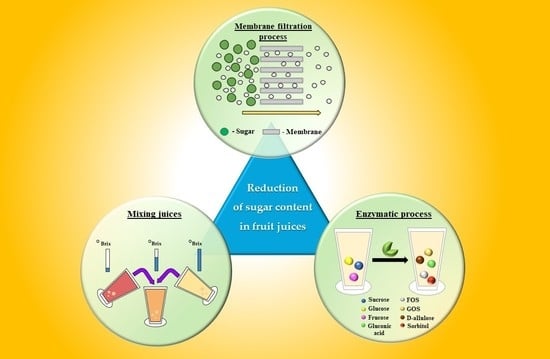
Application of Emerging Techniques in Reduction of the Sugar Content of Fruit Juice: Current Challenges and Future Perspectives
Abstract
:1. Introduction
2. Techniques Used in Reducing the Sugar Content of Juices
2.1. Conventional Techniques
2.2. Membrane Filtration Processes
2.2.1. Microfiltration and Ultrafiltration
2.2.2. Nanofiltration
2.3. Enzymatic Process
2.3.1. Gluconic Acid
2.3.2. Prebiotic Oligosaccharides
2.3.3. Enzyme Treatments and Metal Ion Supplementation
- (a)
- At least 1.5 g/L K+,
- (b)
- At least 0.5 g/L Ca2+,
- (c)
- At least 0.1 g/L Mg2+.
2.3.4. Low-Calorie Compounds
3. Current Challenges and Future Perspectives
4. Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arshad, S.; Rehman, T.; Saif, S.; Rajoka, M.S.R.; Ranjha, M.M.A.N.; Hassoun, A.; Cropotova, J.; Trif, M.; Younas, A.; Aadil, R.M. Replacement of refined sugar by natural sweeteners: Focus on potential health benefits. Heliyon 2022, 8, e10711. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lan, H.; Zhao, L.; Wang, K.; Hu, Z. Characterization and prebiotic potential of longan juice obtained by enzymatic conversion of constituent sucrose into fructo-oligosaccharides. Molecules 2018, 23, 2596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO Reveals Leading Causes of Death and Disability Worldwide: 2000–2019. Available online: https://www.who.int/ (accessed on 20 November 2022).
- Khan, M.R.; Syed, A.; Zia, S.; Ahmed, W.; Aadil, R.M.; Manzoor, M.F.; Inam-Ur-Raheem, M.; Abid, M.; Shabbir, M.A.; Qureshi, S.; et al. Stabilization and attributive amelioration of sugarcane juice by naturally derived preservatives using aonla and moringa extract. Food Sci. Nutr. 2021, 9, 3048–3058. [Google Scholar] [CrossRef]
- Scheffers, F.R.; Boer, J.M.; Verschuren, W.M.; Verheus, M.; van der Schouw, Y.T.; Sluijs, I.; Smit, H.A.; Wijga, A.H. Pure fruit juice and fruit consumption and the risk of CVD: The European Prospective Investigation into Cancer and Nutrition–Netherlands (EPIC-NL) study. Br. J. Nutr. 2019, 121, 351–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, E.A.; Calder, P.C. Effects of citrus fruit juices and their bioactive components on inflammation and immunity: A narrative review. Front. Immunol. 2021, 12, 2558. [Google Scholar] [CrossRef]
- Khomich, L.M.; Kopytko, M.V. Juices in a healthy diet: Recommendations for consumption based on chemical composition data. Vopr. Pitan. 2022, 91, 102–109. [Google Scholar] [CrossRef]
- Gill, J.M.; Sattar, N. Fruit juice: Just another sugary drink? Lancet Diabetes Endocrinol. 2014, 2, 444–446. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Liu, H.; Liu, J.; Jiao, Z. Profiles of sugar and organic acid of fruit juices: A comparative study and implication for authentication. J. Food Qual. 2020, 2020, 7236534. [Google Scholar] [CrossRef]
- Sungur, Ş.; KIlboz, Y. Determination of sugar profiles of sweetened foods and beverages. J. Food Nutr. Res. 2016, 4, 349–354. [Google Scholar]
- Gwóźdź, E.; Gębczyński, P. Prozdrowotne właściwości owoców, warzyw i ich przetworów. Postępy Fitoter. 2015, 16, 268–271. [Google Scholar]
- Goff, H.D.; Repin, N.; Fabek, H.; El Khoury, D.; Gidley, M.J. Dietary fibre for glycaemia control: Towards a mechanistic understanding. Bioact. Carbohydr. Diet. Fibre 2018, 14, 39–53. [Google Scholar] [CrossRef]
- European Parliament. European Parliament Directive 2012/12/EU of the European Parliament and of the Council of 19 April 2012 amending Council Directive 2001/112/EC Relating to Fruit Juices and Certain Similar Products Intended for Human Consumption; European Parliament: Luxembourg, 2012; pp. 1–11. [Google Scholar]
- The AIJN Code of Practice. Available online: https://aijn.eu/en/the-aijn-code-of-practice (accessed on 20 November 2022).
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Tolun, A.; Altintas, Z. Medicinal properties and functional components of beverages. In Functional and Medicinal Beverages; Academic Press: New York, NY, USA, 2019; pp. 235–284. [Google Scholar]
- Olewnik-Mikołajewska, A.; Gutkowska, K.; Sajdakowska, M.; Żakowska-Biemans, S.; Kowalczuk, I. Consumer expectancies towards innovative animal-derived food products. Zesz. Nauk. Szkoły Głównej Gospod. Wiej. Ekon. Organ. Gospod. Żywnościowej 2016, 116, 161–171. [Google Scholar]
- Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2007, 12, 3–18.
