Encapsulation Properties of Mentha piperita Leaf Extracts Prepared Using an Ultrasound-Assisted Double Emulsion Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peppermint Leaf Powder Preparation
2.2. Ultrasonic-Assisted Extraction
Experimental Design for Optimization of UE of Peppermint Leaves
2.3. Analysis of Extract
2.3.1. Determination of TPC
2.3.2. Quantitative Analysis of Antioxidant Activity Using DPPH Method
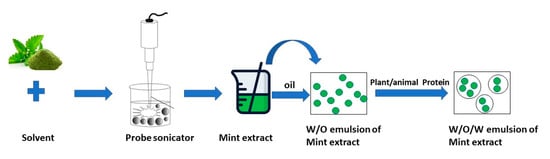
2.4. Encapsulation of MLE Using Double Emulsion Method
2.4.1. Preparation of Inner W1/O Emulsion
2.4.2. Preparation of W1/O/W2 Emulsion
2.5. Characterization of Emulsions
2.5.1. Optical Microscopy and Droplet Size Analysis of Emulsions
2.5.2. Viscosity Measurement
2.5.3. Encapsulation Efficiency (EE)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Optimization of TPC and Percent Inhibition Using RSM
3.2. Encapsulation of Phenolic Compounds
3.2.1. Optical Imaging and Determination of Droplet Size of Double Emulsions
3.2.2. Viscosity Measurements
3.2.3. Encapsulation Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kizil, S.; Hashimi, N.; Tolan, V.; Kilinc, E.; Yuksel, U. Mineral content, essential oil € components and biological activity of two Mentha species (Mentha piperita L., M. spicata L.). Turk. J. Field Crops 2010, 15, 148–153. [Google Scholar]
- Lv, J.; Huang, H.; Yua, L.; Whent, M.; Niu, Y.; Shi, H.; Wang, T.T.; Luthria, D.; Charles, D.; Yu, L.L. Phenolic composition and nutraceutical properties of organic and conventional cinnamon and peppermint. Food Chem. 2012, 132, 1442–1450. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.D.; Blumberg, B.J. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Lopez, V.; Martin, S.; Gomez-Serranillos, M.P.; Carretero, M.E.; Jager, A.K.; Calvo, M.I. Neuroprotective and neurochemical properties of mint extracts. Phytother. Res. 2010, 24, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Areias, F.M.; Valentao, P.; Andrade, P.B.; Ferreres, F.; Seabra, R.M. Phenolic fingerprint of peppermint leaves. Food Chem. 2001, 73, 307–311. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Fecka, I.; Turex, S. Determination of Water-Soluble Polyphenolic Compounds in Commercial Herbal Teas from Lamiaceae: Peppermint, Melissa, and Sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Luque-Garci’a, J.L.; Luque De Castro, M.D. Ultrasound: A powerful tool for leaching, Trend. Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Shotipruk, A.; Kaufman, P.B.; Wang, H.Y. Feasibility study of repeated harvesting of menthol from biologically viable Mentha × piperata using ultrasonic extraction. Biotechnol. Prog. 2001, 17, 924–928. [Google Scholar] [CrossRef]
- Albu, S.; Joyce, E.; Paniwnyk, L.; Lorimer, P.; Mason, J. Potential for the use of ultrasound in the extraction of antioxidants from Rosmarinus officinalis for the food and pharmaceutical industry. Ultrason. Sonochem. 2004, 11, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Riera, E.; Golás, Y.; Blanco, A.; Gallego, A.; Blasco, M.; Mulet, A. Mass transfer enhancement in supercritical fluids extraction by means of power ultrasound. Ultrason. Sonochem. 2004, 11, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Shi, S.; Wan, X. Impact of ultrasonic-assisted extraction on the chemical and sensory quality of tea infusion. J. Food Eng. 2006, 74, 557–560. [Google Scholar] [CrossRef]
