Physical and Chemical Characterization and Bioavailability Evaluation In Vivo of Amaranth Protein Concentrate
Abstract
:1. Introduction
- First, to the identification and quantitative assessment of the macro- and micronutrients and minor biologically active food substances (so-called phytonutrients—secondary metabolites of plant origin) in its composition;
- Second, the development of technological approaches to grain processing, extraction, and concentration of biologically active compounds;
- Third, the development of technology and creation of food products mainly based on flour or concentrates/isolates of amaranth grain protein.
2. Materials and Methods
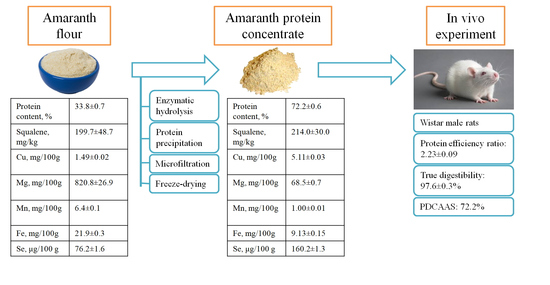
2.1. Materials
2.2. Chemical Composition of Amaranth Flour and Protein Concentrate
2.3. Mineral Composition of Amaranth Flour and Protein Concentrate
2.4. Determination of Lipid Content
2.5. Determination of Flavonoid Profile
2.6. Determination of Hydroxycinnamic Acid Profile
2.7. Determination of Triterpene Saponins Profile
2.8. Determination of Amino Acid Composition
2.9. Preparation of Amaranth Protein Concentrate
2.10. Animal Study
2.11. Comparative Determination of the True Digestibility of Casein and Amaranth Protein Concentrate
2.12. Statistical Analysis
3. Results
3.1. Amaranth Flour and Amaranth Protein Concentrate Characterization
3.2. Results of the In Vivo Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costea, M.; DeMason, D.A. Stem Morphology and Anatomy in Amaranthus L. (Amaranthaceae), Taxonomic Significance. J. Torrey Bot. Soc. 2001, 128, 254. [Google Scholar] [CrossRef]
- Gunina, L.M.; Dmitriev, A.B.; Shustov, E.B.; Kholodkov, A.B.; Golovashchenko, R.B. Prospects of Application of Diet Supplements Based on Amaranth in the Practice of Training Athletes. Ukr. Žurnal Med. Bìol. Sport 2018, 3, 267–277. [Google Scholar] [CrossRef]
- Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; WHO Technical Report Series: Geneva, Switzerland, 2007.
- Balakrishnan, G.; Schneider, R.G. The Role of Amaranth, Quinoa, and Millets for the Development of Healthy, Sustainable Food Products—A Concise Review. Foods 2022, 11, 2442. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Padilla, J.; Centeno-Leija, S.; Bojórquez-Velázquez, E.; Elizalde-Contreras, J.M.; Ruiz-May, E.; Serrano-Posada, H.; Osuna-Castro, J.A. Characterization of the Technofunctional Properties and Three-Dimensional Structure Prediction of 11S Globulins from Amaranth (Amaranthus hypochondriacus L.). Seeds. Foods 2023, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.; Wallace, T. Whole Grains and Their Bioactives; Wiley: New York, NY, USA, 2019. [Google Scholar]
- Zhu, F. Amaranth Proteins and Peptides: Biological Properties and Food Uses. Food Res. Int. 2023, 164, 112405. [Google Scholar] [CrossRef]
- Aguilar, E.G.; de Albarracín, G.J.; Uñates, M.A.; Piola, H.D.; Camiña, J.M.; Escudero, N.L. Evaluation of the Nutritional Quality of the Grain Protein of New Amaranths Varieties. Plant Foods Hum. Nutr. 2015, 70, 21–26. [Google Scholar] [CrossRef]
