Evaluation of Dynamic Changes and Regularity of Volatile Flavor Compounds for Different Green Plum (Prunus mume Sieb. et Zucc) Varieties during the Ripening Process by HS-GC–IMS with PLS-DA
Abstract
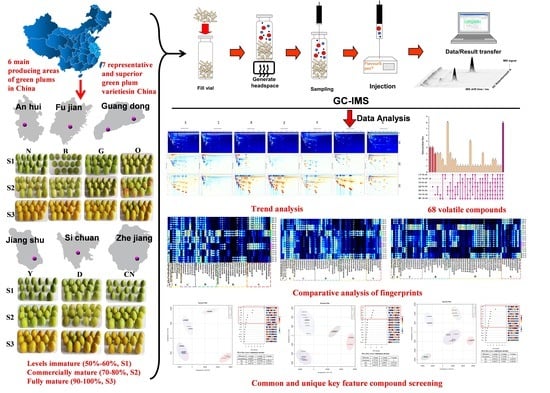
:1. Introduction
2. Materials and Methods
2.1. Samples and Preparation
2.2. HS-GC–IMS Analytical Methods
2.3. Statistical Analysis
3. Results and Discussion
3.1. HS-GC–IMS Visual Topographic Plot Comparison
3.2. Identification of Substances
3.3. Analysis of VOC Fingerprints
3.4. Analysis of Dynamic Changes and Formation Regularity of VOCs
3.5. PLS-DA-Based Fingerprint
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gong, X.P.; Tang, Y.; Song, Y.Y.; Du, G.; Li, J. Comprehensive Review of Phytochemical Constituents, Pharmacological Properties, and Clinical Applications of Prunus mume. Front. Pharmacol. 2021, 12, 679378. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.L.; Wu, C.D.; Huang, J.; Zhou, R.Q.; Jin, Y. Characteristics and Valorization Potential of Fermentation Waste of Greengage (Prunus mume). Appl. Sci. 2021, 11, 8296. [Google Scholar] [CrossRef]
- Tian, T.T.; Cao, H.; Farag, M.A.; Fan, S.T.; Liu, L.X.; Yang, W.J.; Wang, Y.X.; Zou, L.; Cheng, K.W.; Wang, M.F.; et al. Current and potential trends in the bioactive properties and health benefits of Prunus mume Sieb. Et Zucc: A comprehensive review for value maximization. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.T.; Li, W.; Sun, Y.N.; Yang, S.Y.; Lee, S.H.; Chen, J.B.; Jang, H.D.; Kim, Y.H. Identification and biological evaluation of flavonoids from the fruits of Prunus mume. Bioorganic Med. Chem. Lett. 2014, 24, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, Y.P.; Chen, Y.Y.; Meng, A.L.; Liu, P.; Ye, K.Y.; Yuan, A.Q. Effect of Different Sterilization Methods on the Microbial and Physicochemical Changes in Prunus mume Juice during Storage. Molecules 2022, 27, 1197. [Google Scholar] [CrossRef]
- Liu, H.; An, K.; Su, S.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Aromatic Characterization of Mangoes (Mangifera indica L.) Using Solid Phase Extraction Coupled with Gas Chromatography-Mass Spectrometry and Olfactometry and Sensory Analyses. Foods 2020, 9, 75. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Ni, Z.J.; Shi, T.; Tao, R.; Yang, Q.; Luo, C.G.; Li, Y.P.; Li, H.T.; Gao, H.D.; Zhou, X.Y.; et al. Novel insights into the dissemination route of Japanese apricot (Prunus mume Sieb. et Zucc.) based on genomics. Plant J. 2022, 110, 1182–1197. [Google Scholar] [CrossRef]
- Du, D.; Xu, M.; Wang, J.; Gu, S.; Zhu, L.; Hong, X. Tracing internal quality and aroma of a red-fleshed kiwifruit during ripening by means of GC-MS and E-nose. Rsc Adv. 2019, 9, 21164–21174. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Feng, C.; Li, W.; Qu, Z.; Zeng, M.; Xi, W. Transcriptional regulatory networks controlling taste and aroma quality of apricot (Prunus armeniaca L.) fruit during ripening. BMC Genom. 2019, 20, 45. [Google Scholar] [CrossRef] [Green Version]