- Pruksasri, S.; Lanner, B.; Novalin, S. Nanofiltration as a potential process for the reduction of sugar in apple juices on an industrial scale. Lwt 2020, 133, 110118. [Google Scholar] [CrossRef]
- Liu, S.; Munasinghe, L.L.; Ohinmaa, A.; Veugelers, P.J. Added, free and total sugar content and consumption of foods and beverages in Canada. Health Rep. 2020, 31, 14–24. [Google Scholar]
- Rupérez, A.I.; Mesana, M.I.; Moreno, L.A. Dietary sugars, metabolic effects and child health. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 206–216. [Google Scholar] [CrossRef]
- Azaïs-Braesco, V.; Sluik, D.; Maillot, M.; Kok, F.; Moreno, L.A. A review of total & added sugar intakes and dietary sources in Europe. Nutr. J. 2017, 16, 6. [Google Scholar]
- Newens, K.J.; Walton, J.; Newens, K.J.; Walton, J. A review of sugar consumption from nationally representative dietary surveys across the world. J. Hum. Nutr. Diet. 2016, 29, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Liquid Fruit Market Report 2018. Available online: https://aijn.eu/en/publications (accessed on 20 December 2022).
- Sugar Consumption per Capita. Available online: https://www.helgilibrary.com (accessed on 20 December 2022).
- Abdo, E.M.; Shaltout, O.E.S.; Ali, S.; Mansour, H.M. A functional orange juice fortified with beetroot by-products attenuates hyperlipidemia and obesity induced by a high-fat diet. Antioxidants 2022, 11, 457. [Google Scholar] [CrossRef]
- Miskinis, R.D.A.S.; do Nascimento, L.Á.; Colussi, R. Bioactive Compounds from Acerola Pomace: A review. Food Chem. 2022, 404, 134613. [Google Scholar] [CrossRef]
- Pourmasoumi, M.; Hadi, A.; Najafgholizadeh, A.; Joukar, F.; Mansour-Ghanaei, F. The effects of cranberry on cardiovascular metabolic risk factors: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 774–788. [Google Scholar] [CrossRef]
- Yousaf, N.Y.; Tepper, B.J. The Effects of Cranberry Polyphenol Extract (CPE) Supplementation on Astringency and Flavor Perception as a Function of PROP Taster Status and Other Individual Factors. Int. J. Environ. Res. Public Health 2022, 19, 11995. [Google Scholar] [CrossRef] [PubMed]
- Shwe, K.K.W.; Yangon, M.; Win, S.S. Effect of Amylolytic Enzyme Treatment on Banana Juice Preparation, Yield and Clarification: A Preliminary Approach. Int. J. Sci. Eng. Appl. 2019, 8, 75–78. [Google Scholar] [CrossRef]
- Conidi, C.; Castro-Muñoz, R.; Cassano, A. Membrane-based operations in the fruit juice processing industry: A review. Beverages 2020, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 2017, 195, 1–13. [Google Scholar] [CrossRef]
- Echavarría, A.P.; Torras, C.; Pagán, J.; Ibarz, A. Fruit juice processing and membrane technology application. Food Eng. Rev. 2011, 3, 136–158. [Google Scholar] [CrossRef]
- Mirsaeedghazi, H.; Emam-Djomeh, Z. Clarification of Bitter Orange (Citrus aurantium) Juice Using Microfiltration with Mixed Cellulose Esters Membrane. J. Food Process. Preserv. 2017, 41, e12738. [Google Scholar] [CrossRef]
- Cesar, L.T.; de Freitas Cabral, M.; Maia, G.A.; de Figueiredo, R.W.; de Miranda, M.R.A.; de Sousa, P.H.M.; Brasil, I.M.; Gomes, C.L. Effects of Clarification on Physicochemical Characteristics, Antioxidant Capacity and Quality Attributes of Açaí (Euterpe oleracea Mart.) Juice. J. Food Sci. Technol. 2014, 51, 3293–3300. [Google Scholar] [CrossRef]
- Rajendran, S.R.; Mason, B.; Doucette, A.A. Review of membrane separation models and technologies: Processing complex food-based biomolecular fractions. Food Bioprocess Technol. 2021, 14, 415–428. [Google Scholar] [CrossRef]
- Moreno-Vilet, L.; Bonnin-Paris, J.; Bostyn, S.; Ruiz-Cabrera, M.A.; Moscosa-Santillán, M. Assessment of sugars separation from a model carbohydrates solution by nanofiltration using a design of experiments (DoE) methodology. Sep. Purif. Technol. 2014, 131, 84–93. [Google Scholar] [CrossRef]
- Duarah, P.; Mondal, P.; Das, P.P.; Purkait, M.K. Potential of NF Membranes 1 for the Removal of Aquatic Pollutants from Industrial Wastewater A Review. In Membrane and Membrane-Based Processes for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2023; pp. 1–17. [Google Scholar]
- Wilson, D.I. Fouling during food processing–progress in tackling this inconvenient truth. Curr. Opin. Food Sci. 2018, 23, 105–112. [Google Scholar] [CrossRef]
- Lu, C.; Bao, Y.; Huang, J.Y. Fouling in membrane filtration for juice processing. Curr. Opin. Food Sci. 2021, 42, 76–85. [Google Scholar] [CrossRef]
- Panigrahi, C.; Karmakar, S.; Mondal, M.; Mishra, H.N.; De, S. Modeling of permeate flux decline and permeation of sucrose during microfiltration of sugarcane juice using a hollow-fiber membrane module. Innov. Food Sci. Emerg. Technol. 2018, 49, 92–105. [Google Scholar] [CrossRef]
