Influence of Biotreatment on Hordeum vulgare L. Cereal Wholemeal Contamination and Enzymatic Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Barley Grain Samples and Wholemeal Preparation
2.2. Lactic Acid Bacteria
2.3. Biotreatment of Barley Wholemeal Product Samples
2.4. Methods of Analysis
2.4.1. High-Performance Liquid Chromatography
2.4.2. Evaluation of Enzymatic Activities in Barley Wholemeal Product Samples
2.5. Statistical Analysis
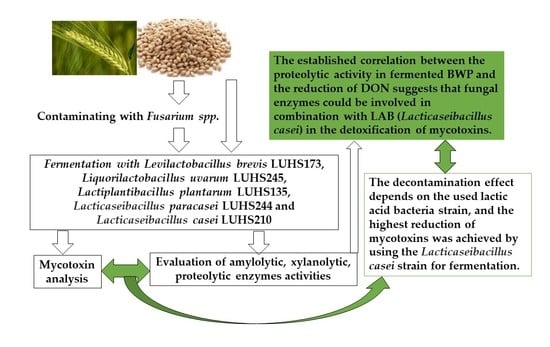
3. Results and Discussion
3.1. Characterization of DON and Its Conjugated Forms in Barley Wholemeal Products
3.2. The Effect of Fermentation on Deoxynivalenol and Its Conjugates Concentrations in Barley Wholemeal Products
3.3. Changes in Barley Wholemeal Products Enzymatic Activities during Fermentation and Their Correlation with Mycotoxin Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janssen, E.M.; Liu, C.; Van der Fels-Klerx, H.J. Fusarium infection and trichothecenes in barley and its comparison with wheat. World Mycotoxin J. 2018, 11, 33–46. [Google Scholar] [CrossRef]
- Mikušová, P.; Šrobárová, A.; Sulyok, M.; Santini, A. Fusarium fungi and associated metabolites presence on grapes from Slovakia. Mycotoxin Res. 2013, 29, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Simsek, S.; Ovando-Martínez, M.; Ozsisli, B.; Whitney, K.; Ohm, J.-B. Occurrence of Deoxynivalenol and Deoxynivalenol-3-glucoside in Hard Red Spring Wheat Grown in the USA. Toxins 2013, 5, 2656–2670. [Google Scholar] [CrossRef] [Green Version]
- JECFA. Evaluation of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives; Technical Report Series 959; WHO: Geneva, Switzerland, 2011.
- EFSA (European Food Safety Authority). Risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, 4718. [Google Scholar]
- EFSA (European Food Safety Authority). Scientific opinion on risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [Google Scholar] [CrossRef]
- EFSA. Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379. [Google Scholar]
- Crews, C.; Macdonald, S.J. Natural Occurrence of Masked Mycotoxins. In Masked Mycotoxins in Food: Formation, Occurrence and Toxicological Relevance; The Royal Society of Chemistry: Cambridge, UK, 2016; pp. 14–31. ISBN 10:1849739722. [Google Scholar]
- Berthiller, F.; Dall’asta, C.; Corradini, R.; Marchelli, R.; Sulyok, M.; Krska, R.; Adam, G.; Schuhmacher, R. Occurrence of deoxynivalenol and its 3-β-Dglucoside in wheat and maize. Food Addit. Contam. 2009, 26, 507–511. [Google Scholar] [CrossRef] [Green Version]
- De Boevre, M.; Jacxsens, L.; Lachat, C.; Eeckhout, M.; Di Mavungu, J.D.; Audenaert, K.; Maene, P.; Haesaert, G.; Kolsteren, P.; De Meulenaer, B.; et al. Human exposure to mycotoxins and their masked forms through cereal-based foods in Belgium. Toxicol. Lett. 2013, 218, 281–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Śliżewska, K.; Chlebicz-Wójcik, A. Growth Kinetics of Probiotic Lactobacillus Strains in the Alternative, Cost-Efficient Semi-Solid Fermentation Medium. Biology 2020, 9, 423. [Google Scholar] [CrossRef]
