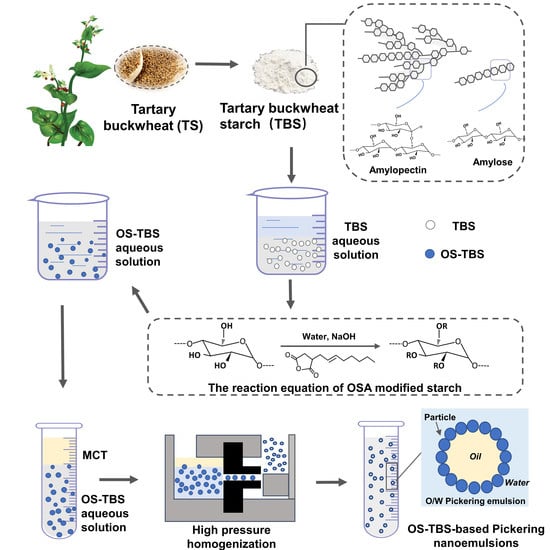
Tartary Buckwheat Starch Modified with Octenyl Succinic Anhydride for Stabilization of Pickering Nanoemulsions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of TBS
2.3. Preparation of OS-TBS
2.4. Determination of the Degree of Substitution (DS) in OS-TBS
2.5. Amylose Content
2.6. Physicochemical Properties of OS-TBS
2.6.1. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.6.2. Particle Size Distribution and Granule Morphology
2.6.3. X-ray Diffractometry (XRD)
2.6.4. Thermal Properties
2.6.5. Solubility Determination
2.7. Contact Angle Measurement
2.8. Preparation and Characterization of Pickering Emulsions
2.8.1. Fabrication
2.8.2. Microstructure and Zeta Potential
2.8.3. Emulsification Index (EI) and Centrifugation Stability
2.9. Statistical Analysis
3. Results and Discussion
3.1. Degree of Substitution (DS) and Amylose Content
3.2. FT-IR Analysis
3.3. Morphology and Particle Size Distribution
3.4. XRD Analysis
3.5. Thermal Properties
3.6. Water Solubility Index (WSI) and Swelling Power (SP)
3.7. Particle Wettability
3.8. Formation and the Storage Stability of OS-TBS-Based Pickering Emulsions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zou, L.; Wu, D.; Ren, G.; Hu, Y.; Peng, L.; Zhao, J.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive compounds, health benefits, and industrial applications of Tartary buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2021, 63, 657–673. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Q.; Xia, M.; Bai, W.; Wang, P.; Gao, X.; Gong, X.; Feng, B.; Gao, L.; Zhou, M.; et al. Effects of phosphate fertiliser on the physicochemical properties of Tartary buckwheat (Fagopyrum tataricum (L.) Gaertn.) starch. Food Chem. 2020, 307, 125543. [Google Scholar] [CrossRef]
- Gao, J.; Kreft, I.; Chao, G.; Wang, Y.; Liu, X.; Wang, L.; Wang, P.; Gao, X.; Feng, B. Tartary buckwheat (Fagopyrum tataricum Gaertn.) starch, a side product in functional food production, as a potential source of retrograded starch. Food Chem. 2016, 190, 552–558. [Google Scholar] [CrossRef]
- Qian, J.Y.; Kuhn, M. Evaluation on gelatinization of buckwheat starch: A comparative study of Brabender viscoamylography, rapid visco-analysis, and differential scanning calorimetry. Eur. Food Res. Technol. 1999, 209, 277–280. [Google Scholar] [CrossRef]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhang, H.; Pei, J.; Liu, H.; Lu, M.; Chen, J.; Wang, M. High-voltage and short-time dielectric barrier discharge plasma treatment affects structural and digestive properties of Tartary buckwheat starch. Int. J. Biol. Macromol. 2022, 213, 268–278. [Google Scholar] [CrossRef]
- Yang, Y.H.; Vong, C.T.; Zeng, S.; Gao, C.F.; Chen, Z.J.; Fu, C.M.; Wang, S.P.; Zou, L.; Wang, A.Q.; Wang, Y.T. Tracking evidences of Coptis chinensis for the treatment of inflammatory bowel disease from pharmacological, pharmacokinetic to clinical studies. J. Ethnopharmacol. 2021, 268, 113573. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liang, X.; Dong, X.; Li, R.; Jiang, G.; Wan, Y.; Fu, G.; Liu, C. Physical modification on the in vitro digestibility of Tartary buckwheat starch: Repeated retrogradation under isothermal and non-isothermal conditions. Int. J. Biol. Macromol. 2021, 184, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Wen, Y.; Xu, Z.; Ma, M.; Li, P.; Brennan, C.; Sui, Z.; Corke, H. Octenylsuccinylation differentially modifies the physicochemical properties and digestibility of small granule starches. Int. J. Biol. Macromol. 2020, 144, 705–714. [Google Scholar] [CrossRef]
