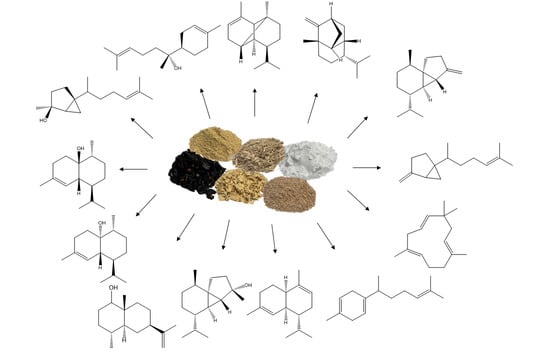
Impact of Agro-Industrial Side-Streams on Sesquiterpene Production by Submerged Cultured Cerrena unicolor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cultivation Conditions
2.3. Extraction of Volatiles
2.4. GOPOD-Assay, Determination of D-Glucose Concentration
2.5. GC-MS Analysis
2.6. Lipid Content Determination
2.7. Fatty Acids Profiling
2.8. Protein Content Determination
3. Results and Discussion
3.1. Formation Kinetics in Standard Nutrient Liquid Medium (SNL)
3.2. Impact of Food and Agro-Industrial Side-Streams
3.3. Impact of Rapeseed Press Cake and Lipidic Supplements
3.4. Labeling Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef]
- Kramer, R.; Abraham, W.-R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar] [CrossRef]
- De Bruyne, M.; Baker, T.C. Odor detection in insects: Volatile codes. J. Chem. Ecol. 2008, 34, 882–897. [Google Scholar] [CrossRef]
- Wu, J.; Kawagishi, H. Plant growth regulators from mushrooms. J. Antibiot. 2020, 73, 657–665. [Google Scholar] [CrossRef]
- Jischa, S.; Sabu, K.; Manjula, S. Multifunctional aspects of Piriformospora indica in plant endosymbiosis. Mycology 2019, 10, 182–190. [Google Scholar] [CrossRef]
- Sprecher, E. Über ätherisches Öl aus Pilzen. Planta Med. 1963, 11, 119–127. [Google Scholar] [CrossRef]
- Abraham, B.G.; Berger, R.G. Higher fungi for generating aroma components through novel biotechnologies. J. Agric Food Chem. 1994, 42, 2344–2348. [Google Scholar] [CrossRef]
- Leonhardt, R.H.; Berger, R.G. Nootkatone. Adv. Biochem. Eng. Biotechnol. 2015, 148, 391–404. [Google Scholar] [CrossRef]
- Grosse, M.; Strauss, E.; Krings, U.; Berger, R.G. Response of the sesquiterpene synthesis in submerged cultures of the Basidiomycete Tyromyces floriformis to the medium composition. Mycologia 2019, 111, 885–894. [Google Scholar] [CrossRef]
- Ohloff, G.; Pickenhagen, W.; Kraft, P. Scent and Chemistry: The Molecular World of Odors; Verl. Helvetica Chimica Acta; Wiley-VCH.: Zurich, Switzerland; Weinheim, Germany, 2012. [Google Scholar]
- Shu, H.Z.; Peng, C.; Bu, L.; Guo, L.; Liu, F.; Xiong, L. Bisabolane-type sesquiterpenoids: Structural diversity and biological activity. Phytochem 2021, 192, 112927. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chiang, C.C.; Chang, Y.S.; Chen, J.J.; Wang, W.H.; Fang, L.S.; Chung, H.M.; Hwang, T.L.; Sung, P.J. Novel Caryophyllane-Related Sesquiterpenoids with Anti-Inflammatory Activity from Rumphella antipathes (Linnaeus, 1758). Mar. Drugs. 2020, 18, 554. [Google Scholar] [CrossRef]
- Tamemoto, K.; Takaishi, Y.; Chen, B.; Kawazoe, K.; Shibata, H.; Higuti, T.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; et al. Sesquiterpenoids from the fruits of Ferula kuhistanica and antibacterial activity of the constituents of F. kuhistanica. Phytochem 2001, 58, 763–767. [Google Scholar] [CrossRef]
- Jang, H.J.; Lee, S.; Lee, S.J.; Lim, H.J.; Jung, K.; Kim, Y.H.; Lee, S.W.; Rho, M.C. Anti-inflammatory Activity of Eudesmane-Type Sesquiterpenoids from Salvia plebeia. J. Nat. Prod. 2017, 80, 2666–2676. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.S.; Yang, B.; Lv, G.P.; Li, S.P. Free radical scavenging activity and characterization of sesquiterpenoids in four species of Curcuma using a TLC bioautography assay and GC-MS analysis. Molecules 2010, 15, 7547–7557. [Google Scholar] [CrossRef]
