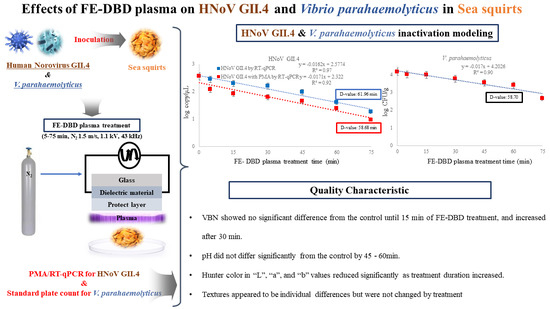
Inactivation of Human Norovirus GII.4 and Vibrio parahaemolyticus in the Sea Squirt (Halocynthia roretzi) by Floating Electrode-Dielectric Barrier Discharge Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. HNoV GII.4 Preparation
2.2. Vibrio parahaemolyticus Preparation
2.3. FE-DBD Plasma Treatment of HNoV GII.4 and Vibrio parahaemolyticus in Sea Squirt
2.4. Propidium Monoazide (PMA) Treatment in HNoV GII.4
2.5. RNA Extraction
2.6. Quantitative Analysis of HNoV GII.4 Infectivity by RT-qPCR
2.7. Quantitative Analysis of V. parahaemolyticus by Standard Plate Count
2.8. Volatile Basic Nitrogen (VBN)
2.9. Hunter Color and pH
2.10. Texture
2.11. Statistical Analysis
3. Results
3.1. Reduction of HNoV GII.4
3.2. Reduction of V. parahaemolyticus
3.3. Effect of DBD Plasma Treatment on D1 Values of HNoV GII.4, HNoV GII.4 with PMA and V. parahaemolyticus in Sea Squirt
3.4. Effect of FE-DBD Plasma on VBN and pH of Sea Squirt
3.5. Effect of FE-DBD Plasma on Hunter Color of Sea Squirt
3.6. Effect of FE-DBD Plasma on Texture of Sea Squirt
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Watanabe, K.; Uehara, H.; Sato, M.; Konosu, S. Seasonal variation of extractive nitrogenous constituents in the muscle of the ascidian Halocynthia roretzi. Bull. Japan. Soc. Sci. Fish. 1985, 51, 1293–1298. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Cho, H.S.; Lee, D.H.; Ryuk, J.H.; Cho, Y.J.; Suh, J.S.; Kim, D.S. Utilization of ascidian, Halocynthia roretzi. 5. Processing and quality evaluation of fermented ascidian (I). Bull. Korean Fish. Soc. 1993, 26, 221–229. [Google Scholar]
- Kim, J.H.; Kim, M.J.; Lee, J.S.; Kim, K.H.; Kim, H.J.; Heu, M.S.; Kim, J.S. Development and characterization of sea squirt Halocynthia roretzi sikhae. Korean J. Fish. Aquat. Sci. 2013, 46, 27–36. [Google Scholar] [CrossRef]
- Hwang, D.W.; Hwang, H.; Lee, G.; Kim, S.; Park, S.; Yoon, S.P. Organic matter and heavy metals pollution assessment of surface sediment from a fish farming area in Tongyoung-Geoje coast of Korea. J. Korean Soc. Mar. Environ. Saf. 2021, 27, 510–520. [Google Scholar] [CrossRef]
- Lee, M.H.; Seo, D.J.; Seo, J.N.; Jeon, S.B.; Oh, H.J.; Lee, J.S.; Joo, I.S.; Lee, H.J.; Choi, C.S. Detection of norovirus using molecular biological method. Safe Food. 2014, 9, 26–32. [Google Scholar]
- Park, K.; Park, Y.S.; Kwon, J.Y.; Yu, H.S.; Lee, H.J.; Kim, J.H.; Lee, T.S.; Kim, P.H. Effect of heat treatment on male specific coliphage and norovirus concentrations in norovirus contaminated oyster Crassostrea gigas. Korean. J. Fish. Aquat. Sci. 2015, 48, 898–903. [Google Scholar] [CrossRef]
- Sala, M.R.; Arias, C.; Mominguez, A.; Bartolome, R.; Muntada, J.M. Foodborne outbreak of gastroenteritis due to Norovirus and Vibrio parahaemolyticus. Epidemiol. Infect. 2009, 137, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Alfano-Sosbey, E.; Sweat, D.; Hall, A.; Breedlove, F.; Rodríguez, R.; Greene, S.; Pierce, A.; Sobsey, M.; Davies, M.; Ledford, S.I. Norovirus outbreak associated with undercooked oysters and secondary household transmission. Epidemiol. Infect. 2012, 140, 276e282. [Google Scholar] [CrossRef] [Green Version]