- Miao, M.; Li, R.; Jiang, B.; Cui, S.W.; Zhang, T.; Jin, Z. Structure and physicochemical properties of octenyl succinic esters of sugary maize soluble starch and waxy maize starch. Food Chem. 2014, 151, 154–160. [Google Scholar] [CrossRef]
- Zainal Abiddin, N.F.; Yusoff, A.; Ahmad, N. Effect of octenylsuccinylation on physicochemical, thermal, morphological and stability of octenyl succinic anhydride (OSA) modified sago starch. Food Hydrocoll. 2018, 75, 138–146. [Google Scholar] [CrossRef]
- Zhang, B.; Mei, J.Q.; Chen, B.; Chen, H.Q. Digestibility, physicochemical and structural properties of octenyl succinic anhydride-modified cassava starches with different degree of substitution. Food Chem. 2017, 229, 136–141. [Google Scholar] [CrossRef]
- Song, X.; Pei, Y.; Qiao, M.; Ma, F.; Ren, H.; Zhao, Q. Preparation and characterizations of Pickering emulsions stabilized by hydrophobic starch particles. Food Hydrocoll. 2015, 45, 256–263. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schafer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Muhammad, Z.; Ramzan, R.; Zhang, R.; Zhao, D.; Khalid, N.; Deng, M.; Dong, L.; Aziz, M.; Batool, R.; Zhang, M. Enhanced bioaccessibility of microencapsulated puerarin delivered by Pickering emulsions stabilized with OSA-modified hydrolyzed Pueraria montana starch: In vitro release, storage stability, and physicochemical properties. Foods 2022, 11, 3591. [Google Scholar] [CrossRef]
- Matos, M.; Marefati, A.; Barrero, P.; Rayner, M.; Gutiérrez, G. Resveratrol loaded Pickering emulsions stabilized by OSA modified rice starch granules. Food Res. Int. 2021, 139, 109837. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, D.; Mackie, A.; Yang, S.; Dai, L.; Zhang, L.; Mao, L.; Gao, Y. Stability, interfacial structure, and gastrointestinal digestion of β-carotene-loaded pickering emulsions co-stabilized by particles, a biopolymer, and a surfactant. J. Agric. Food Chem. 2021, 69, 1619–1636. [Google Scholar] [CrossRef]
- Li, S.; Zhang, B.; Li, C.; Fu, X.; Huang, Q. Pickering emulsion gel stabilized by octenylsuccinate quinoa starch granule as lutein carrier: Role of the gel network. Food Chem. 2020, 305, 125476. [Google Scholar] [CrossRef]
- Ge, S.; Xiong, L.; Li, M.; Liu, J.; Yang, J.; Chang, R.; Liang, C.; Sun, Q. Characterizations of Pickering emulsions stabilized by starch nanoparticles: Influence of starch variety and particle size. Food Chem. 2017, 234, 339–347. [Google Scholar] [CrossRef]
- Lindeboom, N.; Chang, P.R.; Tyler, R.T. Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: A review. Starch-Stärke 2004, 56, 89–99. [Google Scholar] [CrossRef]
- Thompson, K.L.; Cinotti, N.; Jones, E.R.; Mable, C.J.; Fowler, P.W.; Armes, S.P. Bespoke diblock copolymer nanoparticles enable the production of relatively stable oil-in-water Pickering nanoemulsions. Langmuir 2017, 33, 12616–12623. [Google Scholar] [CrossRef]
- Guida, C.; Aguiar, A.C.; Cunha, R.L. Green techniques for starch modification to stabilize Pickering emulsions: A current review and future perspectives. Curr. Opin. Food Sci. 2021, 38, 52–61. [Google Scholar] [CrossRef]
- Saricaoglu, F.T. Application of high-pressure homogenization (HPH) to modify functional, structural and rheological properties of lentil (Lens culinaris) proteins. Int. J. Biol. Macromol. 2020, 144, 760–769. [Google Scholar] [CrossRef]