- Liu, N.; Wu, C.; Yu, J.H.; Zhu, K.K.; Song, M.N.; Yang, F.Y.; Feng, R.L.; Zhang, Y.Y.; Chang, W.Q.; Zhang, H. Germacrane-type sesquiterpenoids with cytotoxic activity from Sigesbeckia orientalis. Bioorg. Chem. 2019, 92, 103196. [Google Scholar] [CrossRef]
- Ziaja-Sołtys, M.; Kołodziej, P.; Stefaniuk, D.; Matuszewska, A.; Jaszek, M.; Bogucka-Kocka, A. Low-Molecular-Weight Secondary Metabolites from Fungi: Cerrena unicolor as a New Proposal of an Effective Preparation against Rhabditis Nematodes. Molecules 2022, 27, 1660. [Google Scholar] [CrossRef]
- Mizerska-Dudka, M.; Jaszek, M.; Błachowicz, A.; Rejczak, T.P.; Matuszewska, A.; Osińska-Jaroszuk, M.; Stefaniuk, D.; Janusz, G.; Sulej, J.; Kandefer-Szerszeń, M. Fungus Cerrena unicolor as an effective source of new antiviral, immunomodulatory, and anticancer compounds. Int. J. Biol. Macromol. 2015, 79, 459–468. [Google Scholar] [CrossRef]
- Michniewicz, A.; Ullrich, R.; Ledakowicz, S.; Hofrichter, M. The white-rot fungus Cerrena unicolor strain 137 produces two laccase isoforms with different physico-chemical and catalytic properties. Appl. Microbiol. Biotechnol. 2006, 69, 682–688. [Google Scholar] [CrossRef]
- Belova, O.V.; Lisov, A.V.; Vinokurova, N.G.; Kostenevich, A.A.; Sapunova, L.I.; Lobanok, A.G.; Leont’evskiĭ, A.A. Xylanase and cellulase of fungus Cerrena unicolor VKM F-3196: Production, properties, and applications for the saccharification of plant material. Prikl. Biokhim. Mikrobiol. 2014, 50, 171–176. [Google Scholar] [CrossRef]
- Kachlishvili, E.; Jokharidze, T.; Kobakhidze, A.; Elisashvili, V. Enhancement of laccase production by Cerrena unicolor through fungal interspecies interaction and optimum conditions determination. Arch. Microbiol. 2021, 203, 3905–3917. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E.; Bakradze, M. Dependence of activities of polysaccharide hydrolases and oxidases from Cerrena unicolor on the source of carbon and aromatic acids in culture media. Prikl. Biokhim. Mikrobiol. 2002, 38, 243–247. [Google Scholar] [PubMed]
- Püth, N.E.F.; Krings, U.; Berger, R.G. Sesquiterpene Cyclases from the Basidiomycete Cerrena unicolor. Catalysts 2021, 11, 1361. [Google Scholar] [CrossRef]
- Strauss, E. Effektoren der Terpenbiosynthese bei Basidiomyceten in Submerskultur. Bachelor Thesis, Leibniz University Hanover, Hannover, Germany, 2018. [Google Scholar]
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef]
- Miller, D.J.; Allemann, R.K. Sesquiterpene synthases: Passive catalysts or active players? Nat. Prod. Rep. 2012, 29, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Orban, A.; Hennicke, F.; Rühl, M. Volatilomes of Cyclocybe aegerita during different stages of monokaryotic and dikaryotic fruiting. Biol. Chem. 2020, 401, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Ezediokpu, M.N.; Krause, K.; Kunert, M.; Hoffmeister, D.; Boland, W.; Kothe, E. Ectomycorrhizal Influence on the Dynamics of Sesquiterpene Release by Tricholoma vaccinum. J. Fungi. 2022, 8, 555. [Google Scholar] [CrossRef] [PubMed]
- Moreno-González, M.; Ottens, M. A Structured Approach to Recover Valuable Compounds from Agri-food Side Streams. Food Bioproc. Technol. 2021, 14, 1387–1406. [Google Scholar] [CrossRef]
- Di Lena, G.; Sanchez Del Pulgar, J.; Lombardi Boccia, G.; Casini, I.; Ferrari Nicoli, S. Corn Bioethanol Side Streams: A Potential Sustainable Source of Fat-Soluble Bioactive Molecules for High-Value Applications. Foods 2020, 9, 1788. [Google Scholar] [CrossRef]