- Endo, H.; Ose, K.; Bai, J.; Imahori, Y. Effect of hot water treatment on chilling injury incidence and antioxidative responses of mature green mume (Prunus mume) fruit during low temperature storage. Sci. Hortic. 2019, 246, 550–556. [Google Scholar] [CrossRef]
- Lin, J.Y.; Wu, Y.J.; Hsiao, Y.T.; Wang, C.Y. Acceleration of the ageing process in mei (Prunus mume Siebold) liqueur by high hydrostatic pressure treatment. Int. J. Food Sci. Technol. 2021, 56, 2030–2040. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Zhu, H.; Zhang, H.; Xu, J.; Fu, Q.; Bao, M.; Zhang, J. Headspace Volatiles and Endogenous Extracts of Prunus mume Cultivars with Different Aroma Types. Molecules 2021, 26, 7256. [Google Scholar] [CrossRef]
- An, K.; Liu, H.; Fu, M.; Qian, M.C.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Identification of the cooked off-flavor in heat-sterilized lychee (Litchi chinensis Sonn.) juice by means of molecular sensory science. Food Chem. 2019, 301, 125282. [Google Scholar] [CrossRef] [PubMed]
- Dursun, A.; Çalışkan, O.; Güler, Z.; Bayazit, S.; Türkmen, D.; Gündüz, K. Effect of harvest maturity on volatile compounds profiling and eating quality of hawthorn (Crataegus azarolus L.) fruit. Sci. Hortic. 2021, 288, 110398. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, L.; Xiao, Z.; Niu, Y. Characterization of the key aroma compounds in mulberry fruits by application of gas chromatography-olfactometry (GC-O), odor activity value (OAV), gas chromatography-mass spectrometry (GC-MS) and flame photometric detection (FPD). Food Chem. 2018, 245, 775–785. [Google Scholar] [CrossRef]
- Li, C.; Xin, M.; Li, L.; He, X.; Yi, P.; Tang, Y.; Li, J.; Zheng, F.; Liu, G.; Sheng, J.; et al. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HS-SPME-GC/MS and RNA sequencing. Food Chem. 2021, 355, 129685. [Google Scholar] [CrossRef]
- Hernandez-Mesa, M.; Ropartz, D.; Garcia-Campana, A.M.; Rogniaux, H.; Dervilly-Pinel, G.; Le Bizec, B. Ion Mobility Spectrometry in Food Analysis: Principles, Current Applications and Future Trends. Molecules 2019, 24, 2706. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Du, H.; Lin, S. Flavor Changes of Tricholoma matsutake Singer under Different Processing Conditions by Using HS-GC-IMS. Foods 2021, 10, 531. [Google Scholar] [CrossRef]