- Medina-Pérez, G.; Estefes-Duarte, J.A.; Afanador-Barajas, L.N.; Fernández-Luqueño, F.; Zepeda-Velázquez, A.P.; FrancoFernández, M.J.; Peláez-Acero, A.; Campos-Montiel, R.G. Encapsulation Preserves Antioxidant and Antidiabetic Activities of Cactus Acid Fruit Bioactive Compounds Under Simulated Digestion Conditions. Molecules 2020, 25, 5736. [Google Scholar] [CrossRef] [PubMed]
- Šaponjac, V.T.; Ćetković, G.; Čanadanović-Brunet, J.; Pajin, B.; Djilas, S.; Petrovi’c, J.; Lončarević, I.; Stajčić, S.; Vulić, J. Sour cherry pomace extract encapsulated in whey and soy proteins: Incorporation in cookies. Food Chem. 2016, 207, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef]
- Surendhiran, D.; Li, C.; Cui, H.; Lin, L. Fabrication of high stability active nanofibers encapsulated with pomegranate peel extract using chitosan/PEO for meat preservation. Food Packag. Shelf Life 2019, 23, 100439. [Google Scholar] [CrossRef]
- Saadat, S.; Emam-Djomeh, Z.; Askari, G. Antibacterial and Antioxidant Gelatin Nanofiber Scaffold Containing Ethanol Extract of Pomegranate Peel: Design, Characterization and In Vitro Assay. Food Bioprocess Technol. 2021, 14, 935–944. [Google Scholar] [CrossRef]
- Sandhya, S.; Khamrui, K.; Prasad, W.; Kumar, M. Preparation of pomegranate peel extract powder and evaluation of its effect on functional properties and shelf life of curd. LWT 2018, 92, 416–421. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Abd-Elgawad, A.E.H.; Soliman, O.A.; Jablonski, M.M. Novel topical ophthalmic formulations for management of glaucoma. Pharm. Res. 2013, 30, 2818–2831. [Google Scholar] [CrossRef]
- Palamoor, M.; Jablonski, M.M. Comparative study on diffusion and evaporation emulsion methods used to load hydrophilic drugs in poly(ortho ester) nanoparticle emulsions. Powder Technol. 2014, 253, 53–62. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef]
- Kim, B.K.; Cho, A.R.; Park, D.J. Enhancing oral bioavailability using preparations of apigenin-loaded W/O/W emulsions: In vitro and in vivo evaluations. Food Chem. 2016, 206, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Flanagan, J.; Hemar, Y.; Singh, H. Synergistic effects of polyglycerol ester of polyricinoleic acid and sodium caseinate on the stabilisation of wateroil-water emulsions. Food Hydrocoll. 2006, 20, 261–268. [Google Scholar] [CrossRef]
- Schmidts, T.; Dobler, D.; Nissing, C.; Runkel, F. Influence of hydrophilic surfactants on the properties of multiple W/O/W emulsions. J. Colloid Interface Sci. 2009, 338, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Muschiolik, G. Factors affecting the droplet size of water-in-oil emulsions (W/O) and the oil globule size in water-in-oil-in-water emulsions (W/O/W). J. Dispers. Sci. Technol. 2007, 28, 703–716. [Google Scholar] [CrossRef]
- Okochi, H.; Nakano, M. Comparative study of two preparation methods of W/O/W emulsions: Stirring and membrane emulsification. Chem. Pharm. Bull. 1997, 45, 1323–1326. [Google Scholar] [CrossRef]
- Benichou, A.; Garti, N.; Aserin, A. Double emulsions stabilized with hybrids of natural polymers for entrapment and slow release of active matters. Adv. Colloid Interface Sci. 2004, 108–109, 29–41. [Google Scholar] [CrossRef]
- Garti, N. Progress in stabilization and transport phenomena of double emulsions in food applications. LWT–Food Sci. Technol. 1997, 30, 222–235. [Google Scholar] [CrossRef]
- Hino, T.; Kawashima, Y.; Shimabayashi, S. Basic study for stabilization of w/o/w emulsion and its application to transcatheter arterial embolization therapy. Adv. Drug Deliv. Rev. 2000, 45, 27–45. [Google Scholar] [CrossRef]
