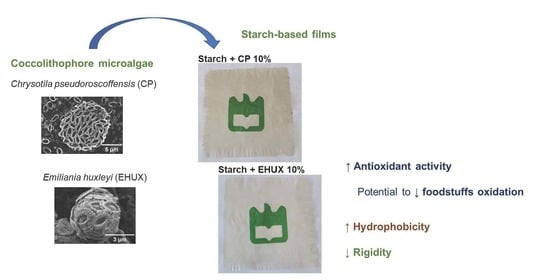
Potential of Coccolithophore Microalgae as Fillers in Starch-Based Films for Active and Sustainable Food Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microalgae Biomass
2.2.1. Culture of E. huxleyi
2.2.2. Culture of C. pseudoroscoffensis
2.3. Elemental Analysis of Microalgae Biomass
2.4. Production of Starch-Based Films
2.5. Starch-Based Films Characterisation
2.5.1. Optical Properties
2.5.2. Thermal Properties
2.5.3. Mechanical Properties
2.5.4. Water Contact Angle
2.5.5. Moisture Content
2.5.6. Solubility in Aqueous Medium
2.5.7. Water Vapor Permeability
2.5.8. Scanning Electron Microscopy (SEM)
2.5.9. Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Starch-Based Films with Microalgae Biomass
3.1.1. Colour and Morphology
3.1.2. Thermal Properties
3.1.3. Mechanical Properties
3.1.4. Wettability, Water Solubility, and Water Vapour Permeability
3.1.5. Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, C.; Moran, F.; Jaiswal, A.K. A review on European Union’s strategy for plastics in a circular economy and its impact on food safety. J. Clean Prod. 2021, 283, 125263. [Google Scholar] [CrossRef]
- Lauer, M.K.; Smith, R.C. Recent advances in starch-based films toward food packaging applications: Physicochemical, mechanical, and functional properties. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3031–3083. [Google Scholar] [CrossRef] [PubMed]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocolloids 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Oyeoka, H.C.; Ewulonu, C.M.; Nwuzor, I.C.; Obele, C.M.; Nwabanne, J.T. Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J. Bioresour. Bioprod. 2021, 6, 168–185. [Google Scholar] [CrossRef]
- Azmin, S.N.H.M.; Hayat, N.A.b.M.; Nor, M.S.M. Development and characterization of food packaging bioplastic film from cocoa pod husk cellulose incorporated with sugarcane bagasse fibre. J. Bioresour. Bioprod. 2020, 5, 248–255. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, protein and lipid -based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Müller, C.M.O.; Laurindo, J.B.; Yamashita, F. Effect of cellulose fibers addition on the mechanical properties and water vapor barrier of starch-based films. Food Hydrocolloids 2009, 23, 1328–1333. [Google Scholar] [CrossRef]
- Sun, Q.; Xi, T.; Li, Y.; Xiong, L. Characterization of corn starch films reinforced with CaCO3 nanoparticles. PLoS ONE 2014, 9, e106727. [Google Scholar] [CrossRef] [Green Version]
- Abdolmohammadi, S.; Siyamak, S.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Rahman, M.Z.A.; Azizi, S.; Fatehi, A. Enhancement of mechanical and thermal properties of polycaprolactone/chitosan blend by calcium carbonate nanoparticles. Int. J. Mol. Sci. 2012, 13, 4508–4522. [Google Scholar] [CrossRef]
- Lopes, J.; Gonçalves, I.; Nunes, C.; Teixeira, B.; Mendes, R.; Ferreira, P.; Coimbra, M.A. Potato peel phenolics as additives for developing active starch-based films with potential to pack smoked fish fillets. Food Packag. Shelf Life 2021, 28, 100644. [Google Scholar] [CrossRef]
- de Medeiros, V.P.B.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef]
- Hamed, I. The evolution and versatility of microalgal biotechnology: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Chacón-Lee, T.L.; González-Mariño, G.E. Microalgae for “healthy” foods—Possibilities and challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Orejuela-Escobar, L.; Gualle, A.; Ochoa-Herrera, V.; Philippidis, G.P. Prospects of microalgae for biomaterial production and environmental applications at biorefineries. Sustainability 2021, 13, 3063. [Google Scholar] [CrossRef]
- Chong, J.W.R.; Tan, X.; Khoo, K.S.; Ng, H.S.; Jonglertjunya, W.; Yew, G.Y.; Show, P.L. Microalgae-based bioplastics: Future solution towards mitigation of plastic wastes. Environ. Res. 2022, 206, 112620. [Google Scholar] [CrossRef]