- Reinholds, I.; Juodeikiene, G.; Bartkiene, E.; Zadeike, D.; Bartkevics, V.; Krungleviciute, V.; Cernauskas, D.; Cizeikiene, D. Evaluation of ozonation as a method for mycotoxins degradation in malting wheat grains. World Mycotoxin J. 2016, 9, 409–417. [Google Scholar] [CrossRef]
- Magnusson, J.; Ström, K.; Roos, S.; Sjögren, J.; Schnürer, J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol. Lett. 2003, 219, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cray, J.A.; Stevenson, A.; Ball, P.; Bankar, S.B.; Eleutherio, E.C.; Ezeji, T.C.; Singhal, R.S.; Thevelein, J.M.; Timson, D.J.; Hallsworth, J.E. Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 2015, 33, 228–259. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.O.; Thrane, U. Fusarium taxonomy with relation to trichothecene formation. Toxicol. Lett. 2004, 153, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trakselyte-Rupsiene, K.; Juodeikiene, G.; Janic Hajnal, E.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Cernauskas, D.; Lele, V.; Zadeike, D.; Bartkiene, E. Challenges of Lactobacillus fermentation in combination with acoustic screening for deoxynivalenol and deoxynivalenol conjugates reduction in contaminated wheat-based products. Food Control 2022, 134, 108699. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Bernatoniene, J.; Jakstas, V.; Viskelis, P.; Zadeike, D.; Juodeikiene, G. Improvement of the antimicrobial activity of lactic acid bacteria in combination with berries/fruits and dairy industry by-products. J. Sci. Food Agric. 2019, 99, 3992–4002. [Google Scholar] [CrossRef]
- Janaviciene, S.; Suproniene, S.; Kadziene, G.; Pavlenko, R.; Berzina, Z.; Bartkevics, V. Toxigenicity of F. graminearum Residing on Host Plants Alternative to Wheat as Influenced by Environmental Conditions. Toxins 2022, 14, 541. [Google Scholar] [CrossRef] [PubMed]
- ICC (International Association for Cereals Science and Technology). Colorimetric Method for the Determination of Alpha-Amylase Activity Method 108; ICC: Washington, DC, USA, 1998. [Google Scholar]
- Bailey, M.J.; Biely, P.; Poutanen, K. Interlaboratory testing of methods for assay of xylanase activity. J. Biotechnol. 1992, 23, 257–270. [Google Scholar] [CrossRef]
- Cupp-Enyard, C. Sigma’s non-specific protease activity assay-casein as a substrate. J. Vis. Exp. 2008, 19, 899. [Google Scholar]
- Gratz, S.W. Do Plant-Bound Masked Mycotoxins Contribute to Toxicity? Toxins 2017, 9, 85. [Google Scholar] [CrossRef]
- Gardiner, S.A.; Boddu, J.; Berthiller, F.; Hametner, C.; Stupar, R.M.; Adam, G.; Muehlbauer, G.J. Transcriptome analysis of the barley-deoxynivalenol interaction: Evidence for a role of glutathione in deoxynivalenol detoxification. Mol. Plant Microbe Interact. 2010, 23, 962–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng-Reiterer, J.; Varga, E.; Nathanail, A.V.; Bueschl, C.; Rechthaler, J.; McCormick, S.P.; Michlmayr, H.; Malachová, A.; Fruhmann, P.; Adam, G.; et al. Tracing the metabolism of HT-2 toxin and T-2 toxin in barley by isotope-assisted untargeted screening and quantitative LC-HRMS analysis. Anal. Bioanal. Chem. 2015, 407, 8019–8033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Z.; Zhou, B.; Gillespie, J.; Gross, T.; Barr, J.; Simsek, S.; Brueggeman, R.; Schwarz, R. Production of deoxynivalenol (DON) and DON-3-glucoside during the malting of Fusarium infected hard red spring wheat. Food Control 2018, 85, 6–10. [Google Scholar] [CrossRef]