- Meher, J.; Durairaj, S.; Dharmadhikari, S.; Meher, R. Future Scope of Membrane Technology in Pineapple Juice Processing: A Review. In Membrane and Membrane-Based Processes for Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2023; pp. 181–197. [Google Scholar]
- Tontul, I. Clarification of fruit juices and wine using membrane processing techniques. In Applications of Membrane Technology for Food Processing Industries; CRC Press: Boca Raton, FL, USA, 2020; pp. 129–154. [Google Scholar]
- Molaee Parvarei, M.; Khorshidian, N.; Yousefi, M.; Zendeboodi, F.; Mirsaeedghazi, H. Effect of membrane clarification on the physicochemical properties of fruit juices: A review. Iran. J. Chem. Chem. Eng. 2022, 41, 3277–3290. [Google Scholar]
- Ghosh, P.; Pradhan, R.C.; Mishra, S. Clarification of Jamun Juice by Centrifugation And Microfiltration: Analysis of Quality Parameters, Operating Conditions, and Resistance. J. Food Process Eng. 2018, 41, e12603. [Google Scholar] [CrossRef]
- Mirsaeedghazi, H.; Emam-Djomeh, Z.; Mousavi, S.M.; Ahmadkhaniha, R.; Shafiee, A. Effect of membrane clarification on the physicochemical properties of pomegranate juice. Int. J. Food Sci. Technol. 2010, 45, 1457–1463. [Google Scholar] [CrossRef]
- Colantuono, A.; Vitaglione, P.; Manzo, N.; Blaiotta, G.; Montefusco, I.; Marrazzo, A.; Pizzolongo, F.; Romano, R. Evaluation of microfiltration and heat treatment on the microbiological characteristics, phenolic composition and volatile compound profile of pomegranate (Punica granatum L.) juice. J. Sci. Food Agric. 2018, 98, 3324–3332. [Google Scholar] [CrossRef]
- Baranska, A.; Kot, A.; Samborska, K. Ultrafiltration as a method to obtain sugar reduced cloudy juices research on juice’s properties. Zesz. Probl. Postępów Nauk. Rol. 2020, 600, 3–11. [Google Scholar] [CrossRef]
- Conidi, C.; Destani, F.; Cassano, A. Performance of hollow fiber ultrafiltration membranes in the clarification of blood orange juice. Beverages 2015, 1, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.; Xie, C.; Lv, Y.; Yang, K.; Sun, P. Changes in physicochemical profiles and quality of apple juice treated by ultrafiltration and during its storage. Food Sci. Nutr. 2020, 8, 2913–2919. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V.; Barragán-Huerta, B.E.; Yáñez-Fernández, J.; Piña-Rosas, J.A.; Arboleda-Mejía, J. Processing of Xoconostle fruit (Opuntia joconostle) Juice for Improving its Commercialization Using Membrane Filtration. J. Food Process. Preserv. 2018, 42, e13394. [Google Scholar] [CrossRef]
- Ilame, S.A.; Singh, S.V. Physico-chemical properties of ultrafiltered kinnow (mandarin) fruit juice. J. Food Sci. Technol. 2018, 55, 2189–2196. [Google Scholar] [CrossRef]
- Samborska, K.; Kamińska, P.; Jedlińska, A.; Matwijczuk, A.; Kamińska-Dwórznicka, A. Membrane processing in the sustainable production of low-sugar apple-cranberry cloudy juice. Appl. Sci. 2018, 8, 1082. [Google Scholar] [CrossRef] [Green Version]
- Arriola, N.A.; dos Santos, G.D.; Prudêncio, E.S.; Vitali, L.; Petrus, J.C.C.; Castanho Amboni, R.D. Potential of nanofiltration for the concentration of bioactive compounds from watermelon juice. Int. J. Food Sci. Technol. 2014, 49, 2052–2060. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A. Recovery of phenolic compounds from bergamot juice by nanofiltration membranes. Desalination Water Treat. 2015, 56, 3510–3518. [Google Scholar] [CrossRef]
- Gaglianò, M.; Conidi, C.; de Luca, G.; Cassano, A. Partial Removal of Sugar from Apple Juice by Nanofiltration and Discontinuous Diafiltration. Membranes 2022, 12, 712. [Google Scholar] [CrossRef]
- Ghosh, P.; Pradhan, R.C.; Mishra, S.; Rout, P.K. Quantification and concentration of anthocyanidin from Indian blackberry (Jamun) by combination of ultra-and nano-filtrations. Food Bioprocess Technol. 2018, 11, 2194–2203. [Google Scholar] [CrossRef]
- Jelemenský, M.; Paulen, R.; Fikar, M.; Kovács, Z. Time-optimal diafiltration in the presence of membrane fouling. IFAC Proc. Vol. 2014, 473, 4897–4902. [Google Scholar] [CrossRef]
- Di Corcia, S.; Dhuique-Mayer, C.; Dornier, M. Concentrates from citrus juice obtained by crossflow microfiltration: Guidance of the process considering carotenoid bioaccessibility. Innov. Food Sci. Emerg. Technol. 2020, 66, 102526. [Google Scholar] [CrossRef]
- Conidi, C.; Castro-Muñoz, R.; Cassano, A. Nanofiltration in beverage industry. In Nanotechnology in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 525–548. [Google Scholar]
- Cao, Y.; Chen, G.; Wan, Y.; Luo, J. Nanofiltration membrane for bio-separation: Process-oriented materials innovation. Eng. Life Sci. 2021, 21, 405–416. [Google Scholar] [CrossRef]
- Morthensen, S.T.; Luo, J.; Meyer, A.S.; Jørgensen, H.; Pinelo, M. High performance separation of xylose and glucose by enzyme assisted nanofiltration. J. Membr. Sci. 2015, 492, 107–115. [Google Scholar] [CrossRef]