- Li, X.; He, Z.; Xu, J.; Su, C.; Xiao, X.; Zhang, L.; Zhang, H.; Li, H. Conformational changes in proteins caused by high-pressure homogenization promote nanoparticle formation in natural bone aqueous suspension. Foods 2022, 11, 2869. [Google Scholar] [CrossRef]
- Luisa Lüdtke, F.; Aparecida Stahl, M.; Grimaldi, R.; Bruno Soares Forte, M.; Lúcia Gigante, M.; Paula Badan Ribeiro, A. Optimization of high pressure homogenization conditions to produce nanostructured lipid carriers using natural and synthetic emulsifiers. Food Res. Int. 2022, 160, 111746. [Google Scholar] [CrossRef]
- Zhang, H.; Schäfer, C.; Wu, P.; Deng, B.; Yang, G.; Li, E.; Gilbert, R.G.; Li, C. Mechanistic understanding of the relationships between molecular structure and emulsification properties of octenyl succinic anhydride (OSA) modified starches. Food Hydrocoll. 2018, 74, 168–175. [Google Scholar] [CrossRef]
- Guo, B.; Liu, C.; Grossmann, L.; Weiss, J. Pickering emulsion stabilized by hydrolyzed starch: Effect of the molecular weight. J. Colloid Interface Sci. 2022, 612, 525–535. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Jiang, S.W.; Zheng, Z.; Cao, X.M.; Hou, Z.G.; Xu, J.J.; Wang, H.L.; Jiang, S.T.; Pan, L.J. Preparation and properties of OSA-modified taro starches and their application for stabilizing Pickering emulsions. Int. J. Biol. Macromol. 2019, 137, 277–285. [Google Scholar] [CrossRef]
- Wu, D.-T.; He, Y.; Yuan, Q.; Wang, S.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Effects of molecular weight and degree of branching on microbial fermentation characteristics of okra pectic-polysaccharide and its selective impact on gut microbial composition. Food Hydrocoll. 2022, 132, 107897. [Google Scholar] [CrossRef]
- Scheffler, S.L.; Wang, X.; Huang, L.; San-Martin Gonzalez, F.; Yao, Y. Phytoglycogen octenyl succinate, an amphiphilic carbohydrate nanoparticle, and epsilon-polylysine to improve lipid oxidative stability of emulsions. J. Agric. Food Chem. 2010, 58, 660–667. [Google Scholar] [CrossRef]
- Ye, F.; Miao, M.; Huang, C.; Lu, K.; Jiang, B.; Zhang, T. Elucidation of substituted ester group position in octenylsuccinic anhydride modified sugary maize soluble starch. J. Agric. Food Chem. 2014, 62, 11696–11705. [Google Scholar] [CrossRef]
- Kaufman, R.C.; Wilson, J.D.; Bean, S.R.; Herald, T.J.; Shi, Y.C. Development of a 96-well plate iodine binding assay for amylose content determination. Carbohydr. Polym. 2015, 115, 444–447. [Google Scholar] [CrossRef]
- Li, W.; Li, J.; Wang, J.; He, Y.; Hu, Y.-C.; Wu, D.-T.; Zou, L. Effects of various degrees of esterification on antioxidant and immunostimulatory activities of okra pectic-polysaccharides. Front. Nutr. 2022, 9, 1025897. [Google Scholar] [CrossRef]
- Li, G.; Zhu, F. Amylopectin molecular structure in relation to physicochemical properties of quinoa starch. Carbohydr. Polym. 2017, 164, 396–402. [Google Scholar] [CrossRef]
- Li, W.; Chen, L.; Gu, Z.; Chen, Z.; Li, H.; Cheng, Z.; Li, H.; Zou, L. Co-delivery of microRNA-150 and quercetin by lipid nanoparticles (LNPs) for the targeted treatment of age-related macular degeneration (AMD). J. Control. Release 2023, 355, 358–370. [Google Scholar] [CrossRef]
- Komiya, T.; Nara, S. Changes in crystallinity and gelatinization phenomena of potato starch by acid treatment. Starch-Stärke 1986, 38, 9–13. [Google Scholar] [CrossRef]