- Czerwonka, M.; Białek, A. Fatty Acid Composition of Pseudocereals and Seeds Used as Functional Food Ingredients. Life 2023, 13, 217. [Google Scholar] [CrossRef]
- Rodríguez, M.; Tironi, V.A. Polyphenols in Amaranth (A. manteggazianus) Flour and Protein Isolate: Interaction with Other Components and Effect of the Gastrointestinal Digestion. Food Res. Int. 2020, 137, 109524. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Kraujalis, P. Nutritional Components of Amaranth Seeds and Vegetables: A Review on Composition, Properties, and Uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Steffensen, S.K.; Rinnan, Å.; Mortensen, A.G.; Laursen, B.; de Troiani, R.M.; Noellemeyer, E.J.; Janovska, D.; Dusek, K.; Délano-Frier, J.; Taberner, A.; et al. Variations in the Polyphenol Content of Seeds of Field Grown Amaranthus Genotypes. Food Chem. 2011, 129, 131–138. [Google Scholar] [CrossRef]
- Mazo, V.K.; Sidorova, Y.S.; Shipelin, V.A.; Petrov, N.A.; Kochetkova, A.A. Polyphenolic Plant Extracts: Effects on Disorders of Carbohydrate and Lipid Metabolism in Laboratory Animals. Probl. Endocrinol. 2016, 62, 38–44. [Google Scholar] [CrossRef]
- Tutelyan, V.A.; Kiseleva, T.L.; Kochetkova, A.A.; Mazo, V.K.; Sarkisyan, V.A.; Glazkova, I.V.; Vorobiova, V.M.; Sidorova, Y.S.; Vorobiova, I.S.; Zorin, S.N.; et al. Plant Sources of Phytonutrients for Antidiabetic Specialized Food Products; Biblio-Globus: Moscow, Russia, 2016; p. 422. [Google Scholar]
- Han, M.-K. Epigallocatechin Gallate, a Constituent of Green Tea, Suppresses Cytokine-Induced Pancreatic β-Cell Damage. Exp. Mol. Med. 2003, 35, 136–139. [Google Scholar] [CrossRef]
- Kubow, S.; Hobson, L.; Iskandar, M.M.; Sabally, K.; Donnelly, D.J.; Agellon, L.B. Extract of Irish Potatoes (Solanum tuberosum L.) Decreases Body Weight Gain and Adiposity and Improves Glucose Control in the Mouse Model of Diet-Induced Obesity. Mol. Nutr. Food Res. 2014, 58, 2235–2238. [Google Scholar] [CrossRef]
- Nascimento, A.C.; Mota, C.; Coelho, I.; Gueifão, S.; Santos, M.; Matos, A.S.; Gimenez, A.; Lobo, M.; Samman, N.; Castanheira, I. Characterisation of Nutrient Profile of Quinoa (Chenopodium quinoa), Amaranth (Amaranthus caudatus), and Purple Corn (Zea mays L.) Consumed in the North of Argentina: Proximates, Minerals and Trace Elements. Food Chem. 2014, 148, 420–426. [Google Scholar] [CrossRef]
- Rizvi, N.B.; Aleem, S.; Khan, M.R.; Ashraf, S.; Busquets, R. Quantitative Estimation of Protein in Sprouts of Vigna radiate (Mung Beans), Lens culinaris (Lentils), and Cicer arietinum (Chickpeas) by Kjeldahl and Lowry Methods. Molecules 2022, 27, 814. [Google Scholar] [CrossRef]
- Rondanelli, M.; Daglia, M.; Meneghini, S.; Di Lorenzo, A.; Peroni, G.; Faliva, M.A.; Perna, S. Nutritional Advantages of Sous-Vide Cooking Compared to Boiling on Cereals and Legumes: Determination of Ashes and Metals Content in Ready-to-Eat Products. Food Sci. Nutr. 2017, 5, 827–833. [Google Scholar] [CrossRef]
- Tutelyan, V.A.; Eller, K.I. Methods for the Analysis of Minor Biologically Active Substances in Food; Dinastiya: Moscow, Russia, 2010; p. 160. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press: Washington, WA, USA, 2011.