- Song, J.; Shao, Y.; Yan, Y.; Li, X.; Peng, J.; Guo, L. Characterization of volatile profiles of three colored quinoas based on GC-IMS and PCA. Lwt 2021, 146, 111292. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Wang, X.; Wang, R.; Ren, F.; Zhang, Q.; Shan, Y.; Ding, S. Characterization of Volatile Component Changes in Jujube Fruits during Cold Storage by Using Headspace-Gas Chromatography-Ion Mobility Spectrometry. Molecules 2019, 24, 3904. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, X.; Ma, Y.; Wang, Y.; Wang, D. Fingerprints and changes analysis of volatile compounds in fresh-cut yam during yellowing process by using HS-GC-IMS. Food Chem. 2022, 369, 130939. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- He, X.; Huang, Y.M.; Gorska-Horczyczak, E.; Wierzbicka, A.; Jelen, H.H. Rapid analysis of Baijiu volatile compounds fingerprint for their aroma and regional origin authenticity assessment. Food Chem. 2021, 337, 128002. [Google Scholar] [CrossRef]
- Song, X.B.; Wang, G.N.; Zhu, L.; Zheng, F.P.; Ji, J.; Sun, J.N.; Li, H.H.; Huang, M.Q.; Zhao, Q.Z.; Zhao, M.M.; et al. Comparison of two cooked vegetable aroma compounds, dimethyl disulfide and methional, in Chinese Baijiu by a sensory-guided approach and chemometrics. Lwt 2021, 146, 111427. [Google Scholar] [CrossRef]
- Pollo, B.J.; Teixeira, C.A.; Belinato, J.R.; Furlan, M.F.; Cunha, I.C.D.; Vaz, C.R.; Volpato, G.V.; Augusto, F. Chemometrics, Comprehensive Two-Dimensional gas chromatography and "omics" sciences: Basic tools and recent applications. Trac-Trends Anal. Chem. 2021, 134, 116111. [Google Scholar] [CrossRef]
- Rocchetti, G.; O'Callaghan, T.F. Application of metabolomics to assess milk quality and traceability. Curr. Opin. Food Sci. 2021, 40, 168–178. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Wu, J.; Wen, J.; Yu, Y.; An, K.; Zou, B. GC-IMS and olfactometry analysis on the tea aroma of Yingde black teas harvested in different seasons. Food Res. Int. 2021, 150, 110784. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Liao, L.; Qin, Y.; Jiang, L.; Liu, Y. Characteristic fingerprints and volatile flavor compound variations in Liuyang Douchi during fermentation via HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2021, 361, 130055. [Google Scholar] [CrossRef]
- Du, X.; Rouseff, R. Aroma active volatiles in four southern highbush blueberry cultivars determined by gas chromatography-olfactometry (GC-O) and gas chromatography-mass spectrometry (GC-MS). J. Agric. Food Chem. 2014, 62, 4537–4543. [Google Scholar] [CrossRef]
- Matheis, K.; Granvogl, M. Characterisation of the key aroma compounds in commercial native cold-pressed rapeseed oil by means of the Sensomics approach. Eur. Food Res. Technol. 2016, 242, 1565–1575. [Google Scholar] [CrossRef]