- Guo, H.; Jian, J.; Wang, J.; Sun, X. Deoxynivalenol: Masked forms, fate during food processing, and potential biological remedies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 895–926. [Google Scholar] [CrossRef] [Green Version]
- Berthiller, F.; Krska, R.; Dall’Asta, C.; Lemmens, M.; Adam, G.; Schuhmacher, R. Determination of DON-3-Glucoside in artificially and naturally contaminated wheat with LC-MS/MS. Mycotoxin Res. 2005, 21, 205–208. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography- tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef]
- Nguyena, Q.D.; Rezessy-Szabóa, J.M.; Claeyssensb, M.; Stalsb, I.; Hoschke, A. Purification and characterisation of amylolytic enzymes from thermophilic fungus Thermomyces lanuginosus strain ATCC 34626. Enzym. Microb. Technol. 2002, 31, 345–352. [Google Scholar] [CrossRef]
- Lemmens, M.; Steiner, B.; Sulyok, M.; Nicholson, P.; Mesterhazy, A.; Buerstmayr, H. Masked mycotoxins: Does breeding enhance Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties? World Mycotoxin J. 2016, 9, 741–754. [Google Scholar] [CrossRef]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins, and mitogenactivated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J. 2014, 12, 3916. [Google Scholar]
- Kooning, H.; Unden, G.; Frolich, J. Biology of Microorganisms on Grapes, in Must and in Wine, Mainz; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-540-85462-3. [Google Scholar]
- Rizzello, C.G.; Cassone, A.; Coda, R.; Gobbetti, M. Antifungal activity of sourdough fermented wheat germ used as an ingredient for bread making. Food Chem. 2011, 127, 952–959. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Saari, N.; Hussin, A.S.M. Review on the biological detoxification of mycotoxins using lactic acid bacteria to enhance the sustainability of foods supply. Molecules 2020, 7, 2655. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Su, R.; Yin, R.; Lai, D.; Wang, M.; Liu, Y.; Zhou, L. Detoxification of mycotoxins through biotransformation. Toxins 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Nezami, H.; Chrevatidis, A.; Auriola, S.; Mykkänen, H. Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit. Contam. 2002, 19, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Zadeike, D.; Vaitkeviciene, R.; Bartkevics, V.; Bogdanova, E.; Bartkiene, E.; Lele, V.; Juodeikiene, G.; Cernauskas, D.; Valatkeviciene, Z. The expedient application of microbial fermentation after whole-wheat milling and fractionation to mitigate mycotoxins in wheat-based products. LWT-Food Sci. Technol. 2021, 137, 110440. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, B.; Dhawan, K.; Vasundhara; Mishra, S.; Kumar, M.; Tripathi, A.D.; et al. Deoxynivalenol: An Overview on Occurrence, Chemistry, Biosynthesis, Health Effects and Its Detection, Management, and Control Strategies in Food and Feed. Microbiol. Res. 2022, 13, 292–314. [Google Scholar] [CrossRef]