- Bonnet, M.; Cansell, M.; Placin, F.; Anton, M.; Leal-Calderon, F. Impact of sodium caseinate concentration and location on magnesium release from multiple W/O/W emulsions. Langmuir 2010, 26, 9250–9260. [Google Scholar] [CrossRef] [PubMed]
- Leal-Calderon, F.; Homer, S.; Goh, A.; Lundin, L. W/O/W emulsions with high internal droplet volume fraction. Food Hydrocoll. 2012, 27, 30–41. [Google Scholar] [CrossRef]
- Chavan, U.D.; McKenzie, D.B.; Shahidi, F. Functional properties of protein isolates from beach pea (Lathyrus maritimus L.). Food Chem. 2001, 74, 177–187. [Google Scholar] [CrossRef]
- Ragab, D.M.; Babiker, E.E.; Eltinay, A.H. Fractionation, solubility and functional properties of cowpea (Vigna unguiculata) proteins as affected by pH and/or salt concentration. Food Chem. 2004, 84, 207–212. [Google Scholar] [CrossRef]
- Livney, Y.D. Milk proteins as vehicles for bioactives. Curr. Opin. Colloid Interface Sci. 2010, 15, 73–83. [Google Scholar] [CrossRef]
- Ghayoura, N.; Hosseini, S.M.H.; Eskandari, M.H.; Esteghlal, S.; Nekoei, A.R.; Gahruie, H.H.; Tatar, M.; Naghibalhossaini, F. Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocoll. 2019, 87, 394–403. [Google Scholar] [CrossRef]
- Świątek, S.; Komorek, P.; Turner, G.; Jachimska, B. β-Lactoglobulin as a potential carrier for bioactive molecules. Bioelectrochemistry 2019, 126, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Somchue, W.; Sermsri, W.; Shiowatana, J.; Siripinyanond, A. Encapsulation of a-tocopherol in protein-based delivery particles. Food Res. Int. 2009, 42, 909–914. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Shi, J.; Huang, X.; Peng, Q.; Xu, F. Encapsulation of tomato oleoresin using soy protein isolate-gum aracia conjugates as emulsifier and coating materials. Food Hydrocoll. 2015, 45, 301–308. [Google Scholar] [CrossRef]
- You, L.; Bin, P.; Yan, L.; Yanan, W.; Zhenqiang, W. Ultrasound extraction of polysaccharides from guava leaves and their antioxidant and antiglycation activity. Process. Biochem. 2018, 73, 228–234. [Google Scholar] [CrossRef]
- Insang, S.; Kijpatanasilp, I.; Jafari, S.; Assatarakul, K. Ultrasound-assisted extraction of functional compound from mulberry (Morus alba L.) leaf using response surface methodology and effect of microencapsulation by spray drying on quality of optimized extract. Ultrason. Sonochemis. 2022, 82, 105806. [Google Scholar] [CrossRef]
- Jabri-Karoui, I.; Bettaieb, I.; Msaada, K.; Hammami, M.; Marzouk, B. Research on the phenolic compounds and antioxidant activities of Tunisian Thymus capitatus. J. Funct. Foods 2012, 4, 661–669. [Google Scholar] [CrossRef]
- Sadef, Y.; Javed, T.; Javed, R.; Mahmood, A.; Alwahibi, M.S.; Elshikh, M.S.; AbdelGawwa, M.R.; Alhaji, J.H.; Rasheed, R.A. Nutritional status, antioxidant activity and total phenolic content of different fruit and vegetable peels. PLoS ONE 2022, 17, e0265566. [Google Scholar] [CrossRef] [PubMed]
- Aditya, N.; Aditya, S.; Yang, H.J.; Kim, H.W.; Park, S.O.; Lee, J.; Ko, S. Curcumin and catechin co-loaded water-in-oil-in-water emulsion and its beverage application. J. Funct. Foods 2015, 15, 35–43. [Google Scholar] [CrossRef]
- Iqbal, S.; Baloch, M.K.; Hameed, G.; McClements, D.J. Controlling W/O/W multiple emulsion microstructure by osmotic swelling and internal protein gelation. Food Res. Int. 2013, 54, 1613–1620. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Acevedo-Fani, A.; González-Aguilar, G.A.; Martín-Belloso, O. Encapsulation and stability of a phenolic-rich extract from mango peel within water-in-oil-in-water emulsions. J. Funct. Foods 2019, 56, 65–73. [Google Scholar] [CrossRef]