- Bertacchi, S.; Pagliari, S.; Cantù, C.; Bruni, I.; Labra, M.; Branduardi, P. Enzymatic Hydrolysate of Cinnamon Waste Material as Feedstock for the Microbial Production of Carotenoids. Int. J. Env. Res. Public Health 2021, 18, 1146. [Google Scholar] [CrossRef]
- Schimanski, S.; Krings, U.; Berger, R.G. Rapid analysis of volatiles in fat-containing matrices for monitoring bioprocesses. Eur. Food Res. Technol. 2013, 237, 739–746. [Google Scholar] [CrossRef]
- Sampedro, M.C.; Goicolea, M.A.; Unceta, N.; Sánchez-Ortega, A.; Barrio, R.J. Sequential stir bar extraction, thermal desorption and retention time locked GC-MS for determination of pesticides in water. J. Sep. Sci. 2009, 32, 3449–3456. [Google Scholar] [CrossRef]
- Mischko, W.; Hirte, M.; Fuchs, M.; Mehlmer, N.; Brück, T.B. Identification of sesquiterpene synthases from the Basidiomycota Coniophora puteana for the efficient and highly selective β-copaene and cubebol production in E. coli. Microb. Cell Factories 2018, 17, 164. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, X.; Duan, Y.; Wang, P.; Qi, J.; Gao, J.M.; Liu, C. Biosynthesis of Sesquiterpenes in Basidiomycetes: A Review. J. Fungi 2022, 8, 913. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.L.; Daramwar, P.P.; Krithika, R.; Pandreka, A.; Shankar, S.S.; Thulasiram, H.V. Functional Characterization of Novel Sesquiterpene Synthases from Indian Sandalwood, Santalum album. Sci. Rep. 2015, 5, 10095. [Google Scholar] [CrossRef]
- Calvo, A.M.; Wilson, R.A.; Bok, J.W.; Keller, N.P. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 2002, 66, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, H.; Ukeba, S.; Kitaoka, T. Latent potentials of the white-rot basidiomycete Phanerochaete chrysosporium responsible for sesquiterpene metabolism: CYP5158A1 and CYP5144C8 decorate (E)-α-bisabolene. Enzym. Microb Technol. 2022, 158, 110037. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Feng, T.; Li, M.; Zhang, X.; He, J.; Hu, B.; Deng, Z.; Liu, T.; Liu, J.K.; Wang, X.; et al. Characterization of Tremulane Sesquiterpene Synthase from the Basidiomycete Irpex lacteus. Org. Lett. 2022, 24, 5669–5673. [Google Scholar] [CrossRef] [PubMed]
- Schüffler, A. Secondary Metabolites of Basidiomycetes. In Physiology and Genetics.The Mycota; Anke, T., Schüffler, A., Eds.; Springer: Cham, Germany, 2018; Volume 15, pp. 231–275. [Google Scholar]
- Östbring, K.; Malmqvist, E.; Nilsson, K.; Rosenlind, I.; Rayner, M. The Effects of Oil Extraction Methods on Recovery Yield and Emulsifying Properties of Proteins from Rapeseed Meal and Press Cake. Foods 2019, 9, 19. [Google Scholar] [CrossRef]
- Andreas Fetzer, T.H.; Stäbler, A.; Menner, M.; Eisner, P. Influence of process conditions during aqueous protein extraction upon yield from pre-pressed and cold-pressed rapeseed press cake. Ind. Crop. Prod. 2018, 112, 236–246. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, M.; Kim, S.H.; Hong, C.Y.; Ryu, S.H.; Choi, I.G. Transcriptomic analysis of the white rot fungus Polyporus brumalis provides insight into sesquiterpene biosynthesis. Microbiol. Res. 2016, 182, 141–149. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, S.; Chowreddy, R.R.; Irmak, S.; Honkapää, K.; Isom, L. Food Industry Co-streams: Potential Raw Materials for Biodegradable Mulch Film Applications. J. Polym. Env. 2017, 25, 1110–1130. [Google Scholar] [CrossRef]
- Piotr Kaczmarek, D.K.; Lipiński, K.; Mazur, M. Chemical Composition of Rapeseed Products and Their Use in Pig Nutrition. Pol. J. Natur. Sc. 2016, 31, 545–562. [Google Scholar]