- Nath, K.; Dave, H.K.; Patel, T.M. Revisiting the recent applications of nanofiltration in food processing industries: Progress and prognosis. Trends Food Sci. Technol. 2018, 73, 12–24. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Castro-Muñoz, R. Current and future applications of nanofiltration in food processing. In Separation of Functional Molecules in Food by Membrane Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 305–348. [Google Scholar]
- Taraboulsi, F.A., Jr.; Tomotani, E.J.; Vitolo, M. Multienzymatic sucrose conversion into fructose and gluconic acid through fed-batch and membrane-continuous processes. Appl. Biochem. Biotechnol. 2011, 165, 1708–1724. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, L.; Zhang, W.; Li, C.; Mao, S.; Lu, F.; Qin, H.M. Improving the enzyme property of D-allulose 3-epimerase from a thermophilic organism of Halanaerobium congolense through rational design. Enzym. Microb. Technol. 2021, 149, 109850. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Bourbon, A.I.; Peixoto, A.R.; Silva, A.S.; Tasso, A.; Almeida, C.; Nobre, C.; Nunes, C.; Sánchez, C.; Gonçalves, D.A.; et al. Strategies for the reduction of sugar in food products. In Food Structure Engineering and Design for Improved Nutrition, Health and Well-Being; Elsevier: Amsterdam, The Netherlands, 2023; pp. 219–241. [Google Scholar]
- Aziz, M.G.; Michlmayr, H.; Kulbe, K.D.; del Hierro, A.M. Biotransformation of pineapple juice sugars into dietetic derivatives by using a cell free oxidoreductase from Zymomonas mobilis together with commercial invertase. Enzym. Microb. Technol. 2011, 48, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Qian, Z.; Ge, J.; Du, R. Glucansucrase Produced by Lactic Acid Bacteria: Structure, Properties, and Applications. Fermentation 2022, 8, 629. [Google Scholar] [CrossRef]
- Vera, C.; Illanes, A.; Guerrero, C. Enzymatic production of prebiotic oligosaccharides. Curr. Opin. Food Sci. 2021, 37, 160–170. [Google Scholar] [CrossRef]
- Minusse, R.C.; Pastore, G.M.; Du’ran, N. Potential applications of laccase in the food industry. Food Sci. Technol. 2002, 13, 205–216. [Google Scholar] [CrossRef]
- Goffin, D.; Delzenne, N.; Blecker, C.; Hanon, E.; Deroanne, C.; Paquot, M. Will isomalto-oligosaccharides, a well-established functional food in Asia, break through the European and American market? The status of knowledge on these prebiotics. Crit. Rev. Food Sci. Nutr. 2011, 51, 394–409. [Google Scholar] [CrossRef]
- Bashir, N.; Sood, M.; Bandral, J.D. Enzyme immobilization and its applications in food processing: A review. Int. J. Chem. Stud. 2020, 8, 254–261. [Google Scholar] [CrossRef]
- Atacan, K.; Cakiroglu, B.; Ozacar, M. Improvement of the stability and activity of immobilized trypsin on modified Fe3O4 magnetic nanoparticles for hydrolysis of bovine serum albumin and its application in the bovine milk. Food Chem. 2016, 212, 460–468. [Google Scholar] [CrossRef]
- Bezborodov, A.M.; Zagustina, N.A. Enzymatic biocatalysis in chemical synthesis of pharmaceuticals. Appl. Biochem. Microbiol. 2016, 52, 237–249. [Google Scholar] [CrossRef]
- Ureta, M.M.; Martins, G.N.; Figueira, O.; Pires, P.F.; Castilho, P.C.; Gomez-Zavaglia, A. Recent advances in β-galactosidase and fructosyltransferase immobilization technology. Crit. Rev. Food Sci. Nutr. 2021, 61, 2659–2690. [Google Scholar] [CrossRef]
- Zdarta, Ł. Enzymes Immobilization onto Selected Organic and Inorganic Carriers. Ph.D. Thesis, Poznan University of Technology, Poznan, Poland, 2017. [Google Scholar]
- Kourkoutas, Y.; Bekatorou, A.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Immobilization technologies and support materials suitable inalcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Wahab, R.A.; Elias, N.; Abdullah, F.; Ghoshal, S.K. On the taught new tricks of enzymes immobilization: An all-inclusive overview. React. Funct. Polym. 2020, 152, 104613. [Google Scholar] [CrossRef]
- Seenuvasan, M.; Kumar, K.S.; Kumar, A.; Parthiban, R. Review on surface modification of nanocarriers to overcome diffusion limitations: An enzyme immobilization aspect. Biochem. Eng. J. 2020, 158, 107574. [Google Scholar]
- Yadav, S.R.; Nainawat, A.K.; Kaushik, S.; Sharma, A.; Sharma, I.K. New Eco-friendly Synthetic Procedures for the Reduction of Carbonyl Compounds. Asian J. Exp. Sci. 2005, 19, 135–141. [Google Scholar]
- Kuo, P.C.; Lin, Z.X.; Wu, T.Y.; Hsu, C.H.; Lin, H.P.; Wu, T.S. Effects of morphology and pore size of mesoporous silicas on the efficiency of an immobilized enzyme. RSC Adv. 2021, 11, 10010–10017. [Google Scholar] [CrossRef] [PubMed]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of enzymes: A literature survey. Immobil. Enzym. Cells 2013, 1051, 15–31. [Google Scholar]
- Better Juice. Available online: https://www.better-juice.com/technology (accessed on 11 October 2022).