- Lyagin, I.; Efremenko, E. Enzymes for Detoxification of Various Mycotoxins: Origins and Mechanisms of Catalytic Action. Molecules 2019, 24, 2362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Zokaityte, E.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Klupsaite, D.; Cernauskas, D.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; et al. Combination of Extrusion and Fermentation with Lactobacillus plantarum and L. uvarum Strains for Improving the Safety Characteristics of Wheat Bran. Toxins 2021, 13, 163. [Google Scholar] [CrossRef]




| Samples | DON, µg/kg | D3G, µg/kg | D3G/DON, % | 15-ADON, µg/kg | 15-ADON/DON, % | 3-ADON, µg/kg | 3-ADON/DON, % |
|---|---|---|---|---|---|---|---|
| BWP1 | 248 ± 12.5 a | 172 ± 8.6 a | 69.35 ± 3.5 a | 84 ± 4.2 a | 33.87 ± 1.7 b | 44 ± 2.2 b | 17.74 ± 0.9 e |
| BWP2 | 223 ± 11.2 a | 107 ± 5.4 a | 47.98 ± 2.4 b | 162 ± 8.1 b | 72.65 ± 3.6 d | 22 ± 1.1 a | 9.80 ± 0.5 c |
| BWP3 | 460 ± 23.0 a | 97 ± 4.9 a | 21.09 ± 1.1 a | 244 ± 12.2 c | 53.04 ± 2.7 c | 72 ± 3.6 c | 15.65 ± 0.8 d |
| BWP4 | 996 ± 49.8 b | 440 ± 22.0 b | 44.18 ± 2.2 b | 278 ± 13.9 c | 27.91 ± 1.4 b | 65 ± 3.3 c | 6.52 ± 0.3 b |
| BWP5 | 6563 ± 328.2 c | 1780 ± 89.0 c | 27.12 ± 1.4 a | 929 ± 46.5 d | 14.12 ± 0.7 a | 209 ± 10.5 d | 3.18 ± 0.2 a |
| Samples | Fermentation Duration | ||||||
|---|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | |||||
| pH | LAB, log10 CFU/g | pH | LAB, log10 CFU/g | pH | LAB, log10 CFU/g | ||
| Lev. brevis (LUHS 173) | BWP1 | 6.37 ± 0.23 a | 7.88 ± 0.21 b | 3.83 ± 0.02 b | 9.64 ± 0.20 d | 3.87 ± 0.06 b | 9.34 ± 0.20 d |
| BWP2 | 5.93 ± 0.04 a | 7.33 ± 0.02 b | 3.90 ± 0.01 b | 9.45 ± 0.18 d | 3.99 ± 0.02 b | 9.36 ± 0.15 d | |
| BWP3 | 5.62 ± 0.02 a | 7.54 ± 0.10 b | 3.86 ± 0.01 b | 9.36 ± 0.15 d | 3.96 ± 0.05 b | 9.35 ± 0.18 d | |
| BWP4 | 5.60 ± 0.02 a | 7.51 ± 0.12 b | 3.85 ± 0.03 b | 9.15 ± 0.10 d | 3.94 ± 0.01 b | 9.38 ± 0.40 d | |
| BWP5 | 5.56 ± 0.07 a | 7.43 ± 0.09 b | 3.92 ± 0.01 b | 9.58 ± 0.16 d | 3.95 ± 0.01 b | 9.37 ± 0.25 d | |
| Liq. uvarum (LUHS 245) | BWP1 | 5.75 ± 0.06 a | 7.11 ± 0.10 b | 3.79 ± 0.03 b | 9.04 ± 0.10 d | 3.57 ± 0.07 b | 8.35 ± 015 c |
| BWP2 | 5.72 ± 0.01 a | 7.14 ± 0.12 b | 3.85 ± 0.01 b | 8.93 ± 0.09 cd | 3.75 ± 0.01 b | 8.57 ± 0.10 c | |
| BWP3 | 5.50 ± 0.02 a | 7.43 ± 0.15 b | 3.86 ± 0.02 b | 8.46 ± 0.12 c | 3.75 ± 0.01 b | 8.42 ± 0.21 c | |
| BWP4 | 5.52 ± 0.01 a | 7.09 ± 0.08 b | 3.87 ± 0.01 b | 8.50 ± 0.16 c | 3.77 ± 0.02 b | 8.50 ± 0.20 c | |
| BWP5 | 5.24 ± 0.08 ab | 7.26 ± 0.12 b | 3.91 ± 0.02 b | 8.52 ± 0.20 c | 3.79 ± 0.03 b | 8.52 ± 0.15 c | |
| Lp. plantarum (LUHS 135) | BWP1 | 6.10 ± 0.05 a | 7.19 ± 0.20 b | 3.53 ± 0.02 b | 7.43 ± 0.15 b | 3.45 ± 0.01 b | 8.05 ± 0.19 bc |