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Sidorova, Y.S.; Mazo, V.K.; Zorin, S.N.; Stefanova, I.L. Evaluation of Biological Value and Antigenicity of Coagulated Chicken Egg Protein. Vopr. Pitan. 2018, 87, 44–50. [Google Scholar]
- Zehring, J.; Reim, V.; Schröter, D.; Neugart, S.; Schreiner, M.; Rohn, S.; Maul, R. Identification of Novel Saponins in Vegetable Amaranth and Characterization of Their Hemolytic Activity. Food Res. Int. 2015, 78, 361–368. [Google Scholar] [CrossRef]
- Das, D.; Mir, N.A.; Chandla, N.K.; Singh, S. Combined Effect of PH Treatment and the Extraction PH on the Physicochemical, Functional and Rheological Characteristics of Amaranth (Amaranthus hypochondriacus) Seed Protein Isolates. Food Chem. 2021, 353, 129466. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sánchez, C.; González-Castañeda, J.; De León-Rodríguez, A.; De La Rosa, A.P.B. Functional and Rheological Properties of Amaranth Albumins Extracted from Two Mexican Varieties. Plant Foods Hum. Nutr. 2004, 59, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Torres, G.; Ontiveros, N.; Lopez-Teros, V.; Ibarra-Diarte, J.; Reyes-Moreno, C.; Cuevas-Rodríguez, E.; Cabrera-Chávez, F. Amaranth Protein Hydrolysates Efficiently Reduce Systolic Blood Pressure in Spontaneously Hypertensive Rats. Molecules 2017, 22, 1905. [Google Scholar] [CrossRef] [PubMed]
- Mokrejs, P.; Janacova, D.; Kolomaznik, K.; Svoboda, P. Modeling Isolation of Amaranth Protein by Enzymatic Breakdown of Polysaccharides. Rasayan J. Chem. 2011, 4, 180–188. [Google Scholar]
- Amare, E.; Mouquet-Rivier, C.; Rochette, I.; Adish, A.; Haki, G.D. Effect of Popping and Fermentation on Proximate Composition, Minerals and Absorption Inhibitors, and Mineral Bioavailability of Amaranthus Caudatus Grain Cultivated in Ethiopia. J. Food Sci. Technol. 2016, 53, 2987–2994. [Google Scholar] [CrossRef]
- Pastor, K. The Chemistry behind Amaranth Grains. J. Nutr. Health Food Eng. 2018, 8, 358–360. [Google Scholar] [CrossRef]
- Castel, V.; Andrich, O.; Netto, F.M.; Santiago, L.G.; Carrara, C.R. Total Phenolic Content and Antioxidant Activity of Different Streams Resulting from Pilot-Plant Processes to Obtain Amaranthus Mantegazzianus Protein Concentrates. J. Food Eng. 2014, 122, 62–67. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in Quinoa and Amaranth Grains and Their Antioxidant, Anti-Inflammatory, and Potential Health Beneficial Effects: A Review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef]
- Mroczek, A. Phytochemistry and Bioactivity of Triterpene Saponins from Amaranthaceae Family. Phytochem. Rev. 2015, 14, 577–605. [Google Scholar] [CrossRef]
- Xu, H.-H.; Jiang, Z.-H.; Sun, Y.-T.; Qiu, L.-Z.; Xu, L.-L.; Tang, X.-L.; Ma, Z.-C.; Gao, Y. Differences in the Hemolytic Behavior of Two Isomers in Ophiopogon Japonicus In Vitro and In Vivo and Their Risk Warnings. Oxid. Med. Cell. Longev. 2020, 2020, 8870656. [Google Scholar] [CrossRef]


| Sample | Content, % | ||||
|---|---|---|---|---|---|
| Fat | Protein | Ash | Moisture | Carbohydrates | |
| Flour (30% protein) | 5.6 ± 0.1 | 28.7 ± 2.8 | 6.12 ± 0.06 | 4.1 ± 0.1 | 50.4 ± 2.6 including dietary fiber (unsoluble/soluble) 15.8 ± 1.5/10.2 ± 1.0 |
| Concentrate | 15.0 ± 1.0 | 72.2 ± 0.6 | 1.8 ± 0.2 | 3.4 ± 0.1 | 7.6 ± 0.8 |