- Shapira, R.; Balachinsky, E. Low Sugar Food Products with Higher Fiber Content. U.S. Patent Application No. 16/343,840, 8 August 2019. [Google Scholar]
- Ricca, E.; Brucher, B.; Schrittwieser, J.H. Multi-enzymatic cascade reactions: Overview and perspectives. Adv. Synth. Catal. 2011, 353, 2239–2262. [Google Scholar] [CrossRef]
- Vitolo, M. Overview on invertase. World J. Pharm. Pharm. Sci. 2021, 10, 49–73. [Google Scholar]
- Khandekar, D.C.; Palai, T.; Agarwal, A.; Bhattacharya, P.K. Kinetics of sucrose conversion to fructo-oligosaccharides using enzyme (invertase) under free condition. Bioprocess Biosyst. Eng. 2014, 37, 2529–2537. [Google Scholar] [CrossRef]
- Kornecki, J.F.; Carballares, D.; Tardioli, P.W.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Alcantara, A.R.; Fernandez-Lafuente, R. Enzyme production of D-gluconic acid and glucose oxidase: Successful tales of cascade reactions. Catal. Sci. Technol. 2020, 10, 5740–5771. [Google Scholar] [CrossRef]
- Trawczyńska, I. New method of determining kinetic parameters for decomposition of hydrogen peroxide by catalase. Catalysts 2020, 10, 323. [Google Scholar] [CrossRef] [Green Version]
- Laue, C.; Ballance, S.; Knutsen, S.H.; Papazova, E.; Soeth, E.; Pannenbeckers, A.; Schrezenmeir, J. Glycemic response to low sugar apple juice treated with invertase, glucose oxidase and catalase. Eur. J. Clin. Nutr. 2019, 73, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Kherade, M.; Solanke, S.; Tawar, M.; Wankhede, S. Fructooligosaccharides: A comprehensive review. J. Ayurvedic Herb. Med. 2021, 7, 193–200. [Google Scholar] [CrossRef]
- Shahbaz, M.U.; Arshad, M.; Mukhtar, K.; Nabi, B.G.; Goksen, G.; Starowicz, M.; Nawaz, A.; Ahmad, I.; Walayat, N.; Manzoor, M.F.; et al. Natural Plant Extracts: An Update about Novel Spraying as an Alternative of Chemical Pesticides to Extend the Postharvest Shelf Life of Fruits and Vegetables. Molecules 2022, 27, 5152. [Google Scholar] [CrossRef]
- Toledo, L.E.T.; García, D.M.; Cruz, E.P.; Intriago, L.M.R.; Pérez, J.N.; Chanfrau, J.M.P. Fructosyltransferases and invertases: Useful enzymes in the food and feed industries. Enzym. Food Biotechnol. 2019, 451–469. [Google Scholar] [CrossRef]
- Son, G.; Nguyen, T.T.H.; Park, B.; Kwak, S.; Jin, J.; Kim, Y.-M.; Moon, Y.-H.; Park, S.; Kim, S.-B.; Kim, D. Synthesis and characterization of stevioside having low degree polymerized glucosides using dextransucrase and dextranase. Enzym. Microb. Technol. 2020, 132, 109412. [Google Scholar] [CrossRef]
- Hoshino, T.; Fujiwara, T. The findings of glucosyltransferase enzymes derived from oral streptococci. Jpn. Dent. Sci. Rev. 2022, 58, 328–335. [Google Scholar] [CrossRef]
- Besrour-Aouam, N.; Fhoula, I.; Hernández-Alcántara, A.M.; Mohedano, M.L.; Najjari, A.; Prieto, A.; Ruas-Madiedo, P.; López, P.; Ouzari, H.-I. The role of dextran production in the metabolic context of Leuconostoc and Weissella Tunisian strains. Carbohydr. Polym. 2021, 253, 117254. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Kim, Y.J.; Baek, N.I.; Mathiyalagan, R.; Wang, C.; Jin, Y.; Xu, X.Y.; Yang, D.C. Glycosyltransformation of ginsenoside Rh2 into two novel ginsenosides using recombinant glycosyltransferase from Lactobacillus rhamnosus and its in vitro applications. J. Ginseng Res. 2021, 45, 48–57. [Google Scholar] [CrossRef]
- Tingirikari, J.M.R.; Gomes, W.F.; Rodrigues, S. Efficient production of prebiotic gluco-oligosaccharides in orange juice using immobilized and co-immobilized dextransucrase. Appl. Biochem. Biotechnol. 2017, 183, 1265–1281. [Google Scholar] [CrossRef] [PubMed]
- Vafeiadi, C.; Johansson, S.; Sanz-Valero, J.; Pu-Sheng, C.; Gancel, C.; Austin, S.C.; Bourdin, G. Sugar Reduction of Food Products. U.S. Patent Application No. 15/569,139, 3 November 2016. [Google Scholar]
- Sans-Valero, J.; Cheng, P.S. Intrinsic Sugar Reduction of Juices and Ready to Drink Products. U.S. Patent Application No. 13/882,697, 22 August 2013. [Google Scholar]
- Hajar-Azhari, S.; Abd Rahim, M.H.; Wan, W.A.A.Q.I.; Sarbini, S.R.; Saari, N. Novel fructooligosaccharide conversion from sugarcane syrup using a specialised enzymatic pH-stat bioreactor. Process Biochem. 2020, 95, 55–63. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Feng, Z.; Guan, L.; Lu, F.; Qin, H.M. Two-step biosynthesis of D-allulose via a multienzyme cascade for the bioconversion of fruit juices. Food Chem. 2021, 357, 129746. [Google Scholar] [CrossRef]
- Novozymes. Viscozyme® L. Available online: https://biosolutions.novozymes.com/en/juice-fruit-vegetables/products/olive-oil/viscozyme-l (accessed on 21 February 2023).