- Wang, S.; Chen, H.; Sun, B. Recent progress in food flavor analysis using gas chromatography-ion mobility spectrometry (GC-IMS). Food Chem. 2020, 315, 126158. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. J. Chromatogr. B 2018, 1099, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Shi, D.; Sun, J.; Li, A.; Sun, B.; Zhao, M.; Chen, F.; Sun, X.; Li, H.; Huang, M.; et al. Characterization of key aroma compounds in Gujinggong Chinese Baijiu by gas chromatography-olfactometry, quantitative measurements, and sensory evaluation. Food Res. Int. 2018, 105, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Mitalo, O.W.; Tokiwa, S.; Kondo, Y.; Otsuki, T.; Galis, I.; Suezawa, K.; Kataoka, I.; Doan, A.T.; Nakano, R.; Ushijima, K.; et al. Low Temperature Storage Stimulates Fruit Softening and Sugar Accumulation without Ethylene and Aroma Volatile Production in Kiwifruit. Front. Plant Sci. 2019, 10, 888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Wang, H.; Wang, Z.; Huang, P.; Kan, J. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2022, 375, 131671. [Google Scholar] [CrossRef]
- Wang, X.; Rogers, K.M.; Li, Y.; Yang, S.; Chen, L.; Zhou, J. Untargeted and Targeted Discrimination of Honey Collected by Apis cerana and Apis mellifera Based on Volatiles Using HS-GC-IMS and HS-SPME-GC-MS. J. Agric. Food Chem. 2019, 67, 12144–12152. [Google Scholar] [CrossRef]
- De Géa Neves, M.; Poppi, R.J.; Breitkreitz, M.C. Authentication of plant-based protein powders and classification of adulterants as whey, soy protein, and wheat using FT-NIR in tandem with OC-PLS and PLS-DA models. Food Control 2022, 132, 108489. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Du, W.; Jin, L.; Ren, P.; Ren, F.; Xie, J.C. Comparative analysis of aroma compounds in Chinese traditional dry-rendered fat by HS/GC-IMS, SPME/GC-MS, and SPME/GC-O. J. Food Compos. Anal. 2022, 107, 104378. [Google Scholar] [CrossRef]







| Count | Compounds | CAS# | Formula | MW | RI a | Rt b | Dt c | Identification Approach |
|---|---|---|---|---|---|---|---|---|
| Aldehydes | ||||||||
| A1 | (E)-2-nonenal | 18829-56-6 | C9H16O | 140.2 | 1167 | 901.514 | 1.40456 | RI, Dt |
| A2 | (E)-2-octenal | 2548-87-0 | C8H14O | 126.2 | 1035 | 655.16 | 1.82104 | RI, Dt |
| A3 | (E)-2-heptenal | 18829-55-5 | C7H12O | 112.2 | 954.3 | 504.555 | 1.25426 | RI, Dt |
| A4 | 3-methylbutanal | 590-86-3 | C5H10O | 86.1 | 658.7 | 173.618 | 1.41089 | RI, Dt |
| A5-1 | benzaldehyde M d | 100-52-7 | C7H6O | 106.1 | 955 | 505.944 | 1.46953 | RI, Dt |
| A5-2 | benzaldehyde D e | 100-52-7 | C7H6O | 106.1 | 955 | 505.944 | 1.14839 | RI, Dt |
| A6 | heptanal | 111-71-7 | C7H14O | 114.2 | 899.8 | 403.046 | 1.33324 | RI, Dt |
| A7 | hexanal | 66-25-1 | C6H12O | 100.2 | 790.4 | 273.455 | 1.25514 | RI, Dt |
| A8 | nonanal | 124-19-6 | C9H18O | 142.2 | 1106.1 | 787.759 | 1.47369 | RI, Dt |
| A9 | octanal | 124-13-0 | C8H16O | 128.2 | 1009.9 | 608.339 | 1.40676 | RI, Dt |
| A10-1 | pentanal D | 110-62-3 | C5H10O | 86.1 | 694.1 | 191.548 | 1.42316 | RI, Dt |
| A10-2 | pentanal M | 110-62-3 | C5H10O | 86.1 | 694.5 | 191.892 | 1.18296 | RI, Dt |
| A11 | phenylacetaldehyde | 122-78-1 | C8H8O | 120.2 | 991.4 | 573.768 | 1.53538 | RI, Dt |
| Alcohols | ||||||||