- Sindhu, R.; Khatkar, B.S. Thermal, structural and textural properties of amaranth and buckwheat starches. J. Food Sci. Technol. 2018, 55, 5153–5160. [Google Scholar] [CrossRef]
- Hu, Y.-C.; Hu, J.-L.; Li, J.; Wang, J.; Zhang, X.-Y.; Wu, X.-Y.; Li, X.; Guo, Z.-B.; Zou, L.; Wu, D.-T. Physicochemical characteristics and biological activities of soluble dietary fibers isolated from the leaves of different quinoa cultivars. Food Res. Int. 2023, 163, 112166. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Zhou, Y. Study on the structure and digestibility of high amylose Tartary buckwheat (Fagopyrum tataricum Gaertn.) starch-flavonoid prepared by different methods. J. Food Sci. 2021, 86, 1463–1474. [Google Scholar] [CrossRef]
- Song, X.; Gong, H.; Zhu, W.; Wang, J.; Zhai, Y.; Lin, S. Pickering emulsion stabilized by composite-modified waxy corn starch particles. Int. J. Biol. Macromol. 2022, 205, 66–75. [Google Scholar] [CrossRef]
- Ko, E.B.; Kim, J.-Y. Application of starch nanoparticles as a stabilizer for Pickering emulsions: Effect of environmental factors and approach for enhancing its storage stability. Food Hydrocoll. 2021, 120, 106984. [Google Scholar] [CrossRef]
- Wang, P.P.; Luo, Z.G.; Chun, C.; Xiong, F.; Tamer, T.M. Effects of octenyl succinic anhydride groups distribution on the storage and shear stability of Pickering emulsions formulated by modified rice starch. Carbohydr. Polym. 2020, 228, 115389. [Google Scholar] [CrossRef]
- Tao, S.; Jiang, H.; Gong, S.; Yin, S.; Li, Y.; Ngai, T. Pickering emulsions simultaneously stabilized by starch nanocrystals and zein nanoparticles: Fabrication, characterization, and application. Langmuir 2021, 37, 8577–8584. [Google Scholar] [CrossRef]
- Lopez-Silva, M.; Bello-Perez, L.A.; Castillo-Rodriguez, V.M.; Agama-Acevedo, E.; Alvarez-Ramirez, J. In vitro digestibility characteristics of octenyl succinic acid (OSA) modified starch with different amylose content. Food Chem. 2020, 304, 125434. [Google Scholar] [CrossRef]
- Qin, P.; Wang, Q.; Shan, F.; Hou, Z.; Ren, G. Nutritional composition and flavonoids content of flour from different buckwheat cultivars. Int. J. Food Sci. Technol. 2010, 45, 951–958. [Google Scholar] [CrossRef]
- Gao, L.; Bai, W.; Xia, M.; Wan, C.; Wang, M.; Wang, P.; Gao, X.; Gao, J. Diverse effects of nitrogen fertilizer on the structural, pasting, and thermal properties of common buckwheat starch. Int. J. Biol. Macromol. 2021, 179, 542–549. [Google Scholar] [CrossRef]
- Wang, C.; He, X.; Huang, Q.; Fu, X.; Luo, F.; Li, L. Distribution of octenylsuccinic substituents in modified A and B polymorph starch granules. J. Agric. Food Chem. 2013, 61, 12492–12498. [Google Scholar] [CrossRef]
- Gao, L.; Xia, M.; Li, Z.; Wang, M.; Wang, P.; Yang, P.; Gao, X.; Gao, J. Common buckwheat-resistant starch as a suitable raw material for food production: A structural and physicochemical investigation. Int. J. Biol. Macromol. 2020, 145, 145–153. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, B.; Li, J.; Shu, Z.; Wang, P.; Zeng, X. Structure and properties of octenyl succinic anhydride-modified high-amylose japonica rice starches. Polymers 2021, 13, 1325. [Google Scholar] [CrossRef]
- Bhosale, R.; Singhal, R. Effect of octenylsuccinylation on physicochemical and functional properties of waxy maize and amaranth starches. Carbohydr. Polym. 2007, 68, 447–456. [Google Scholar] [CrossRef]