- Karagiannis, L.; Detsis, M.; Gkolfinopoulou, K.; Pervanidou, D.; Panagiotopoulos, T.; Bonovas, S. An outbreak of gastroenteritis linked to seafood consumption in a remote Northern Aegean island, February-March 2010. Rural Remote Health 2010, 10, 53–59. [Google Scholar] [CrossRef]
- Zhang, L.; Orth, K. Virulence determinants for Vibrio parahaemolyticus infection. Curr. Opin Microbiol. 2013, 16, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Padovan, A.; Kennedy, K.; Rose, D.; Gibb, K. Microbial quality of wild shellfish in a tropical estuary subject to treated effluent discharge. Environ. Res. 2020, 181, 108921. [Google Scholar] [CrossRef] [PubMed]
- Rince, A.; Ballere, C.; Hervio-Heath, D.; Cozien, J.; Lozach, S.; Parnaudeau, S.; Le Guyader, F.S.; Le Hello, S.; Giard, J.C.; Sauvageot, N.; et al. Occurrence of bacterial pathogens and human noroviruses in shellfish-harvesting areas and their catchments in France. Front. Microbiol. 2018, 9, 2443. [Google Scholar] [CrossRef] [PubMed]
- Suffredini, E.; Mioni, R.; Mazzette, R.; Bordin, P.; Serratore, P.; Fois, F.; Piano, A.; Cozzi, L.; Croci, L. Detection and quantification of Vibrio parahaemolyticus in shellfish Italian production areas. Int. J. Food Microbiol. 2014, 184, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Lee, J.; Oh, H.; Shin, I.S.; Kim, Y.M.; Park, K.S.; Yoon, Y. Quantitative microbial risk assessment of pathogenic Vibrio through sea squirt consumption in Korea. J. Food Hyg. Saf. 2020, 35, 51–59. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med. Oncol. 2018, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Patil, S.; Boehm, D.; Milosavljevic, V.; Cullen, P.J.; Bourke, P. Mechanisms of inactivation by high-voltage atmospheric cold plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2015, 82, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Lopez, M.; Calvo, T.; Prieto, M.; Mugica-Vidal, R.; Muro-Fragus, L.; Alba-Elias, F.; Alvarez-Ordonez, A. A Review on non-thermal atmospheric plasma for food preservation: Mode of action, determinants of effectiveness, and applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, H.J.; Park, S.; Alahakoon, A.U.; Kim, K.; Choe, W.; Jo, C. Evaluation of pathogen inactivation on sliced cheese induced by encapsulated atmospheric pressure dielectric barrier discharge plasma. Food Microbiol. 2015, 46, 46–50. [Google Scholar] [CrossRef]
- Kim, K.Y.; Paik, N.W.; Kim, Y.H.; Yoo, K.H. Bactericidal efficacy of non-thermal DBD plasma on Staphylococcus aureus and Escherichia coli. J. Korean Soc. Occup. Environ. Hyg. 2018, 28, 61–79. [Google Scholar] [CrossRef]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Brooks, A.D. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents Chemother. 2011, 55, 1053–1062. [Google Scholar] [CrossRef] [Green Version]