- Ureta, M.M.; Romano, N.; Kakisu, E.; Gómez-Zavaglia, A. Synthesis of fructo-oligosaccharides using grape must and sucrose as raw materials. Food Res. Int. 2019, 123, 166–171. [Google Scholar] [CrossRef]
- Özgür, M.; Özgür, E.B.; Dinçoğlu, A.H. Prebiotic Effect of D-Allulose (D-Psicose): Traditional Review. Turk. Klin. J. Health Sci. 2022, 7, 573–577. [Google Scholar]
- Renuka, B.; Kulkarni, S.G.; Vijayanand, P.; Prapulla, S.G. Fructooligosaccharide fortification of selected fruit juice beverages: Effect on the quality characteristics. LWT-Food Sci. Technol. 2009, 42, 1031–1033. [Google Scholar] [CrossRef]
- Schrezenmeir, J.; Knutsen, S.H.; Ballance, S. Improved Sugar-Depleted Fruit or Vegetable Juice and Juice-Retaining Fruit or Vegetable Derived Matter, Methods of Producing the Same and the Use Thereof to Maintain Health and to Treat and Prevent Medical Ailments. International Patent Application No. PCT/GB2015/052880, 12 November 2019. [Google Scholar]
- Regulation (EC) No 1925/2006 of the European Parliament and of the Council of 20 December 2006 on the addition of vitamins and minerals and of certain other substances to foods. Off. J. Eur. Union OJ 2006, 50, 26–38.
- Jürkenbeck, K.; Haarhoff, T.; Spiller, A.; Schulze, M. Does Allulose Appeal to Consumers? Results from a Discrete Choice Experiment in Germany. Nutrients 2022, 14, 3350. [Google Scholar] [CrossRef]
- Niibo, M.; Kanasaki, A.; Iida, T.; Ohnishi, K.; Ozaki, T.; Akimitsu, K.; Minamino, T. D-allulose protects against diabetic nephropathy progression in Otsuka Long-Evans Tokushima Fatty rats with type 2 diabetes. PLoS ONE 2022, 17, e0263300. [Google Scholar] [CrossRef] [PubMed]
- GRAS Notice 000400: D-Psicose. Available online: https://www.regulations.gov/ (accessed on 30 November 2022).
- Daniel, H.; Hauner, H.; Hornef, M.; Clavel, T. Allulose in human diet: The knowns and the unknowns. Br. J. Nutr. 2022, 128, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Xu, W.; Zhang, W.; Chen, Y.; Mu, W. Efficient Utilization of Fruit Peels for the Bioproduction of D-Allulose and D-Mannitol. Foods 2022, 11, 3613. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hong, S.H.; Kim, K.R.; Oh, D.K. High-yield production of pure tagatose from fructose by a three-step enzymatic cascade reaction. Biotechnol. Lett. 2017, 39, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Sokołowska, E.; Sadowska, A.; Sawicka, D.; Kotulska-Bąblińska, I.; Car, H. A head-to-head comparison review of biological and toxicological studies of isomaltulose, d-tagatose, and trehalose on glycemic control. Crit. Rev. Food Sci. Nutr. 2022, 62, 5679–5704. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, H.; Okuma, K.; Iida, T.; Izumori, K.; Tokuda, M.; Fukada, K. Sweetener Containing D-Psicose and Foods and Drinks Obtained by Using the Same. U.S. Patent No. 10,869,494, 22 December 2020. [Google Scholar]
- Awuchi, C.G.; Echeta, K.C. Current developments in sugar alcohols: Chemistry, nutrition, and health concerns of sorbitol, xylitol, glycerol, arabitol, inositol, maltitol, and lactitol. Int. J. Adv. Acad. Res. 2019, 5, 1–33. [Google Scholar]
- Zhang, Z.H.; Zeng, X.A.; Brennan, C.S.; Ma, H.; Aadil, R.M. Preparation and characterisation of novelty food preservatives by Maillard reaction between ε-polylysine and reducing sugars. Int. J. Food Sci. Technol. 2019, 54, 1824–1835. [Google Scholar] [CrossRef]
- Prasetijo, L.D.; Trisnawati, C.Y.; Srianta, I. Physicochemical and sensory characteristics of reduced sugar starfruit juice. Food Res. 2017, 1, 114–117. [Google Scholar] [CrossRef]
- Charcosset, C. Classical and recent applications of membrane processes in the food industry. Food Eng. Rev. 2021, 13, 322–343. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Gontarek, E. Nanofiltration in the food industry. In Handbook of Food Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 73–106. [Google Scholar]
- Bhattacharjee, C.; Saxena, V.K.; Dutta, S. Fruit juice processing using membrane technology: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 136–153. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of microbial enzymes in food industry. Food Technol. Biotechnol. 2018, 56, 16. [Google Scholar] [CrossRef] [PubMed]