- Fabra, M.J.; Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Coll-Marqués, J.M.; Martínez, J.C.; López-Rubio, A. Development and characterisation of hybrid corn starch-microalgae films: Effect of ultrasound pre-treatment on structural, barrier and mechanical performance. Algal Res. 2017, 28, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Carissimi, M.; Flôres, S.H.; Rech, R. Effect of microalgae addition on active biodegradable starch film. Algal Res. 2018, 32, 201–209. [Google Scholar] [CrossRef]
- Tedeschi, A.M.; Di Caprio, F.; Piozzi, A.; Pagnanelli, F.; Francolini, I. Sustainable bioactive packaging based on thermoplastic starch and microalgae. Int. J. Mol. Sci. 2022, 23, 178. [Google Scholar] [CrossRef] [PubMed]
- Aveiro, S.S.; Melo, T.; Figueiredo, A.; Domingues, P.; Pereira, H.; Maia, I.B.; Silva, J.; Domingues, M.R.; Nunes, C.; Moreira, A.S.P. The polar lipidome of cultured Emiliania huxleyi: A source of bioactive lipids with relevance for biotechnological applications. Biomolecules 2020, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.P.; Gonçalves, J.; Conde, T.A.; Couto, D.; Melo, T.; Maia, I.B.; Pereira, H.; Silva, J.; Domingues, M.R.; Nunes, C. Chrysotila pseudoroscoffensis as a source of high-value polar lipids with antioxidant activity: A lipidomic approach. Algal Res. 2022, 66, 102756. [Google Scholar] [CrossRef]
- Reifel, K.M.; McCoy, M.P.; Tiffany, M.A.; Rocke, T.E.; Trees, C.C.; Barlow, S.B.; Faulkner, D.J.; Hurlbert, S.H. Pleurochrysis pseudoroscoffensis (Prymnesiophyceae) blooms on the surface of the Salton Sea, California. Hydrobiologia 2001, 466, 177–185. [Google Scholar] [CrossRef]
- Brownlee, C.; Wheeler, G.L.; Taylor, A.R. Coccolithophore biomineralisation: New questions, new answers. Semin. Cell Dev. Biol. 2015, 46, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.R.; Brownlee, C.; Wheeler, G. Coccolithophore cell biology: Chalking up progress. Annu. Rev. Mar. Sci. 2017, 9, 283–310. [Google Scholar] [CrossRef] [Green Version]
- Jakob, I.; Chairopoulou, M.A.; Vučak, M.; Posten, C.; Teipel, U. Biogenic calcite particles from microalgae-Coccoliths as a potential raw material. Eng. Life Sci. 2017, 17, 605–612. [Google Scholar] [CrossRef] [Green Version]
- Schultz, L.N.; Andersson, M.P.; Dalby, K.N.; Müter, D.; Okhrimenko, D.V.; Fordsmand, H.; Stipp, S.L.S. High surface area calcite. J. Cryst. Growth 2013, 371, 34–38. [Google Scholar] [CrossRef]
- Sheyn, U.; Rosenwasser, S.; Lehahn, Y.; Barak-Gavish, N.; Rotkopf, R.; Bidle, K.D.; Koren, I.; Schatz, D.; Vardi, A. Expression profiling of host and virus during a coccolithophore bloom provides insights into the role of viral infection in promoting carbon export. ISME J. 2018, 12, 704–713. [Google Scholar] [CrossRef] [Green Version]
- Boyjoo, Y.; Pareek, V.K.; Liu, J. Synthesis of micro and nano-sized calcium carbonate particles and their applications. J. Mater. Chem. A 2014, 2, 14270–14288. [Google Scholar] [CrossRef]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Pereira, H.; Barreira, L.; Mozes, A.; Florindo, C.; Polo, C.; Duarte, C.V.; Custódio, L.; Varela, J. Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnol. Biofuels 2011, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Lourenço, S.O.; Barbarino, E.; Lavín, P.L.; Lanfer Marquez, U.M.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; Silva, J.A.L.d.; Coimbra, M.A. Chitosan–caffeic acid–genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef]
- Gonçalves, I.; Lopes, J.; Barra, A.; Hernández, D.; Nunes, C.; Kapusniak, K.; Kapusniak, J.; Evtyugin, D.V.; Lopes da Silva, J.A.; Ferreira, P.; et al. Tailoring the surface properties and flexibility of starch-based films using oil and waxes recovered from potato chips byproducts. Int. J. Biol. Macromol. 2020, 163, 251–259. [Google Scholar] [CrossRef]
- Python Software Foundation, Python Language Reference (version 3.9). Available online: http://www.python.org (accessed on 17 January 2023).