- Lewinska, A.Z.J.; Duda, M.; Gorka, A.; Wnuk, M. Fatty Acid Profile and Biological Activities of Linseed and Rapeseed Oils. Molecules 2015, 20, 22872–22880. [Google Scholar] [CrossRef]
- Shimizu, M.; Yuda, N.; Nakamura, T.; Tanaka, H.; Wariishi, H. Metabolic regulation at the tricarboxylic acid and glyoxylate cycles of the lignin-degrading basidiomycete Phanerochaete chrysosporium against exogenous addition of vanillin. Proteomics 2005, 5, 3919–3931. [Google Scholar] [CrossRef]
- Balaeș, T.T.C. Basidiomycetes as potential biocontrol agents against nematodes. Rom. Biotechnol. Let. 2016, 21, 11185–11193. [Google Scholar]
- Quin, M.B.; Flynn, C.M.; Schmidt-Dannert, C. Traversing the fungal terpenome. Nat. Prod. Rep. 2014, 31, 1449–1473. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, R.; Zhang, Y.; Qi, P.; Wang, L.; Fang, S. Biosynthesis and regulation of terpenoids from basidiomycetes: Exploration of new research. AMB Express 2021, 11, 150. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E. Physiological regulation of laccase and manganese peroxidase production by white-rot Basidiomycetes. J. Biotechnol 2009, 144, 37–42. [Google Scholar] [CrossRef]
- Strong, P.J.; Self, R.; Allikian, K.; Szewczyk, E.; Speight, R.; O’Hara, I.; Harrison, M.D. Filamentous fungi for future functional food and feed. Curr. Opin. Biotechnol. 2022, 76, 102729. [Google Scholar] [CrossRef] [PubMed]
- Román, S.; Sánchez-Siles, L.M.; Siegrist, M. The importance of food naturalness for consumers: Results of a systematic review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]







| Clade | Substance | RI Polar a (b) | RI Non-Polar a (b) | Identification |
|---|---|---|---|---|
| II | α-Copaene | 1462 (1502) | 1349 (1375) | A, B, C, E |
| I | Isosativene | 1492 | 1390 (1417) | B, C |
| I | β-Cubebene | 1544 (1540) | 1390 (1388) | A, B, C, E |
| III | Sesquisabinene | 1663 (1648) | 1454 (1465) | A, B, C, E |
| IV | Humulene | 1693 (1670) | 1460 (1455) | A, B, C, E |
| II | α-Muurolene | 1729 (1727) | 1501 (1499) | A, B, C, E |
| III | β-Curcumene | 1743 (1734) | 1510 (1516) | A, B, C |
| II | Cubebol | 1956 (1951) | 1521 (1531) | A, B, C, E |
| II | 5β,7βH,10α-Eudesm-11-en-1α-ol | 2001 | 1544 | C |
| II | Cubenol | 2074 (2063) | 1641 (1642) | A, B, C, E |
| II | Epicubenol | 2083 (2072) | 1635 (1625) | A, B, C |
| III | Sesquisabinene hydrate | 2114 (2092) | 1596 (1581) | A, B, C |
| III | α-Bisabolol | 2223 (2232) | 1694 (1697) | A, B, C, D, E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Püth, N.; Ersoy, F.; Berger, R.G.; Krings, U. Impact of Agro-Industrial Side-Streams on Sesquiterpene Production by Submerged Cultured Cerrena unicolor. Foods 2023, 12, 668. https://doi.org/10.3390/foods12030668
Püth N, Ersoy F, Berger RG, Krings U. Impact of Agro-Industrial Side-Streams on Sesquiterpene Production by Submerged Cultured Cerrena unicolor. Foods. 2023; 12(3):668. https://doi.org/10.3390/foods12030668
Chicago/Turabian StylePüth, Nils, Franziska Ersoy, Ralf G. Berger, and Ulrich Krings. 2023. "Impact of Agro-Industrial Side-Streams on Sesquiterpene Production by Submerged Cultured Cerrena unicolor" Foods 12, no. 3: 668. https://doi.org/10.3390/foods12030668
APA StylePüth, N., Ersoy, F., Berger, R. G., & Krings, U. (2023). Impact of Agro-Industrial Side-Streams on Sesquiterpene Production by Submerged Cultured Cerrena unicolor. Foods, 12(3), 668. https://doi.org/10.3390/foods12030668