| Amino Acid | Amino Acid Scale of Ideal Protein (FAO/WHO), g/100 g | Amino Acid Content in Amaranth Protein Concentrate, g/100 g | Amino Acid Score, % |
|---|---|---|---|
| Threonine | 2.3 | 4.5 | 196 |
| Cysteine + Methionine | 2.2 | 4.0 | 182 |
| Valine | 3.9 | 2.9 | 74 |
| Isoleucine | 3.0 | 2.7 | 90 |
| Leucine | 5.9 | 6.8 | 115 |
| Tyrosine + Phenylalanine | 3.8 | 7.7 | 203 |
| Lysine | 4.5 | 6.9 | 153 |
| Tryptophan | 0.6 | 2.1 | 350 |
| Element | Daily Norm for Men/Women, mg/day | Flour Content, mg/100 g | Percentage of Daily Requirement, % | Protein Content in Amaranth Protein Concentrate, mg/100 g | Percentage of Daily Requirement, % |
|---|---|---|---|---|---|
| Ca | 1000 | 269.5 ± 19.7 | 27 | 75.7 ± 4.2 | 8 |
| Cu | 1 | 1.49 ± 0.02 | 149 | 5.11 ± 0.03 | 511 |
| Fe | 10/18 | 21.9 ± 0.3 | 219/122 | 9.13 ± 0.15 | 91/51 |
| K | 3500 | 2633.6 ± 17.2 | 75 | 586.9 ± 8.3 | 17 |
| Mg | 420 | 820.8 ± 26.9 | 195 | 68.5 ± 0.7 | 16 |
| Mn | 2 | 6.4 ± 0.1 | 320 | 1.00 ± 0.01 | 50 |
| Na | 1300 | 6.7 ± 0.2 | 0.5 | 255.6 ± 5.1 | 20 |
| P | 700 | 1592.1 ± 51.3 | 227 | 566.0 ± 10.7 | 81 |
| Zn | 12 | 4.71 ± 0.04 | 39 | 2.95 ± 0.01 | 25 |
| Element | Daily Norm for Men/Women μg/day | Flour Content, μg/100 g | Percentage of Daily Requirement, % | Protein Content in Amaranth Protein Concentrate, μg/100 g | Percentage of Daily Requirement, % |
| Co | 10 | 13.0 ± 0.2 | 130 | 23.3 ± 0.1 | 233 |
| Cr | 40 | 49.1 ± 1.5 | 123 | 183.6 ± 11.7 | 459 |
| Se | 70/55 | 76.2 ± 1.6 | 109/139 | 160.2 ± 1.3 | 229/291 |
| Fatty Acid | Fatty Acid Index | Fatty Acid Content, % of Total Fatty Acids | |
|---|---|---|---|
| Original Flour | Amaranth Protein Concentrate | ||
| Lauric | 12:0 | 0.05 | 0.02 |
| Myristic | 14:0 | 0.26 | 0.21 |
| Pentadecanoic | 15:0 | 0.11 | 0.09 |
| Pentadecene | 15:1 | 0.00 | 0.06 |
| Palmitic | 16:0 | 19.76 | 20.09 |
| Hexadecene | 16:1 | 0.06 | 0.05 |
| Palmitoleic | 16:1 9-cis | 0.10 | 0.10 |
| Margarine | 17:0 | 0.13 | 0.12 |
| Heptadecene | 17:1 | 0.04 | 0.00 |
| Stearic | 18:0 | 3.50 | 3.43 |
| Oleic | 18:1 9-cis | 23.41 | 22.95 |
| Vaccenic | 18:1 11-trans | 1.12 | 1.05 |
| Iso-octadecadienoic | 18:2 9-trans, 12-trans | 0.16 | 0.19 |
| Cis, translinolic | 18:2 9-cis, 12-trans | 0.08 | 0.10 |
| Linoleic | 18:2 | 48.39 | 48.89 |
| α-Linolenic | 18:3 ω-3 | 0.95 | 0.99 |
| Arachidic | 20:0 | 0.76 | 0.70 |
| Gondoinic (total isomers) | 20:1 | 0.25 | 0.27 |
| Eicosadienoic | 20:2 | 0.04 | 0.04 |
| Behenic | 22:0 | 0.35 | 0.34 |
| Lignoceric | 24:0 | 0.30 | 0.27 |
| Total fatty acids | 99.86 | 99.96 | |
| Content, µg/100 g | Sample | |
|---|---|---|
| Amaranth Flour | Amaranth Protein Concentrate | |
| Caffeic acid | 610 ± 30 | n/d |
| Ferulic acid | 900 ± 50 | 3090 ± 100 |
| p-Coumaric acid | 130 ± 10 | n/d |
| Total hydroxycinnamic acid derivatives | 7570 ± 280 | 3090 ± 100 |
| Rutin | 1370 ± 80 | n/d |
| Nicotiflorin (kaempferol-3-rutinoside) | 800 ± 30 | n/d |
| Total flavonoids | 2170 ± 120 | - |
| No | Saponin | [M − H]- | Amaranth Flour | Amaranth Protein Concentrate |
|---|---|---|---|---|
| 1 | 28-O-β-D-glucopyranosyl ester of 3-O-α-L-rhamnopyranosyl(1→3)-β-D-glucuronopyranosyl-2β,3β-dihydroxyolean-12-en-28-oic acid | 955.54 | n/d | |
| 2 | 28-O-β-D-glucopyranosyl ester of 3-O-β-D-glucuronopyranosyl-2β,3β,6α-trihydroxyolean-12-en-23-al-28-oic acid | 823.48 | ||