| B1 | (E)-2-hexen-1-ol | 928-95-0 | C6H12O | 100.2 | 902.3 | 407.734 | 1.51487 | RI, Dt |
| B2 | 1-butanol | 71-36-3 | C4H10O | 74.1 | 657 | 172.876 | 1.37972 | RI, Dt |
| B3 | (E)-3-hexen-1-ol | 928-97-2 | C6H12O | 100.2 | 864.6 | 357.634 | 1.52914 | RI, Dt |
| B4 | 1-octen-3-ol | 3391-86-4 | C8H16O | 128.2 | 982.5 | 557.24 | 1.15661 | RI, Dt |
| B5-1 | 1-pentanol D | 71-41-0 | C5H12O | 88.1 | 759.1 | 245.531 | 1.50921 | RI, Dt |
| B5-2 | 1-pentanol M | 71-41-0 | C5H12O | 88.1 | 761.6 | 247.643 | 1.25143 | RI, Dt |
| B6 | 1-propanol | 71-23-8 | C3H8O | 60.1 | 580.1 | 138.542 | 1.11011 | RI, Dt |
| B7 | 2-hexanol | 626-93-7 | C6H14O | 102.2 | 768.4 | 253.22 | 1.5696 | RI, Dt |
| B8 | 2-methyl-1-propanol | 78-83-1 | C4H10O | 74.1 | 621 | 156.782 | 1.36365 | RI, Dt |
| B9 | 2-hexen-1-ol | 2305-21-7 | C6H12O | 100.2 | 849.3 | 340.218 | 1.17979 | RI, Dt |
| B10 | 2-methylbutanol | 137-32-6 | C5H12O | 88.1 | 766.1 | 251.358 | 1.47465 | RI, Dt |
| B11 | 2-methylisoborneol | 2371-42-8 | C11H20O | 168.3 | 1171.1 | 909.235 | 1.26369 | RI, Dt |
| B12 | 3-methylbutan-1-ol | 123-51-3 | C5H12O | 88.1 | 731 | 222.234 | 1.48826 | RI, Dt |
| B13 | cumin alcohol | 536-60-7 | C10H14O | 150.2 | 1296 | 1142.267 | 1.32661 | RI, Dt |
| B14 | linalool | 78-70-6 | C10H18O | 154.3 | 1062.1 | 705.745 | 1.21707 | RI, Dt |
| B15-1 | maltol D | 118-71-8 | C6H6O3 | 126.1 | 1092.3 | 762.012 | 1.60266 | RI, Dt |
| B15-2 | maltol M | 118-71-8 | C6H6O3 | 126.1 | 1091.5 | 760.659 | 1.21276 | RI, Dt |
| B16-1 | n-hexanol D | 111-27-3 | C6H14O | 102.2 | 868 | 361.45 | 1.63935 | RI, Dt |
| B16-2 | n-hexanol M | 111-27-3 | C6H14O | 102.2 | 868.7 | 362.253 | 1.32543 | RI, Dt |
| Ketones | ||||||||
| C1 | 1-octen-3-one | 4312-99-6 | C8H14O | 126.2 | 979.5 | 551.692 | 1.2718 | RI, Dt |
| C2 | 2,3-pentanedione | 600-14-6 | C5H8O2 | 100.1 | 705.7 | 201.203 | 1.21544 | RI, Dt |
| C3-1 | 2-heptanone D | 110-43-0 | C7H14O | 114.2 | 893.1 | 390.601 | 1.63177 | RI, Dt |
| C3-2 | 2-heptanone M | 110-43-0 | C7H14O | 114.2 | 894.3 | 392.798 | 1.26173 | RI, Dt |
| C4-1 | 2-hexanone M | 591-78-6 | C6H12O | 100.2 | 784.3 | 266.503 | 1.18638 | RI, Dt |
| C4-2 | 2-hexanone D | 591-78-6 | C6H12O | 100.2 | 781.9 | 264.491 | 1.50278 | RI, Dt |
| C5-1 | 2-nonanone D | 821-55-6 | C9H18O | 142.2 | 1096.9 | 770.615 | 1.87878 | RI, Dt |
| C5-2 | 2-nonanone M | 821-55-6 | C9H18O | 142.2 | 1100.4 | 777.192 | 1.40774 | RI, Dt |
| C6-1 | 2-octanone D | 111-13-7 | C8H16O | 128.2 | 997.1 | 584.423 | 1.75543 | RI, Dt |
| C6-2 | 2-octanone M | 111-13-7 | C8H16O | 128.2 | 997.3 | 584.748 | 1.33435 | RI, Dt |
| C7 | 2-pentanone | 107-87-9 | C5H10O | 86.1 | 686.8 | 186.181 | 1.37047 | RI, Dt |
| C8 | 2-propanone | 67-64-1 | C3H6O | 58.1 | 520.3 | 111.855 | 1.1139 | RI, Dt |
| C9-1 | 3-octanone D | 106-68-3 | C8H16O | 128.2 | 990.7 | 572.555 | 1.71809 | RI, Dt |