- Jeon, E.; Choi, M.S.; Kim, J.; Choi, E.; Lim, J.; Choi, J.; Ha, K.; Kwon, J.; Jeong, S.; Park, S. Assessment of potential infectivity of human norovirus in the traditional Korean salted clam product “Jogaejeotgal” by floating electrode-dielectric barrier discharge plasma. Int. Food Res. J. 2021, 141, 110107. [Google Scholar] [CrossRef]
- Abdi, S.; Hossein, A.; Moselehishad, M.; Dorranian, D. Decontamination of red pepper using cold atmospheric prfessure plasma as alternative technique. Appl. Food Biotechnol. 2019, 6, 247–254. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation mechanisms of non-thermal plasma on microbes: A review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly reactive and highly sensitive assay for norwalk-like viruses based on real-time quantitative reverse transcription-pcr. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef] [Green Version]
- Miao, L.; Walton, W.C.; Wang, L.; Li, L.; Wang, Y. Characterization of polylactic acids-polyhydroxybutyrate based packaging film with fennel oil, and its application on oysters. Food Packag. Shelf Life 2019, 22, 100388. [Google Scholar] [CrossRef]
- Zhang, J.; Walton, W.C.; Wang, Y. Quantitative quality evaluation of eastern oyster (Crassostrea virginica) cultured by two different methods. Aquac. Res. 2017, 48, 2934–2944. [Google Scholar] [CrossRef]
- Lester, S.E.; Gentry, R.R.; Kappel, C.V.; Gaines, S.D. Offshore aquaculture in the United States: Untapped potential in need of smart policy. Proc. Natl. Acad. Sci. USA 2018, 115, 7162–7165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; Bae, Y.M.; Oh, S.W.; Lee, S.Y. Current microbiological safety of sliced raw fish. J. Food Hyg. Saf. 2016, 11, 3–9. [Google Scholar]
- Kwon, R.W.; Shin, M.C.; Hwang, S.W.; Kim, D.H.; Lee, S.H.; Park, J.H.; Kim, J.G. Processing and quality characteristic of high value-added low-salt fermented sea squirt Halocynthia roretzi with yujacheong. J. Korean Soc. Fish. Mar. Educ. 2021, 33, 1013–1026. [Google Scholar] [CrossRef]
- Shin, S.; Oh, E.G.; Lee, H.J.; Kim, Y.; Lee, T.; Kim, J.H. Norovirus quantification in oyster Crassostrea gigas collected from Tongyeoung, Korea. Korean J. Fish. Aquat. Sci. 2014, 47, 501–507. [Google Scholar] [CrossRef] [Green Version]
- Vincent-Hubert, F.; Wacrenier, C.; Morga, B.; Lozach, S.; Quenot, E.; Mege, M.; Lecadet, C.; Gourmelon, M.; Hervio-Heath, D.; Guyader, F. Passive samplers, a powerful tool to detect viruses and bacteria in marine coastal areas. Front. Microbiol. 2021, 12, 631174. [Google Scholar] [CrossRef]
- Elbashir, S.; Parveen, S.; Schwarz, J.; Rippen, T.; Jahncke, M.; Depaola, A. Seafood pathogens and information on antimicrobial resistance: A review. Food Microbiol. 2018, 70, 85–93. [Google Scholar] [CrossRef]
- Nam, S.J.; Park, P.H.; Bang, S.J.; Huh, J.W.; Yun, H.J.; Park, K.H.; Yoon, M.H. Molecular epidemiological study of norovirus gastroenteritis outbreaks in Gyeonggi-Do from 2014 to 2015. Korean J. Microbiol. 2018, 54, 24–30. [Google Scholar] [CrossRef]
- Ministry of Food and Drug Safety. Food Poisoning Statistics. Available online: https://www.foodsafetykorea.go.kr/portal/healthyfoodlife/foodPoisoningStat.do?menu_no=3724&menu_grp=MENU_NEW02 (accessed on 9 March 2022).