- Rafiee, Z.S.M.; Jafari, M.; Alami, M.; Khomeiri, M. Antioxidant effect of microwave-assisted extracts of olive leaves on sunflower oil. J. Agric. Sci. Technol. 2012, 14, 1497–1509. [Google Scholar]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Borrás-Linares, I.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A.; Souto, E.B. Polyphenols-enriched Hibiscus sabdariffa extract-loaded nanostructured lipid carriers (NLC): Optimization by multi-response surface methodology. J. Drug Deliv. Sci. Technol. 2019, 49, 660–667. [Google Scholar] [CrossRef]
- Oh, J.; Jo, H.; Cho, A.R.; Kim, S.J.; Han, J. Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control 2013, 31, 403–409. [Google Scholar] [CrossRef]
- Yi, W.; Wetzstein, H.Y. Effects of Drying and Extraction Conditions on the Biochemical Activity of Selected Herbs. HortScience 2011, 46, 70–73. [Google Scholar] [CrossRef]
- Uribe, E.; Marín, D.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Rodríguez, A. Assessment of vacuum-dried peppermint (Mentha piperita L.) as a source of natural antioxidants. Food Chem. 2016, 190, 559–565. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Pressurized liquid extraction of isoflavones from soybeans. Anal. Chim. Acta 2004, 522, 169–177. [Google Scholar] [CrossRef]
- Uma, D.B.; Ho, C.W.; Wan Aida, W.M. Optimization of extraction parameters of total phenolic compounds from henna (Lawsonia inermis) leaves. Sains Malays. 2010, 39, 119–128. [Google Scholar]
- Verma, B.; Hucl, P.; Chibbar, R.N. Phenolic content and antioxidant properties of bran in 51 wheat cultivars. Cereal Chem. 2008, 85, 544–549. [Google Scholar] [CrossRef]
- Canistro, D.; Boccia, C.; Falconi, R.; Bonamassa, B.; Valgimigli, L.; Vivarelli, F.; Soleti, A.; Genova, M.L.; Lenaz, G.; Sapone, A.; et al. Redox-based flagging of the global network of oxidative stress greatly promotes longevity. J. Gerontolog. A Biol. Sci. Med. 2015, 70, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Suslick, K.S.; Price, G. Applications of ultrasound to materials chemistry. Annu. Rev. Mater. Sci. 1999, 29, 295–326. [Google Scholar] [CrossRef]
- Kaderides, K.; Goula, A.M.; Adamopoulos, K.G. A process for turning pomegranate peels into a valuable food ingredient using ultrasound-assisted extraction and encapsulation. Innov. Food Sci. Emerg. Technol. 2015, 31, 204–215. [Google Scholar] [CrossRef]
- Hilares, R.T.; Ramos, L.; da Silva, S.S.; Dragone, G.; Mussatto, S.I.; Santos, J.C.D. Hydrodynamic cavitation as a strategy to enhance the efficiency of lignocellulosic biomass pretreatment. Crit. Rev. Biotechnol. 2018, 38, 483–493. [Google Scholar] [CrossRef]
- Wu, Z.; Ferreira, D.F.; Crudo, D.; Bosco, V.; Stevanato, L.; Costale, A.; Cravotto, G. Plant and Biomass Extraction and Valorisation under Hydrodynamic Cavitation. Processes 2019, 7, 965. [Google Scholar] [CrossRef]
- Sapei, L.; Naqvi, M.A.; Rousseau, D. Stability and release properties of double emulsions for food applications. Food Hydrocoll. 2012, 27, 316–323. [Google Scholar] [CrossRef]
- Dickinson, E. Double emulsions stabilized by food biopolymers. Food Biophys. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, T.G.; Choi, H.K. Development of oral drug delivery system using floating microspheres. J. Microencap. 1999, 16, 715–717. [Google Scholar] [CrossRef]
