± 0.71 | 9.52 ± 1.22 | 6.02 ± 0.07 | 6.38 ± 0.61 | 6.35 ± 0.57 | 0.001 | 1.17 |
| Ketones | 10.12 ± 1.26 | 12.22 ± 1.22 | 11.2 ± 0.6 | 11.35 ± 0.81 | 11.28 ± 1.30 | |||||
| 54 | 6-Methyl-5-heptene-2-one | CDV | 50 [15,31] | 0.41 ± 0.03 | 0.93 ± 0.07 | 1.06 ± 0.06 | 0.83 ± 0.14 | 0.87 ± 0.08 | 0.000 | 1.03 |
| 55 | 5-Ethyl-6-methyl-3-hepten-2-one | 0.53 ± 0.16 | 1.08 ± 0.60 | 0.81 ± 0.12 | 1.07 ± 0.13 | 0.79 ± 0.11 | 0.198 | 0.71 | ||
| 56 | d-Verbenone | VT | 0.89 ± 0.34 | 1.77 ± 0.32 | 1.22 ± 0.15 | 1.27 ± 0.35 | 1.15 ± 0.23 | 0.041 | 0.88 | |
| 57 | β-Damascenone | CDV | 0.002 [7] | 0.79 ± 0.1 | 1.12 ± 0.25 | 1.14 ± 0.21 | 1.19 ± 0.32 | 0.94 ± 0.14 | 0.212 | 0.93 |
| 58 | α-Ionone | CDV | 0.4 [8] | 1.70 ± 0.29 | 1.17 ± 0.11 | 0.82 ± 0.02 | 0.63 ± 0.15 | 0.74 ± 0.14 | 0.000 | 0.88 |
| 59 | Geranylacetone | CDV | 60 [15,33] | 0.73 ± 0.13 | 2.22 ± 0.20 | 2.23 ± 0.25 | 2.33 ± 0.37 | 2.19 ± 0.27 | 0.000 | 0.82 |
| 60 | β-Ionone | CDV | 0.007 [7] | 5.08 ± 0.58 | 3.93 ± 0.60 | 3.92 ± 0.45 | 4.04 ± 0.75 | 4.61 ± 0.61 | 0.151 | 0.68 |
| Esters | 87.13 ± 9.65 | 134.82 ± 10.94 | 147.22 ± 3.69 | 126.51 ± 10.63 | 99.88 ± 7.98 | |||||
| 61 | Vinyl hexanoate | FADV | 5 [33] | 0.63 ± 0.07 | 0.17 ± 0.02 | 0.24 ± 0.02 | 0.17 ± 0.01 | 0.26 ± 0.05 | 0.000 | 0.88 |
| 62 | (E)-3-Hexenyl butyrate | FADV | 1.32 ± 0.40 | 2.02 ± 0.17 | 2.59 ± 0.63 | 1.89 ± 0.60 | 0.67 ± 0.08 | 0.003 | 1.13 | |
| 63 | Methyl salicylate | AADV | 40 [3,15] | 75.47 ± 8.69 | 117.86 ± 8.34 | 133.83 ± 3.14 | 113.58 ± 8.45 | 91.66 ± 7.57 | 0.000 | 1.10 |
| 64 | cis-3-Hexenyl isovalerate | FADV/AADV | 0.13 [15] | 2.83 ± 0.67 | 2.36 ± 0.43 | 2.63 ± 0.30 | 2.47 ± 0.47 | 2.57 ± 0.19 | 0.761 | 0.43 |
| 65 | Methyl geranate | VT | 3.27 ± 0.57 | 4.25 ± 0.73 | 2.62 ± 0.15 | 2.03 ± 0.34 | 2.61 ± 0.24 | 0.002 | 1.18 | |
| 66 | cis-3-Hexenyl hexanoate | FADV | 16 [32] | 1.25 ± 0.22 | 4.82 ± 0.87 | 2.93 ± 0.23 | 3.39 ± 0.56 | 1.09 ± 0.15 | 0.000 | 1.05 |
| 67 | Cyclohexyl hexanoate | FADV | 1.61 ± 0.34 | 1.76 ± 0.45 | 0.78 ± 0.10 | 1.21 ± 0.35 | nd | 0.028 | 1.05 | |
| 68 | (E)-2-Hexenyl caproate | FADV | 0.76 ± 0.20 | 1.59 ± 0.36 | 1.60 ± 0.17 | 1.77 ± 0.24 | 1.02 ± 0.08 | 0.001 | 0.94 | |
| Heterocyclic compounds | 2.72 ± 0.27 | 0.87 ± 0.04 | 1.10 ± 0.12 | 0.69 ± 0.09 | 1.14 ± 0.13 | |||||
| 69 | 2-Ethylfuran | 8000 [33] | 0.43 ± 0.11 | 0.46 ± 0.02 | 0.61 ± 0.06 | 0.31 ± 0.06 | 0.42 ± 0.09 | 0.009 | 1.47 | |
| 70 | (E)-2-(2-Pentenyl)furan | 2.28 ± 0.24 | 0.42 ± 0.03 | 0.49 ± 0.06 | 0.38 ± 0.02 | 0.72 ± 0.08 | 0.000 | 0.88 | ||
| Sulfur compounds | 3.28 ± 0.78 | 7.97 ± 1.00 | 5.26 ± 0.87 | 4.96 ± 0.44 | 6.57 ± 0.50 | |||||
| 71 | Dimethyl sulfide | AADV | 1.1 [31] | 3.28 ± 0.78 | 7.97 ± 1.00 | 5.26 ± 0.87 | 4.96 ± 0.44 | 6.57 ± 0.50 | 0.000 | 1.13 |
| Total | 497.58 ± 38.91 | 690.51 ± 34.09 | 715.27 ± 25.98 | 678.23 ± 33.05 | 596.61 ± 14.73 |
| No. | Volatile Compound | Odor Description | OAV | ||||
|---|---|---|---|---|---|---|---|
| ZYQ-1 | ZYQ-2 | ZYQ-3 | ZYQ-4 | ZYQ-5 | |||
| 1 | Naphthalene I | Pungent [32] | 6.2 | 2.7 | 6.2 | 6.8 | 4.1 |
| 2 | (E)-β-Ocimene II | Warm, floral, herbal, sweet [34] | 115.5 | 206.3 | 223.4 | 311.8 | 205.4 |