| Juice 1 (°Brix Value) | Juice 2 (°Brix Value) | Volume Ratio Juice 2/Juice 1 (v/v) | The Rate of Juice 2 °Brix Value Depletion |
|---|---|---|---|
| Coconut water (°Brix value equal to 3.6) | Apple/orange juice (°Brix = 10.0) | 1:1 | 32% |
| 1:2 | 43% | ||
| Grape juice (°Brix = 13.5) | 1:1 | 37% | |
| 1:2 | 49% | ||
| Mango juice/puree (°Brix = 14.0) | 1:1 | 37% | |
| 1:2 | 49% | ||
| Banana juice/puree (°Brix = 20.0) | 1:1 | 49% | |
| 1:2 | 55% | ||
| Acerola juice/puree or Cranberry juice (°Brix value equal to 6.0) | Apple/orange juice (°Brix = 10.0) | 1:1 | 20% |
| 1:2 | 27% | ||
| Grape juice (°Brix = 13.5) | 1:1 | 28% | |
| 1:2 | 37% | ||
| Mango juice/puree (°Brix = 14.0) | 1:1 | 29% | |
| 1:2 | 38% | ||
| Banana juice/puree (°Brix = 20.0) | 1:1 | 35% | |
| 1:2 | 47% | ||
| Strawberry juice/puree or Raspberry juice (°Brix value equal to 7.0) | Apple/orange juice (°Brix = 10.0) | 1:1 | 15% |
| 1:2 | 20% | ||
| Grape juice (°Brix = 13.5) | 1:1 | 24% | |
| 1:2 | 32% | ||
| Mango juice/puree (°Brix = 14.0) | 1:1 | 25% | |
| 1:2 | 33% | ||
| Banana juice/puree (°Brix = 20.0) | 1:1 | 33% | |
| 1:2 | 43% |
| Process | Membrane and Pore Size | Type of Fruit Juice | Operating Conditions | A Degree of TS and TSS Reduction | Ref. |
|---|---|---|---|---|---|
| MF | Polyacrylonitrile-based MF-grade hollow fibers. Pore size: 0.1 μm | Sugarcane juice | T = 20 °C CFR = 30 L/h TMP = 104 kPa | TSS = 9.0% | [41] |
| MF | Mixed cellulose esters membrane (MCE), 0.45 µm | Pomegranate juice | - | TSS = 8.1% | [47] |
| MF | Hollow fiber membrane, 0.45 µm | Jamun (Syzygium cumini) juice | T = 30 °C CFR = 10 L/h TMP = 137.8 kPa | In retentate: TSS = 8.1% | [44] |
| MF | Mixed cellulose esters (MCE) membrane, 0.22 µm | Pomegranate juice | - | TSS = 24.6% | [45] |
| UF | Mixed cellulose esters (MCE) membrane, 0.025 µm | Pomegranate juice | - | TSS = 20.4% | [45] |
| MF/UF | Mixed cellulose esters (MCE) MF membrane, 0.22 µm Mixed cellulose esters (MCE) UF membrane, 0.025 µm | Pomegranate juice | - | TSS = 40.4% | [42] |
| UF | Ceramic membrane, 15 kDa | Cloudy apple juice | TMP = 0.35 MPa | In permeate: TS (after UF) = 21.2% TSS (after UF) = 20.0% In retentate: TS (after UF) = 15.4% TSS (after UF) = 16.4% | [48] |
| UF | Three hollow fiber membranes:
| Red-colored blood oranges (Citrus sinensis) juice | T = 20 °C TMP = 50 kPa Qf = 140 L/h |
| [49] |
| UF | Polyethersulfone, 10 kDa (PES-10 kDa) | Apple juice | T = 25 °C CFR = 30 L/h TMP = 0.75 MPa | TS = 54.3% TSS = 31.7% | [50] |
| UF | Polysulfone, 100 Da | The Xoconostle fruits | T = 25 °C TMP = 138 kPa Qf = 58 L/h | TSS = 10.2% | [51] |
| Primary clarification MF + UF | Hollow fiber polyacrylonitrile (PAN) MF membrane Polymeric hollow fiber membrane made of polysulfone, 30 kDa (PSU-30 kDa) UF membrane | Kinnow (mandarin) juice | T = 25 °C TMP = 69 kPa CFR = 20 L/h | TS = 1.4% TSS = 13.4% | [52] |
| UF/DF | Ceramic tubular membrane, 15 kDa | Cloudy apple-cranberry juice | TMP = 0.35 MPa | In permeate: TS (after UF) = 25.5% TSS (after UF) = 22.6% TS (after DF) = 31.6% TSS (after DF) = 45.2% In retentate: TS (after UF) = 23.5% TSS (after UF) = 16.1% TS (after DF) = 41.8% TSS (after DF) = 34.7% | [53] |
| NF/DF | A flat sheet polyamide-thin film composite NF membrane, 150–300 Da | Apple juice | T = 25 °C Pressure = 50 bar flow rate = 40 L/h | TS = 94.9% | [19] |
| NF | Spiral polyvinylidene difluoride (PVDF) membrane, 150–300 Da | Watermelon juice | T = 25 °C pressure = 600 kPa flow rate = 1 m/s | TSS = 29.4% | [54] |
| Clarified by UF + NF | Microdyn Nadir Polyethersulfone, 1000 Da | Bergamot juice | TMP = 6 bar T = 20 °C | TSS = 33.6% | [55] |
| Microdyn Nadir Polyethersulfone, 400 Da | TSS = 52.7% | ||||
| Semi-aromatic piperazine-based polyamide layer on top of a polysulphone microporous support, 150–250 Da | TSS = 75.3% | ||||
| Clarified by UF + NF/DF | Spiral-wound membranes: TFC 200–300 Da | Apple juice | T = 25 °C TMP = 25 bar Qf = 7 L/min | TS = 60% | [56] |
| MF/UF/NF | Hollow fibre MF/UF membranes, 0.45 µm (MF) and 50 kDa (UF). Spiral wound NF membrane, 300 Da | Indian blackberry juice | MF/UF: TMP = 0.137 mPa NF: TMP = 2.5 mPa | TSS = 16.7% | [57] |
| Basic Methods of Enzyme/ Cell Immobilization | Characteristics of the Method | Examples of Carriers |
|---|---|---|