- Jaya, B.N.; Hoffmann, R.; Kirchlechner, C.; Dehm, G.; Scheu, C.; Langer, G. Coccospheres confer mechanical protection: New evidence for an old hypothesis. Acta Biomater. 2016, 42, 258–264. [Google Scholar] [CrossRef]
- Marsh, M.E. Polyanion-mediated mineralisation—Assembly and reorganisation of acidic polysaccharides in the Golgi system of a coccolithophorid alga during mineral deposition. Protoplasma 1994, 177, 108–122. [Google Scholar] [CrossRef]
- Chen, X.; Kameshwar, A.K.S.; Chio, C.; Lu, F.; Qin, W. Effect of KNO3 on lipid synthesis and CaCO3 accumulation in Pleurochrysis dentata coccoliths with a special focus on morphological characters of coccolithophores. Int. J. Biol. Sci. 2019, 15, 2844–2858. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Pan, Y.; Yao, C.; Wang, H.; Cao, X.; Xue, S. Determination of ash content and concomitant acquisition of cell compositions in microalgae via thermogravimetric (TG) analysis. Algal Res. 2015, 12, 149–155. [Google Scholar] [CrossRef]
- Chairopoulou, M.A.; Kratzer, F.; Gross, R.; Herrmann, M.; Teipel, U. Influence of the temperature on coccolith-containing systems from Emiliania huxleyi cultivations. Chem. Eng. Technol. 2020, 43, 904–912. [Google Scholar] [CrossRef] [Green Version]
- Shapi’i, R.A.; Othman, S.H.; Basha, R.K.; Naim, M.N. Mechanical, thermal, and barrier properties of starch films incorporated with chitosan nanoparticles. Nanotechnol. Rev. 2022, 11, 1464–1477. [Google Scholar] [CrossRef]







| L* | a* | b* | ∆E | |
|---|---|---|---|---|
| Starch (control) | 90.52 ± 0.34 | 2.05 ± 0.03 | −3.49 ± 0.05 | - |
| Starch + CaCO3 2.5% | 90.77 ± 0.37 | 2.10 ± 0.01 | −3.44 ± 0.03 | 0.37± 0.33 |
| Starch + CaCO3 5% | 90.42 ± 0.26 | 2.11 ± 0.08 | −3.27 ± 0.23 | 0.36 ± 0.19 |
| Starch + CaCO3 10% | 90.33 ± 0.31 | 2.08 ± 0.05 | −3.34 ± 0.05 | 0.32 ± 0.22 |
| Starch + CaCO3 20% | 90.39 ± 0.21 | 2.08 ± 0.06 | −3.44 ± 0.08 | 0.23 ± 0.12 |
| Starch + EHUX 2.5% | 89.97 ± 0.25 | 1.89 ± 0.03 | −2.52 ± 0.06 | 1.14 ± 0.13 |
| Starch + EHUX 5% | 89.78 ± 0.51 | 1.52 ± 0.08 | −0.65 ± 0.23 | 3.01 ± 0.21 |
| Starch + EHUX 10% | 88.69 ± 0.55 | 0.90 ± 0.05 | 2.67 ± 0.19 | 6.54 ± 0.30 |
| Starch + EHUX 20% | 84.19 ± 0.25 | −0.98 ± 0.23 | 11.44 ± 0.26 | 16.50 ± 0.33 |
| Starch + CP 2.5% | 88.76 ± 0.25 | 1.82 ± 0.03 | −0.90 ± 0.11 | 3.14 ± 0.21 |
| Starch + CP 5% | 87.54 ± 0.39 | 1.18 ± 0.03 | 1.34 ± 0.21 | 5.75 ± 0.36 |
| Starch + CP 10% | 82.58 ± 0.48 | 0.12 ± 0.09 | 6.49 ± 0.33 | 12.90 ± 0.53 |
| Starch + CP 20% | 77.54 ± 0.39 | −0.05 ± 0.03 | 12.40 ± 0.17 | 20.62 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, A.S.P.; Gonçalves, J.; Sousa, F.; Maia, I.; Pereira, H.; Silva, J.; Coimbra, M.A.; Ferreira, P.; Nunes, C. Potential of Coccolithophore Microalgae as Fillers in Starch-Based Films for Active and Sustainable Food Packaging. Foods 2023, 12, 513. https://doi.org/10.3390/foods12030513
Moreira ASP, Gonçalves J, Sousa F, Maia I, Pereira H, Silva J, Coimbra MA, Ferreira P, Nunes C. Potential of Coccolithophore Microalgae as Fillers in Starch-Based Films for Active and Sustainable Food Packaging. Foods. 2023; 12(3):513. https://doi.org/10.3390/foods12030513
Chicago/Turabian StyleMoreira, Ana S. P., Joana Gonçalves, Francisco Sousa, Inês Maia, Hugo Pereira, Joana Silva, Manuel A. Coimbra, Paula Ferreira, and Cláudia Nunes. 2023. "Potential of Coccolithophore Microalgae as Fillers in Starch-Based Films for Active and Sustainable Food Packaging" Foods 12, no. 3: 513. https://doi.org/10.3390/foods12030513
APA StyleMoreira, A. S. P., Gonçalves, J., Sousa, F., Maia, I., Pereira, H., Silva, J., Coimbra, M. A., Ferreira, P., & Nunes, C. (2023). Potential of Coccolithophore Microalgae as Fillers in Starch-Based Films for Active and Sustainable Food Packaging. Foods, 12(3), 513. https://doi.org/10.3390/foods12030513