| C9-2 | 3-octanone M | 106-68-3 | C8H16O | 128.2 | 991.2 | 573.358 | 1.30758 | RI, Dt |
| C10-1 | 3-pentanone D | 96-22-0 | C5H10O | 86.1 | 694 | 191.444 | 1.35447 | RI, Dt |
| C10-2 | 3-pentanone M | 96-22-0 | C5H10O | 86.1 | 696 | 193.097 | 1.10848 | RI, Dt |
| C11 | 6-methyl-5-hepten-2-one | 110-93-0 | C8H14O | 126.2 | 992.4 | 575.635 | 1.17731 | RI, Dt |
| C12 | cyclohexanone | 108-94-1 | C6H10O | 98.1 | 896.4 | 396.71 | 1.15243 | RI, Dt |
| Esters | ||||||||
| D1-1 | amyl acetate D | 628-63-7 | C7H14O2 | 130.2 | 916.1 | 433.442 | 1.764 | RI, Dt |
| D1-2 | amyl acetate M | 628-63-7 | C7H14O2 | 130.2 | 916.7 | 434.541 | 1.31285 | RI, Dt |
| D2-1 | benzyl acetate D | 140-11-4 | C9H10O2 | 150.2 | 1168.9 | 904.987 | 1.76778 | RI, Dt |
| D2-2 | benzyl acetate M | 140-11-4 | C9H10O2 | 150.2 | 1168.2 | 903.709 | 1.32639 | RI, Dt |
| D3 | butyl acetate | 123-86-4 | C6H12O2 | 116.2 | 804.6 | 289.538 | 1.61954 | RI, Dt |
| D4 | ethyl 2-methylbutyrate | 7452-79-1 | C7H14O2 | 130.2 | 844 | 334.251 | 1.65277 | RI, Dt |
| D5 | butyl butyrate | 109-21-7 | C8H16O2 | 144.2 | 1009.9 | 608.224 | 1.82457 | RI, Dt |
| D6-1 | butyl hexanoate D | 626-82-4 | C10H20O2 | 172.3 | 1209.6 | 980.988 | 2.05481 | RI, Dt |
| D6-2 | butyl hexanoate M | 626-82-4 | C10H20O2 | 172.3 | 1208.8 | 979.538 | 1.46908 | RI, Dt |
| D7-1 | butyl propanoate D | 590-01-2 | C7H14O2 | 130.2 | 909.3 | 420.78 | 1.72179 | RI, Dt |
| D7-2 | butyl propanoate M | 590-01-2 | C7H14O2 | 130.2 | 909.6 | 421.354 | 1.2867 | RI, Dt |
| D8 | ethyl acetate | 141-78-6 | C4H8O2 | 88.1 | 605.8 | 150.027 | 1.33841 | RI, Dt |
| D9 | ethyl butyrate | 105-54-4 | C6H12O2 | 116.2 | 793 | 276.356 | 1.56065 | RI, Dt |
| D10-1 | ethyl hexanoate D | 123-66-0 | C8H16O2 | 144.2 | 1009.2 | 607.007 | 1.79623 | RI, Dt |
| D10-2 | ethyl hexanoate M | 123-66-0 | C8H16O2 | 144.2 | 1011 | 610.302 | 1.34063 | RI, Dt |
| D11 | ethyl lactate | 97-64-3 | C5H10O3 | 118.1 | 844.9 | 335.177 | 1.53478 | RI, Dt |
| D12 | ethyl pentanoate | 539-82-2 | C7H14O2 | 130.2 | 901.3 | 405.905 | 1.68137 | RI, Dt |
| D13 | ethyl propanoate | 105-37-3 | C5H10O2 | 102.1 | 705.4 | 200.94 | 1.45255 | RI, Dt |
| D14 | ethyl-2-methylpropanoate | 97-62-1 | C6H12O2 | 116.2 | 750.6 | 238.502 | 1.56531 | RI, Dt |
| D15 | hexyl acetate | 142-92-7 | C8H16O2 | 144.2 | 1042.9 | 669.943 | 1.41084 | RI, Dt |
| D16-1 | hexyl butanoate D | 2639-63-6 | C10H20O2 | 172.3 | 1210.2 | 982.075 | 2.0827 | RI, Dt |
| D16-2 | hexyl butanoate M | 2639-63-6 | C10H20O2 | 172.3 | 1207.8 | 977.727 | 1.48683 | RI, Dt |
| D17 | isoamyl acetate | 123-92-2 | C7H14O2 | 130.2 | 877.3 | 371.926 | 1.74845 | RI, Dt |
| D18 | isobutyl acetate | 110-19-0 | C6H12O2 | 116.2 | 768.3 | 253.199 | 1.6155 | RI, Dt |
| D19 | methyl 3-methylbutanoate | 556-24-1 | C6H12O2 | 116.2 | 765.8 | 251.078 | 1.53391 | RI, Dt |