- Park, S.; Chung, M.-G.; Yoo, B. Effect of octenylsuccinylation on rheological properties of corn starch pastes. Starch-Stärke 2004, 56, 399–406. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Chen, M.; Xu, F.; Zhong, F. Stabilizing oil-in-water emulsion with amorphous and granular octenyl succinic anhydride modified starches. J. Agric. Food Chem. 2018, 66, 9301–9308. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Food-grade particles for emulsion stabilization. Trends Food Sci. Technol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Yang, Y.; He, X.; Zhang, B.; Fu, X.; Tan, C.P.; Huang, Q. Starch granules as Pickering emulsifiers: Role of octenylsuccinylation and particle size. Food Chem. 2019, 283, 437–444. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Liu, H.; Gao, Y. Stabilizing emulsions using high-amylose maize starch treated by solvothermal process. Carbohydr. Polym. 2022, 284, 119190. [Google Scholar] [CrossRef] [PubMed]






| Sample | Amylose Content (%) | DS | To (°C) | Tp (°C) | Tc (°C) | ΔH (J/g) |
|---|---|---|---|---|---|---|
| TBS | 12.11 ± 0.60 a | NA | 61.90 ± 0.08 a | 66.63 ± 0.09 a | 72.40 ± 0.68 a | 11.89 ± 0.99 a |
| OS-TBS-3 | 9.61 ± 0.36 b | 0.0184 ± 0.0017 c | 60.83 ± 0.17 b | 65.57 ± 0.12 b | 71.47 ± 0.24 b | 8.90 ± 1.24 a |
| OS-TBS-5 | 8.48 ± 0.13 c | 0.0251 ± 0.0010 b | 60.37 ± 0.19 c | 64.77 ± 0.05 c | 71.37 ± 0.19 b | 8.71 ± 0.22 a |
| OS-TBS-7 | 7.73 ± 0.10 d | 0.0312 ± 0.0007 a | 58.83 ± 0.23 d | 63.47 ± 0.32 d | 70.63 ± 0.20 b | 8.79 ± 0.17 a |
| Parameter | Sample | Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| 50 °C | 60 °C | 70 °C | 80 °C | 90 °C | ||
| WSI (%) | TBS | 0.91 ± 0.01 d | 1.06 ± 0.01 d | 4.75 ± 0.27 d | 7.20 ± 0.37 d | 9.99 ± 0.53 d |
| OS-TBS-3 | 1.06 ± 0.01 c | 2.03 ± 0.11 c | 8.18 ± 0.08 c | 31.36 ± 1.55 c | 76.55 ± 1.19 c | |
| OS-TBS-5 | 1.52 ± 0.07 b | 5.73 ± 0.81 b | 26.66 ± 0.80 b | 50.81 ± 1.88 b | 78.37 ± 1.89 b | |
| OS-TBS-7 | 1.91 ± 0.31 a | 8.39 ± 0.58 a | 53.53 ± 0.48 a | 65.75 ± 0.50 a | 80.50 ± 0.31 a | |
| SP (%) | TBS | 3.55 ± 0.89 c | 2.47 ± 0.11 d | 7.39 ± 0.07 d | 8.49 ± 0.42 d | 8.53 ± 0.29 d |
| OS-TBS-3 | 3.55 ± 0.84 c | 4.42 ± 0.27 c | 10.70 ± 0.65 c | 14.19 ± 1.18 c | 38.72 ± 0.30 c | |
| OS-TBS-5 | 3.83 ± 0.25 b | 5.82 ± 1.16 b | 22.12 ± 0.82 b | 31.48 ± 2.8 b | 52.84 ± 5.15 b | |
| OS-TBS-7 | 4.29 ± 0.41 a | 10.36 ± 0.25 a | 36.18 ± 2.97 a | 52.41 ± 4.49 a | 78.63 ± 2.44 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Fan, S.; Ruan, Y.; Wu, D.; Yang, T.; Hu, Y.; Li, W.; Zou, L. Tartary Buckwheat Starch Modified with Octenyl Succinic Anhydride for Stabilization of Pickering Nanoemulsions. Foods 2023, 12, 1126. https://doi.org/10.3390/foods12061126
Lin J, Fan S, Ruan Y, Wu D, Yang T, Hu Y, Li W, Zou L. Tartary Buckwheat Starch Modified with Octenyl Succinic Anhydride for Stabilization of Pickering Nanoemulsions. Foods. 2023; 12(6):1126. https://doi.org/10.3390/foods12061126
Chicago/Turabian StyleLin, Jie, Shasha Fan, Yuyue Ruan, Dingtao Wu, Ting Yang, Yichen Hu, Wei Li, and Liang Zou. 2023. "Tartary Buckwheat Starch Modified with Octenyl Succinic Anhydride for Stabilization of Pickering Nanoemulsions" Foods 12, no. 6: 1126. https://doi.org/10.3390/foods12061126
APA StyleLin, J., Fan, S., Ruan, Y., Wu, D., Yang, T., Hu, Y., Li, W., & Zou, L. (2023). Tartary Buckwheat Starch Modified with Octenyl Succinic Anhydride for Stabilization of Pickering Nanoemulsions. Foods, 12(6), 1126. https://doi.org/10.3390/foods12061126