- Kang, D.H. Current thermal/non-thermal technologies to control foodborne pathogens. Korean J. Food Sci. Technol. 2012, 45, 48–59. [Google Scholar] [CrossRef]
- Csadek, I.; Paulsen, P.; Weidinger, P.; Bak, K.H.; Bauer, S.; Pilz, B.; Nowontny, N.; Smulders, F.J.M. Nitrogen accumulation in oyster (Crassostrea gigas) slurry exposed to virucidal cold atmospheric plasma treatment. Life 2021, 11, 1333. [Google Scholar] [CrossRef] [PubMed]
- Filipic, A.; Gutierrez-Aguirre, I.; Primc, G.; Mozetic, M.; Dobnik, D. Cold plasma, a new hope in the field of virus inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef]
- Cliver, D. Capsid and infectivity in virus detection. Food Environ. Virol. 2009, 1, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Domonkos, M.; Ticha, P.; Demo, P. Applications of cold atmospheric pressure plasma technology in medicine, agriculture and food industry. Appl. Sci. 2021, 11, 4809. [Google Scholar] [CrossRef]
- Bosch, A.; Sanchez, G.; Abbaszadegan, M.; Carducci, A.; Guix, S.; Le Guyader, F. Analytical methods for virus detection in water and food. Food Anal. Methods 2011, 4, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Coudray-Meunier, C.; Fraisse, A.; Martin-Latil, S.; Guillier, L.; Delannoy, S.; Fach, P.; Perelle, S. A comparative study of digital RT-PCR and RT-qPCR for quantification of hepatitis A virus and norovirus in lettuce and water samples. Int. J. Food Microbiol. 2015, 201, 17–26. [Google Scholar] [CrossRef]
- Knight, A.; Haines, J.; Stals, A.; Li, D.; Uyttendaele, M.; Knight, A.; Jakus, L.A. A system review of human norovirus survival reveals a greater persistence of human norovirus RT-qPCR signals compared to those of cultivable surrogate viruses. Int. J. Food Microbiol. 2016, 216, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, M.; Rodriguez, N.J.P.; Codony, F.; Adrados, B.; Penuela, G.A.; Morato, J. Discrimination of infectious bacteriophage T4 virus by propidium monoazide real-time pcr. J. Virol. Methods. 2010, 168, 228–232. [Google Scholar] [CrossRef]
- Fuster, N.; Pinto, R.M.; Fuentes, C.; Beguiristain, N.; Bosch, A.; Guix, S. Propidium monoazide RTqPCR assays for the assessment of hepatitis A inactivation and for a better estimation of the health risk of contaminated waters. Water Res. 2016, 101, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Jeon, E.B.; Choi, M.S.; Park, S.Y. Inactivation of human norovirus GII.4 on oyster Crassostrea gigas by electron beam irradiation. Korean J. Fish. Aquat. Sci. 2021, 54, 16–22. [Google Scholar] [CrossRef]
- Choi, M.S.; Jeon, E.B.; Kim, J.Y.; Choi, E.H.; Lim, J.S.; Choi, J.S.; Ha, K.S.; Kwon, J.Y.; Jeong, S.H.; Park, S.Y. Virucidal effects of dielectric barrier discharge plasma on human norovirus infectivity in fresh oysters (Crassostrea gigas). Foods 2020, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Park, B.; Jin, S.; Kim, D.; Han, I.; Kim, J.; Hyun, S.; Chung, K.H.; Park, J.C. Removal and sterilization of biofilms and planktonic bacteria by microwave-induced argon plasma at atmospheric pressure. New J. Phys. 2009, 11, 115022. [Google Scholar] [CrossRef]