| 3 | Ocimene II | Herbal, green [15] | 1.3 | 1.3 | 1.0 | ||
| 4 | δ-Cadinene II | Herbal, woody [32] | 1.0 | 3.5 | 2.2 | 3.7 | 3.0 |
| 5 | cis-Linalool oxide (furanoid) II | Fruity [15] | 1.3 | 1.8 | 2.0 | ||
| 6 | trans-Linalool oxide (furanoid) II | Fruity, fresh [15] | 1.8 | 3.3 | 3.2 | 4.0 | |
| 7 | Linalool II | Floral, sweet [33] | 284.6 | 278.4 | 403.6 | 586.9 | 510.7 |
| 8 | Geraniol II | Rose-like, sweet [7] | 63.3 | 100.0 | 87.1 | 63.8 | 37.3 |
| 9 | Nerolidol II | Floral, woody [31] | 1.3 | 1.4 | |||
| 10 | 3-Methylbutanal II | Malt [31] | 4.4 | 13.1 | 8.7 | 5.7 | 17.7 |
| 11 | 2-Methylbutanal II | Cocoa, almond [31] | 3.7 | 3.5 | 2.3 | 7.9 | |
| 12 | Hexanal I | Green, leafy, grassy [10,33] | 2.0 | ||||
| 13 | Benzene acetaldehyde II | Woody, sweet, honey [3,8,31] | 1.0 | 2.4 | 2.3 | 3.9 | 5.8 |
| 14 | (E)-2-Octenal I | Fresh, cucumber-like, fatty, green [10] | 1.1 | ||||
| 15 | Nonanal I | Waxy, fresh, orange-like [10] | 13.9 | 6.3 | 6.9 | 6.7 | 15.6 |
| 16 | (E)-2-Nonenal I | Watermelon, cucumber-like [15] | 24.1 | 17.2 | 11.0 | 13.9 | 14.8 |
| 17 | Safranal II | Woody, spicy, medicinal [33] | 1.1 | 1.0 | 2.8 | ||
| 18 | (E,E)-2,4-Nonadienal I | Cucumber-like [8] | 95.9 | 56.9 | 52.6 | 40.2 | 64.2 |
| 19 | β-Cyclocitral II | Herbal, clean, rose-like, fruity [33] | 1.2 | 0.9 | 1.6 | ||
| 20 | β-Damascenone II | Fruity, apple-like [7] | 392.7 | 558.3 | 569.2 | 593.7 | 469.0 |
| 21 | α-Ionone II | Woody, violet-like, floral [8,33] | 4.2 | 2.9 | 2.0 | 1.6 | 1.8 |
| 22 | β-Ionone II | Woody, violet, floral [7,33] | 725.5 | 561.1 | 560.0 | 576.8 | 657.9 |
| 23 | Methyl salicylate I | Vanilla flavor [3,15] | 1.9 | 2.9 | 3.3 | 2.8 | 2.3 |
| 24 | cis-3-Hexenyl isovalerate II | Fresh, green [15] | 21.7 | 18.2 | 20.2 | 19.0 | 19.8 |
| 25 | Dimethyl sulfide II | Cabbage, sulfur, corn, molasses [31] | 3.0 | 7.2 | 4.8 | 4.5 | 6.0 |
| Sum | 1762.1 | 1844.4 | 1975.2 | 2252.5 | 2055.1 | ||
| Group I | 145.1 | 86.0 | 80.0 | 70.4 | 101.1 | ||
| Group II | 1617.0 | 1758.3 | 1895.2 | 2182.1 | 1954.0 | ||
| Group II/Group I | 11.1 | 20.4 | 23.7 | 31.0 | 19.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Huang, Y.; Li, Z.; Zhao, X.; Huang, H.; Zhong, N.; Zheng, H.; Chen, Q. Difference in Aroma Components of Black Teas Processed on Different Dates in the Spring Season. Foods 2023, 12, 4368. https://doi.org/10.3390/foods12234368
Yu P, Huang Y, Li Z, Zhao X, Huang H, Zhong N, Zheng H, Chen Q. Difference in Aroma Components of Black Teas Processed on Different Dates in the Spring Season. Foods. 2023; 12(23):4368. https://doi.org/10.3390/foods12234368
Chicago/Turabian StyleYu, Penghui, Yingjie Huang, Ziyi Li, Xi Zhao, Hao Huang, Ni Zhong, Hongfa Zheng, and Qincao Chen. 2023. "Difference in Aroma Components of Black Teas Processed on Different Dates in the Spring Season" Foods 12, no. 23: 4368. https://doi.org/10.3390/foods12234368
APA StyleYu, P., Huang, Y., Li, Z., Zhao, X., Huang, H., Zhong, N., Zheng, H., & Chen, Q. (2023). Difference in Aroma Components of Black Teas Processed on Different Dates in the Spring Season. Foods, 12(23), 4368. https://doi.org/10.3390/foods12234368