| Immobilization on the surface of a solid carrier | Consists of the creation of a covalent bond between the cell membrane of the microorganism/the enzyme and the carrier or is the result of electrostatic forces on the carrier, causing physical adsorption | Cellulosic materials: DEAE-cellulose, wood, delignified sawdust, sawdust; inorganic materials: porous porcelain, hydromica, porous glass, palygorskite, montmorillonites |
| Entrapment within a porous matrix | The porous material is formed into the cell culture, and the cells or the enzyme are allowed to penetrate the porous matrix until other cells/enzymes restrict their mobility | polysaccharide gels: chitosan, polygalacturonic acid, alginates, κ-carrageenan, agar; polymeric matrixes: polyvinyl alcohol, collagen, gelatin |
| Cell/Enzymes flocculation (Aggregation) | It is a cell/enzyme aggregation of the microorganism/enzyme: physical or chemical cross-linking. It is an aggregation of cells/enzymes to form a larger unit. Because of the large aggregates, they can be used as a method of immobilization | Mainly molds, fungi, and plant cells are capable of forming aggregates |
| Mechanical containment behind a barrier | Using microporous membrane filters, we obtained immobilization of a cell/enzyme into a microcapsule or entrapment of a cell/enzyme on the interaction surface of two liquids that are not miscible. An appropriate type of immobilization is when little transfer of compounds and cell-free products is expected. The main disadvantage of this method is that the growth of cells can fill the filter and the limitation of mass transfer | Chitosan, k-carrageenan, and collagen can be used as polymers’ porous networks for entrapment |
| Sugar | Enzyme | Product | Type of Juice | Additional Compounds | Ref. |
|---|---|---|---|---|---|
| Sucrose Glucose | Invertase; Glucose oxidase; catalase | Gluconic Acid | Apple juice | KOH/Ca(OH)2 to optimize organoleptic properties | [91] |
| Sucrose Glucose | Dextransucrase from L. mesenteroides and dextranase from C. erraticum | GOS | Orange juice | Co-immobilization of enzymes on alginate beads | [99] |
| Sucrose Glucose | Dextransucrase from L. mesenteroides | GOS | Concentrated orange juice | 1% Ca(OH)2 to improve transferase activity, hydrolytic activity, and the total activity of the enzyme | [26] |
| Sucrose Glucose | Glucosyltransferase that comprises an amino acid sequence at least 95% identical to SEQ ID NO: 1 | GOS | Fruit juice containing sucrose, and glucose/fructose | 1 mM CaCl2 to improve transferase activity, hydrolytic activity, and the total activity of the enzyme | [100] |
| Sucrose Glucose | Glucosyltransferase (such as dextransucrase) | GOS | Fruit juice containing sucrose and glucose | - | [101] |
| Sucrose | Fructosyltransferase | FOS | Fruit juice containing sucrose | ||
| Sucrose | Viscozyme L (Novozymes, Denmark) | FOS | Sugarcane syrup | - | [102] |
| Sucrose Glucose Fructose | Invertase (INV) GFOR from Z. mobilis | Gluconic acid; Sorbitol | Pineapple juice | - | [68] |
| Sucrose Glucose Fructose | Invertase (INV); D-glucose isomerase (GI); D-allulose 3-epimerase from Pirellula sp. (DAE) | D-Allulose | Mango, orange, and sugar cane juices | GI and DAE were immobilized on epoxy support | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cywińska-Antonik, M.; Chen, Z.; Groele, B.; Marszałek, K. Application of Emerging Techniques in Reduction of the Sugar Content of Fruit Juice: Current Challenges and Future Perspectives. Foods 2023, 12, 1181. https://doi.org/10.3390/foods12061181
Cywińska-Antonik M, Chen Z, Groele B, Marszałek K. Application of Emerging Techniques in Reduction of the Sugar Content of Fruit Juice: Current Challenges and Future Perspectives. Foods. 2023; 12(6):1181. https://doi.org/10.3390/foods12061181
Chicago/Turabian StyleCywińska-Antonik, Magdalena, Zhe Chen, Barbara Groele, and Krystian Marszałek. 2023. "Application of Emerging Techniques in Reduction of the Sugar Content of Fruit Juice: Current Challenges and Future Perspectives" Foods 12, no. 6: 1181. https://doi.org/10.3390/foods12061181
APA StyleCywińska-Antonik, M., Chen, Z., Groele, B., & Marszałek, K. (2023). Application of Emerging Techniques in Reduction of the Sugar Content of Fruit Juice: Current Challenges and Future Perspectives. Foods, 12(6), 1181. https://doi.org/10.3390/foods12061181