- Korachi, M.; Turan, Z.; Senturk, K.; Sahin, F.; Aslan, N. An investigation into biocidal effect of high voltage AC/DC atmospheric corona discharge on bacteria, yeast, fungi and algae. J. Electrost. 2009, 67, 678–685. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, M.G.; Jeon, E.B.; Kim, J.S.; Lee, J.S.; Choi, E.H.; Lim, J.S.; Choi, J.S.; Park, S.Y. Antibacterial effects of non-thermal dielectric barrier discharge plasma against Escherichia coli and Vibrio parahaemolyticus on the surface of wooden chopping board. Innov. Food Sci. Emerg. Technol. 2021, 73, 102784. [Google Scholar] [CrossRef]
- Eun, J.B.; Lee, J.C.; Chung, D.O. Chemical changes of low salt-dried yellow corvenia muscle during frozen storage. Korean J. Fish. Aquat. Sci. 1997, 30, 660–666. [Google Scholar]
- Song, H.N.; Lee, D.G.; Han, S.W.; Yoon, H.K.; Hwang, I.K. Quality changes of salted and semi-dried mackerel fillets by UV treatment during refrigerated storage. Korean J. Food Cook Sci. 2005, 21, 662–668. [Google Scholar]
- Shin, M.C.; Kwon, R.W.; Hwang, S.W.; Kim, H.J.; Kim, D.H.; Lee, S.H.; Park, J.H.; Kim, J.G. Processing and quality characteristics of high value-added low-salt fermented sea squirt Halocynthia roretzi with maesil extracts. J. Fish. Mar. Sci. Edu. 2021, 33, 1065–1079. [Google Scholar] [CrossRef]
- Oh, S.C. The change of volatile basic nitrogen and browing in salt fermented squid affected by adding to squid ink. J. Korean Oil Chem. Soc. 2012, 29, 631–637. [Google Scholar] [CrossRef]
- Choi, M.S.; Jeon, E.B.; Kim, J.Y.; Choi, E.H.; Lim, J.S.; Choi, J.S.; Park, S.Y. Application of dielectric barrier discharge plasma for the reduction of non-pathogenic Escherichia coli and E. coli O157:H7 and the quality stability of fresh oysters (Crassostrea gigas). LWT 2022, 154, 112698. [Google Scholar] [CrossRef]


| Genotype | Type | Component | Sequence (5′→3′) |
|---|---|---|---|
| GII | Primer | COG1F | 5′-CAR GAR BCN ATG TTY AGR TGG ATG AG–3′ |
| COG2R | 5′-TCG ACG CCA TCT TCA TTC ACA-3′ | ||
| Probe | RING2 | 5′-TGG GAG GGC GAT CGC AAT CT-3′ |
| FE-DBD Plasma (min) | Non-PMA/RT-qPCR | PMA/RT-qPCR | Before/After Using PMA to HNoV Reduction Difference (log Copy Number/µL) |
|---|---|---|---|
| log Copy Number/µL | log Copy Number/µL | ||
| 0 | 2.55 ± 0.16 a | 2.55 ± 0.16 a | |
| 5 | 2.44 ± 0.07 Aa | 2.07 ± 0.04 Bb | (2.44 − 2.07) = 0.37 |
| 15 | 2.29 ± 0.03 Ab | 1.92 ± 0.09 Bc | (2.29 − 1.92) = 0.37 |
| 30 | 2.20 ± 0.01 Ab | 1.79 ± 0.01 Bc | (2.20 − 1.79) = 0.41 |
| 45 | 1.98 ± 0.01 Ac | 1.65 ± 0.00 Bd | (1.98 − 1.65) = 0.33 |
| 60 | 1.60 ± 0.02 Ad | 1.39 ± 0.06 Be | (1.60 − 1.39) = 0.21 |
| 75 | 1.26 ± 0.07 Ae | 0.96 ± 0.06 Bf | (1.26 − 0.96) = 0.30 |
| FE-DBD Plasma (min) | V. parahaemolyticus |
|---|---|
| log CFU/g | |
| 0 | 4.16 ± 0.03 a |
| 5 | 4.00 ± 0.06 b |