| BWP2 | 6.15 ± 0.11 a | 7.07 ± 0.08 b | 3.55 ± 0.01 b | 7.29 ± 0.12 b | 3.48 ± 0.02 b | 8.08 ± 0.20 bc | |
| BWP3 | 5.89 ± 0.04 a | 7.12 ± 0.05 b | 3.58 ± 0.05 b | 7.25 ± 0.09 b | 3.50 ± 0.02 b | 8.85 ± 0.26 cd | |
| BWP4 | 5.80 ± 0.05 a | 7.10 ± 0.09 b | 3.60 ± 0.01 b | 7.48 ± 0.20 b | 3.51 ± 0.01 b | 7.85 ± 0.12 b | |
| BWP5 | 5.81 ± 0.01 a | 6.66 ± 0.05 ab | 3.57 ± 0.01 b | 8.03 ± 0.13 bc | 3.48 ± 0.11 b | 7.94 ± 0.09 bc | |
| Lc. paracasei (LUHS 244) | BWP1 | 5.81 ± 0.04 a | 5.40 ± 0.07 a | 3.66 ± 0.02 b | 7.91 ± 0.15 bc | 3.52 ± 0.01 b | 7.42 ± 0.14 b |
| BWP2 | 5.63 ± 0.01 a | 5.53 ± 0.10 a | 3.71 ± 0.04 b | 7.38 ± 0.10 b | 3.54 ± 0.03 b | 7.46 ± 0.20 b | |
| BWP3 | 5.59 ± 0.12 a | 5.47 ± 0.15 a | 3.72 ± 0.02 b | 7.52 ± 0.25 b | 3.53 ± 0.01 b | 7.36 ± 0.14 b | |
| BWP4 | 5.65 ± 0.08 a | 5.52 ± 0.12 a | 3.72 ± 0.01 b | 7.42 ± 0.40 b | 3.55 ± 0.01 b | 7.52 ± 0.09 b | |
| BWP5 | 5.56 ± 0.09 a | 5.79 ± 0.20 a | 3.73 ± 0.05 b | 7.41 ± 0.12 b | 3.56 ± 0.05 b | 7.64 ± 0.18 b | |
| Lc. casei (LUHS 210) | BWP1 | 6.00 ± 0.03 a | 7.01 ± 0.09 b | 3.70 ± 0.01 b | 8.09 ± 0.20 bc | 3.58 ± 0.07 b | 8.31 ± 0.22 c |
| BWP2 | 6.02 ± 0.03 a | 7.25 ± 0.10 b | 3.71 ± 0.01 b | 7.87 ± 0.20 b | 3.58 ± 0.01 b | 7.83 ± 0.09 b | |
| BWP3 | 5.84 ± 0.02 a | 7.23 ± 0.15 b | 3.72 ± 0.01 b | 7.81 ± 0.15 b | 3.59 ± 0.02 b | 8.13 ± 0.21 bc | |
| BWP4 | 5.90 ± 0.03 a | 7.12 ± 0.09 b | 3.71 ± 0.02 b | 7.72 ± 0.20 b | 3.60 ± 0.01 b | 8.02 ± 0.19 bc | |
| BWP5 | 5.66 ± 0.01 a | 7.16 ± 0.20 b | 3.71 ± 0.05 b | 7.73 ± 0.18 b | 3.59 ± 0.01 b | 8.27 ± 0.23 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juodeikiene, G.; Trakselyte-Rupsiene, K.; Reikertaite, K.; Janic Hajnal, E.; Bartkevics, V.; Pugajeva, I.; Gruzauskas, V.; Švazas, M.; Gruzauskas, R.; Santini, A.; et al. Influence of Biotreatment on Hordeum vulgare L. Cereal Wholemeal Contamination and Enzymatic Activities. Foods 2023, 12, 1050. https://doi.org/10.3390/foods12051050
Juodeikiene G, Trakselyte-Rupsiene K, Reikertaite K, Janic Hajnal E, Bartkevics V, Pugajeva I, Gruzauskas V, Švazas M, Gruzauskas R, Santini A, et al. Influence of Biotreatment on Hordeum vulgare L. Cereal Wholemeal Contamination and Enzymatic Activities. Foods. 2023; 12(5):1050. https://doi.org/10.3390/foods12051050
Chicago/Turabian StyleJuodeikiene, Grazina, Karolina Trakselyte-Rupsiene, Karolina Reikertaite, Elizabet Janic Hajnal, Vadims Bartkevics, Iveta Pugajeva, Valentas Gruzauskas, Mantas Švazas, Romas Gruzauskas, Antonello Santini, and et al. 2023. "Influence of Biotreatment on Hordeum vulgare L. Cereal Wholemeal Contamination and Enzymatic Activities" Foods 12, no. 5: 1050. https://doi.org/10.3390/foods12051050
APA StyleJuodeikiene, G., Trakselyte-Rupsiene, K., Reikertaite, K., Janic Hajnal, E., Bartkevics, V., Pugajeva, I., Gruzauskas, V., Švazas, M., Gruzauskas, R., Santini, A., Rocha, J. M., & Bartkiene, E. (2023). Influence of Biotreatment on Hordeum vulgare L. Cereal Wholemeal Contamination and Enzymatic Activities. Foods, 12(5), 1050. https://doi.org/10.3390/foods12051050