| D20 | methyl benzoate | 93-58-3 | C8H8O2 | 136.1 | 1054.9 | 692.207 | 1.20739 | RI, Dt |
| D21 | methyl salicylate | 119-36-8 | C8H8O3 | 152.1 | 1192.8 | 949.699 | 1.19979 | RI, Dt |
| D22-1 | n-propyl acetate D | 109-60-4 | C5H10O2 | 102.1 | 709 | 203.911 | 1.47959 | RI, Dt |
| D22-2 | n-propyl acetate M | 109-60-4 | C5H10O2 | 102.1 | 709.7 | 204.539 | 1.16613 | RI, Dt |
| D23 | propyl butyrate | 105-66-8 | C7H14O2 | 130.2 | 915.3 | 432.035 | 1.68986 | RI, Dt |
| Terpenes | ||||||||
| E1 | α-phellandrene | 99-83-2 | C10H16 | 136.2 | 993.6 | 577.939 | 1.21878 | RI, Dt |
| E2 | α-pinene | 80-56-8 | C10H16 | 136.2 | 974 | 541.289 | 1.21564 | RI, Dt |
| E3-1 | limonene D | 138-86-3 | C10H16 | 136.2 | 1033.9 | 653.05 | 1.29495 | RI, Dt |
| E3-2 | limonene M | 138-86-3 | C10H16 | 136.2 | 1034.4 | 653.972 | 1.21703 | RI, Dt |
| Others | ||||||||
| F1-1 | 2-methylbutanoic acid D | 116-53-0 | C5H10O2 | 102.1 | 844.5 | 334.793 | 1.47419 | RI, Dt |
| F1-2 | 2-methylbutanoic acid M | 116-53-0 | C5H10O2 | 102.1 | 846.8 | 337.429 | 1.20282 | RI, Dt |
| F2 | 2,4,5-trimethyl-thiazole | 13623-11-5 | C6H9NS | 127.2 | 955.3 | 506.511 | 1.56675 | RI, Dt |
| F3 | ethyl pyrazine | 13925-00-3 | C6H8N2 | 108.1 | 923.3 | 446.836 | 1.5138 | RI, Dt |
| Cultivar | Aldehydes | Alcohols | Ketones | Esters | Terpenes | Others | Total |
|---|---|---|---|---|---|---|---|
| Peak Intensity | |||||||
| BF S1 | 985.34 ± 86.33 | 3043.29 ± 184.72 | 5919.63 ± 398.66 | 8236.98 ± 313.37 | 1026.19 ± 98.42 | 61.57 ± 1.76 | 19,273 ± 1083.26 |
| BF S2 | 6674.48 ± 136.04 | 1200.21 ± 43.6 | 1385.02 ± 130.58 | 5229.23 ± 573.34 | 828.76 ± 216.49 | 918.07 ± 2.84 | 16,235.77 ± 1102.89 |
| BF S3 | 1361.29 ± 174.67 | 4641.56 ± 89.67 | 17,825.5 ± 552.55 | 59,926.1 ± 920.71 | 544.62 ± 84.93 | 80.63 ± 6.33 | 84,379.7 ± 1828.86 |
| QZ S1 | 2042.13 ± 373.82 | 2163.97 ± 137.18 | 4652.09 ± 372.82 | 10,210.29 ± 357.07 | 840.7 ± 97.3 | 96.2 ± 7.22 | 20,005.38 ± 1345.41 |
| QZ S2 | 6963.52 ± 247.39 | 1372.2 ± 95.86 | 1858.84 ± 114.55 | 10,271.08 ± 1394.71 | 1185.12 ± 19.29 | 564.42 ± 18.47 | 22,215.18 ± 1890.27 |
| QZ S3 | 5842.44 ± 132.97 | 1442.62 ± 88.76 | 1907.61 ± 81.35 | 13,676.02 ± 336.03 | 1030.24 ± 68.82 | 436.16 ± 13.98 | 24,335.09 ± 721.91 |
| YS S1 | 1995.28 ± 276.7 | 5195.08 ± 61.51 | 503.05 ± 18.55 | 114,268.68 ± 502.48 | 684.4 ± 127.63 | 63.62 ± 2.32 | 122,710.11 ± 989.19 |
| YS S2 | 1581.34 ± 56.45 | 5936.45 ± 70.78 | 788.69 ± 32.07 | 97,161.86 ± 524.26 | 708.84 ± 60.52 | 26.57 ± 3.09 | 106,203.75 ± 747.17 |
| YS S3 | 1766.35 ± 124.74 | 5548.3 ± 89.53 | 679.17 ± 42.73 | 165,322.85 ± 506.43 | 790.83 ± 11.66 | 151.64 ± 3.61 | 174,259.14 ± 778.7 |
| NG S1 | 458.45 ± 43.88 | 11,997.74 ± 251.77 | 3937.94 ± 479.05 | 15,616.84 ± 864.18 | 1398.19 ± 203.08 | 168.74 ± 12.13 | 33,577.9 ± 1854.09 |