| 3 | 28-O-β-D-glucopyranosyl ester of 3-O-α-L-rhamnopyranosyl(1→3)-β-D-glucuronopyranosyl-2β,3β-dihydroxy-30-norolean-12,20(29)-diene-23-al-28-oic acid | 953.47 | ||
| 4 | Nonidentified saponin * | 962.46 | ||
| 5 | 28-O-β-D-glucopyranosyl ester of 3-O-β-D-glucopyranosyl(1→3)-β-D-glucuronopyranosyl-2β,3β,6α-trihydroxyolean-4-desmethyl-12-en-23-al-28-oic acid | 971.55 | ||
| 6 | 28-O-β-D-glucopyranosyl(1→3)-β-D-glucuronopyranosyl ester of 2β,3β,6α-trihydroxyolean-12,20(29)-diene-28-oic acid | 825.54 | n/d | |
| 7 | 28-O-β-D-glucopyranosyl ester of 3-O-β-D-glucuronopyranosyl-2β,3β,6α-trihydroxyolean-12,20(29)-dien-23-al-28-oic acid | 839.44 | ||
| 8 | 28-O-β-D-glucopyranosyl ester of 3-O-α-L-rhamnopyranosyl(1→3)-β-D-glucuronopyranosyl-2β,3β-dihydroxy-30-norolean-12,20(29)-diene-28-oic acid | 939.45 | ||
| 9 | 28-O-β-D-glucopyranosyl ester of 3-O-α-L-rhamnopyranosyl(1→3)-β-D-glucuronopyranosyl-2β,3β-dihydroxyolean-12,20(29)-dien-23-al-28-oic acid | 969.58 | ||
| 10 | 28-O-β-D-glucopyranosyl ester 3-O-α-L-rhamnopyranosyl(1→3)-β-D-glucuronopyranosyl-2β,3β-dihydroxyolean-12,20(29)-diene-28-oic acid | 955.53 | ||
| 11 | 28-O-β-D-glucopyranosyl ester of 3-O-α-L-rhamnopyranosyl(1→3)-β-D-glucuronopyranosyl-3β-hydroxyolean-12-en-28-oic acid | 939.56 | ||
| 12 | 3-O-α-L-rhamnopyranosyl(1→3)-β-D-glucuronopyranosyl-2β,3β-dihydroxyolean-12,20(29)-diene-28-oic acid | 793.35 | ||
| 13 | 3-O-β-D-glucuronopyranosyl-2β,3β,6α-trihydroxyolean-12-en-23-al-28-oic acid | 661.38 | ||
| 14 | 3-O-β-D-glucuronopyranosyl-2β,3β,6α-trihydroxyolean-23-al-28-oic acid | 677.28 | ||
| 15 | 28-O-β-D-glucuronopyranosyl ester of 2β,3β,6α-trihydroxyolean-12,20(29)-diene-28-oic acid | 663.56 | n/d | |
| 16 | 3-O-α-L-rhamnopyranosyl(1→3)-β-D-glucuronopyranosyl-2β,3β-dihydroxyolean-12-en-23-al-28-oic acid | 807.43 |
| Parameter | Animal Groups | |
|---|---|---|
| Control | Amaranth | |
| Feces, wet weight, mg | 1.0 ± 0.1 | 2.3 ± 0.2 * |
| Protein in feces, % | 4.7 ± 0.2 | 5.4 ± 0.2 * |
| Nitrogen in feces, mg per wet weight | 46.7 ± 6.7 | 121.8 ± 12.7 * |
| Food intake, g | 20.7 ± 0.9 | 20.7 ± 1.2 |
| Protein intake, g | 4.5 ± 0.2 | 4.5 ± 0.3 |
| True digestibility, % | 99.3 ± 0.2 | 97.6 ± 0.3 * |
| PDCAAS, % | 100.0 | 72.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorova, Y.S.; Petrov, N.A.; Perova, I.B.; Kolobanov, A.I.; Zorin, S.N. Physical and Chemical Characterization and Bioavailability Evaluation In Vivo of Amaranth Protein Concentrate. Foods 2023, 12, 1728. https://doi.org/10.3390/foods12081728
Sidorova YS, Petrov NA, Perova IB, Kolobanov AI, Zorin SN. Physical and Chemical Characterization and Bioavailability Evaluation In Vivo of Amaranth Protein Concentrate. Foods. 2023; 12(8):1728. https://doi.org/10.3390/foods12081728
Chicago/Turabian StyleSidorova, Yuliya S., Nikita A. Petrov, Irina B. Perova, Alexey I. Kolobanov, and Sergey N. Zorin. 2023. "Physical and Chemical Characterization and Bioavailability Evaluation In Vivo of Amaranth Protein Concentrate" Foods 12, no. 8: 1728. https://doi.org/10.3390/foods12081728
APA StyleSidorova, Y. S., Petrov, N. A., Perova, I. B., Kolobanov, A. I., & Zorin, S. N. (2023). Physical and Chemical Characterization and Bioavailability Evaluation In Vivo of Amaranth Protein Concentrate. Foods, 12(8), 1728. https://doi.org/10.3390/foods12081728