| 15 | 3.98 ± 0.03 b |
| 30 | 3.76 ± 0.08 c |
| 45 | 3.54 ± 0.08 d |
| 60 | 3.41 ± 0.01 e |
| 75 | 2.66 ± 0.05 f |
| Quantify | D-Value (min) | R2 | y = −ax + b | |
|---|---|---|---|---|
| HNoV GII.4 | RT-qPCR | 61.96 ± 3.28 | 0.97 | y= −0.016x + 2.577 |
| PMA with RT-qPCR | 58.68 ± 2.74 | 0.92 | y= −0.017x + 2.322 | |
| V. parahaemolyticus | Standard plate count | 58.70 ± 0.20 | 0.90 | y= −0.017x + 4.202 |
| FE-DBD Plasma (min) | VBN (mg/100 g) | pH |
|---|---|---|
| 0 | 8.3 ± 0.1 c | 5.78 ± 0.04 c |
| 5 | 8.3 ± 0.1 c | 5.88 ± 0.04 ab |
| 15 | 8.2 ± 0.2 c | 5.88 ± 0.04 ab |
| 30 | 11.0 ± 0.1 b | 5.94 ± 0.05 a |
| 45 | 12.3 ± 0.1 a | 5.80 ± 0.00 c |
| 60 | 12.4 ± 0.2 a | 5.84 ± 0.05 bc |
| 75 | 12.4 ± 0.2 a | 5.94 ± 0.05 a |
| FE-DBD Plasma (min) | Color | ||
|---|---|---|---|
| L’ Value | a’ Value | b’ Value | |
| 0 | 46.16 ± 0.02 a | 20.22 ± 0.02 a | 27.05 ± 0.01 a |
| 5 | 46.09 ± 0.01 b | 19.85 ± 0.01 b | 26.97 ± 0.01 b |
| 15 | 45.92 ± 0.01 c | 19.77 ± 0.02 c | 26.87 ± 0.01 c |
| 30 | 45.85 ± 0.01 d | 19.41 ± 0.02 d | 26.79 ± 0.01 d |
| 45 | 44.73 ± 0.02 e | 19.37 ± 0.01 e | 26.75 ± 0.01 e |
| 60 | 44.44 ± 0.01 f | 19.20 ± 0.01 f | 26.53 ± 0.01 f |
| 75 | 43.67 ± 0.01 g | 19.16 ± 0.01 g | 26.34 ± 0.01 g |
| Time (min) | Texture | |
|---|---|---|
| Hardness | Chewiness | |
| 0 | 233 ± 3.2 | 14 ± 0.5 |
| 5 | 261 ± 3.8 | 20 ± 0.8 |
| 15 | 255 ± 2.7 | 18 ± 0.9 |
| 30 | 234 ± 6.9 | 14 ± 0.5 |
| 45 | 240 ± 3.5 | 15 ± 0.7 |
| 60 | 250 ± 8.1 | 11 ± 0.3 |
| 75 | 265 ± 1.2 | 19 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.G.; Kim, S.H.; Jeon, E.B.; Ha, K.S.; Cho, S.R.; Jung, Y.J.; Choi, E.H.; Lim, J.S.; Choi, J.; Park, S.Y. Inactivation of Human Norovirus GII.4 and Vibrio parahaemolyticus in the Sea Squirt (Halocynthia roretzi) by Floating Electrode-Dielectric Barrier Discharge Plasma. Foods 2023, 12, 1030. https://doi.org/10.3390/foods12051030
Song MG, Kim SH, Jeon EB, Ha KS, Cho SR, Jung YJ, Choi EH, Lim JS, Choi J, Park SY. Inactivation of Human Norovirus GII.4 and Vibrio parahaemolyticus in the Sea Squirt (Halocynthia roretzi) by Floating Electrode-Dielectric Barrier Discharge Plasma. Foods. 2023; 12(5):1030. https://doi.org/10.3390/foods12051030
Chicago/Turabian StyleSong, Min Gyu, So Hee Kim, Eun Bi Jeon, Kwang Soo Ha, Sung Rae Cho, Yeoun Joong Jung, Eun Ha Choi, Jun Sup Lim, Jinsung Choi, and Shin Young Park. 2023. "Inactivation of Human Norovirus GII.4 and Vibrio parahaemolyticus in the Sea Squirt (Halocynthia roretzi) by Floating Electrode-Dielectric Barrier Discharge Plasma" Foods 12, no. 5: 1030. https://doi.org/10.3390/foods12051030
APA StyleSong, M. G., Kim, S. H., Jeon, E. B., Ha, K. S., Cho, S. R., Jung, Y. J., Choi, E. H., Lim, J. S., Choi, J., & Park, S. Y. (2023). Inactivation of Human Norovirus GII.4 and Vibrio parahaemolyticus in the Sea Squirt (Halocynthia roretzi) by Floating Electrode-Dielectric Barrier Discharge Plasma. Foods, 12(5), 1030. https://doi.org/10.3390/foods12051030