| NG S2 | 326.46 ± 19.18 | 10,800.05 ± 115.55 | 3211.11 ± 239.55 | 11,227.39 ± 199.9 | 1188.26 ± 243.21 | 148.41 ± 30.08 | 26,901.68 ± 847.47 |
| NG S3 | 1707.45 ± 124.88 | 6174.68 ± 209.84 | 10,698.35 ± 731.89 | 116,221.99 ± 509.95 | 919.1 ± 54.81 | 232.98 ± 5.02 | 135,954.55 ± 1636.39 |
| DZ S1 | 821.23 ± 100.06 | 13,209.91 ± 113.87 | 1082.07 ± 22.78 | 10,511.61 ± 410.34 | 992.94 ± 10.09 | 63.6 ± 18.15 | 26,681.36 ± 675.29 |
| DZ S2 | 9955.28 ± 396.93 | 1947.84 ± 476.79 | 659.41 ± 28.73 | 11,251.03 ± 690.35 | 1163.73 ± 142 | 1071.24 ± 166.37 | 26,048.53 ± 1901.17 |
| DZ S3 | 1837.69 ± 564.91 | 7150.64 ± 401.4 | 1168.35 ± 80.86 | 110,524.83 ± 1369.02 | 774.07 ± 33.61 | 224.84 ± 65.65 | 121,680.42 ± 2515.45 |
| CN S1 | 139.45 ± 47.07 | 14,392.84 ± 122.98 | 1217.91 ± 112.41 | 5936.16 ± 255.15 | 1181.34 ± 133.07 | 532.22 ± 68.14 | 23,399.92 ± 738.82 |
| CN S2 | 113.13 ± 20.69 | 9834.38 ± 50.79 | 2028.3 ± 272.07 | 2011.28 ± 69.95 | 939.72 ± 16.36 | 303.05 ± 69.78 | 15,229.86 ± 499.64 |
| CN S3 | 72.01 ± 6.57 | 9786.64 ± 22.18 | 2827.46 ± 55.88 | 10,245.83 ± 32.53 | 713.37 ± 20.72 | 185.3 ± 14.54 | 23,830.61 ± 152.42 |
| GC S1 | 1679.03 ± 239.49 | 3989.75 ± 186.25 | 878.52 ± 44.99 | 82,941.45 ± 293.88 | 1276.94 ± 97.44 | 0 | 90,765.69 ± 862.05 |
| GC S2 | 1093.02 ± 65.17 | 2142.51 ± 68.9 | 328.1 ± 14.1 | 51,224.58 ± 427.59 | 1253.39 ± 7.97 | 0 | 56,041.6 ± 583.73 |
| GC S3 | 2421.12 ± 431.3 | 4907.02 ± 419.92 | 1035.85 ± 15.24 | 119,377.73 ± 836.67 | 1238.32 ± 112.33 | 0 | 128,980.04 ± 1815.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Yu, Y.; Zou, B.; Yu, Y.; Yang, J.; Xu, Y.; Chen, X.; Yang, F. Evaluation of Dynamic Changes and Regularity of Volatile Flavor Compounds for Different Green Plum (Prunus mume Sieb. et Zucc) Varieties during the Ripening Process by HS-GC–IMS with PLS-DA. Foods 2023, 12, 551. https://doi.org/10.3390/foods12030551
Liu H, Yu Y, Zou B, Yu Y, Yang J, Xu Y, Chen X, Yang F. Evaluation of Dynamic Changes and Regularity of Volatile Flavor Compounds for Different Green Plum (Prunus mume Sieb. et Zucc) Varieties during the Ripening Process by HS-GC–IMS with PLS-DA. Foods. 2023; 12(3):551. https://doi.org/10.3390/foods12030551
Chicago/Turabian StyleLiu, Haocheng, Yuanshan Yu, Bo Zou, Yangyang Yu, Jiguo Yang, Yujuan Xu, Xiaowei Chen, and Fan Yang. 2023. "Evaluation of Dynamic Changes and Regularity of Volatile Flavor Compounds for Different Green Plum (Prunus mume Sieb. et Zucc) Varieties during the Ripening Process by HS-GC–IMS with PLS-DA" Foods 12, no. 3: 551. https://doi.org/10.3390/foods12030551
APA StyleLiu, H., Yu, Y., Zou, B., Yu, Y., Yang, J., Xu, Y., Chen, X., & Yang, F. (2023). Evaluation of Dynamic Changes and Regularity of Volatile Flavor Compounds for Different Green Plum (Prunus mume Sieb. et Zucc) Varieties during the Ripening Process by HS-GC–IMS with PLS-DA. Foods, 12(3), 551. https://doi.org/10.3390/foods12030551