- Sriprablom, J.; Luangpituksa, P.; Wongkongkatep, J.; Pongtharangkul, T.; Suphantharika, M. Influence of pH and ionic strengthon the physical and rheological properties and stability of whey protein stabilized o/w emulsions containing xanthan gum. J. Food Eng. 2018, 242, 141–152. [Google Scholar] [CrossRef]
- Wang, J.; Jing, H.; Wang, Y. Possible effects of complex internal structures on the apparent viscosity of multiple emulsions. Chem. Eng. Sci. 2015, 135, 381–392. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Assadpour, E.; Esfanjani, A.F. Nano-encapsulation of olive leaf phenolic compounds through WPC–pectin complexes and evaluating their release rate. Int. J. Biol. Macromol. 2016, 82, 816–822. [Google Scholar] [CrossRef]
- Cortesi, R.; Esposito, E. Pharmaceutical and biotechnological applications of multiple emulsions. In Colloids in Drug Delivery, 1st ed.; Fanun, M., Ed.; CRC Press: Boca Raton, FL, USA, 2010; pp. 203–220. [Google Scholar]
- Cuevas-Bernardino, J.C.; Leyva-Gutierrez, F.M.A.; Vernon-Carter, E.J.; Lobato-Calleros, C.; Roman-Guerrero, A.; Davidov-Pardo, G. Formation of biopolymer complexes composed of pea protein and mesquite gum-Impact of quercetin addition on their physical and chemical stability. Food Hydrocoll. 2018, 77, 736–745. [Google Scholar] [CrossRef]





| Coded Levels | Uncoded Levels | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| Experiment No. | Xa | Xt | Xe | Xa | Xt | Xe | TPC * (mg Gallic Acid Equivalent/g Mint Powder) | Inhibition (%) |
| 1 | −1 | 0 | −1 | 20 | 20 | 70 | 49.94 ± 1.67 | 26.44 ± 0.95 |
| 2 | 0 | −1 | 1 | 35 | 10 | 90 | 28.93 ± 2.54 | 17.26 ± 1.70 |
| 3 | 1 | 0 | 1 | 50 | 20 | 90 | 36.16 ± 2.9 | 27.45 ± 2.24 |
| 4 | 0 | 0 | 0 | 35 | 20 | 80 | 56.83 ± 1.38 | 31.68 ± 3.60 |
| 5 | −1 | −1 | 0 | 20 | 10 | 80 | 57.85 ± 3.05 | 16.88 ± 3.20 |
| 6 | 0 | −1 | −1 | 35 | 10 | 70 | 61.56 ± 3.05 | 45.77 ± 1.63 |
| 7 | −1 | 0 | 1 | 20 | 20 | 90 | 31.23 ± 1.09 | 11.92 ± 3.67 |
| 8 | −1 | 1 | 0 | 20 | 30 | 80 | 56.16 ± 4.14 | 27.21 ± 1.90 |
| 9 | 0 | 1 | 1 | 35 | 30 | 90 | 48.52 ± 2.01 | 21.63 ± 1.63 |
| 10 | 0 | 0 | 0 | 35 | 20 | 80 | 60.86 ± 2.29 | 32.14 ± 0.01 |
| 11 | 0 | 1 | −1 | 35 | 30 | 70 | 66.34 ± 1.3 | 29.62 ± 2.31 |
| 12 | 0 | 0 | 0 | 35 | 20 | 80 | 61.02 ± 5.45 | 28.08 ± 0.03 |
| 13 | 1 | 1 | 0 | 50 | 30 | 80 | 58.63 ± 2.62 | 19.52 ± 2.31 |
| 14 | 1 | −1 | 0 | 50 | 10 | 80 | 60.09 ± 5.45 | 35.19 ± 0.03 |
| 15 | 1 | 0 | −1 | 50 | 20 | 70 | 58.15 ± 2.18 | 45.67 ± 4.90 |
| Constant | Xa | Xt | Xe | Xa * Xa | Xt * Xt | Xe * Xe | Xa * Xt | Xa * Xe | Xt * Xe | R2 Value | Model p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total phenolic content | 58.78 *** | NS | NS | −11.39 *** | NS | NS | −11.27 ** | NS | NS | NS | 93% | <0.05 |
| % Inhibition | 30.63 | 5.67 ** | NS | −8.65 ** | NS | NS | NS | −6.5 ** | NS | 5.13 * | 86% | <0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobti, B.; Kamal-Eldin, A.; Rasul, S.; Alnuaimi, M.S.K.; Alnuaimi, K.J.J.; Alhassani, A.A.K.; Almheiri, M.M.A.; Nazir, A. Encapsulation Properties of Mentha piperita Leaf Extracts Prepared Using an Ultrasound-Assisted Double Emulsion Method. Foods 2023, 12, 1838. https://doi.org/10.3390/foods12091838
Sobti B, Kamal-Eldin A, Rasul S, Alnuaimi MSK, Alnuaimi KJJ, Alhassani AAK, Almheiri MMA, Nazir A. Encapsulation Properties of Mentha piperita Leaf Extracts Prepared Using an Ultrasound-Assisted Double Emulsion Method. Foods. 2023; 12(9):1838. https://doi.org/10.3390/foods12091838
Chicago/Turabian StyleSobti, Bhawna, Afaf Kamal-Eldin, Sanaa Rasul, Mariam Saeed Khalfan Alnuaimi, Khulood Jaber Jasim Alnuaimi, Alia Ali Khsaif Alhassani, Mariam M. A. Almheiri, and Akmal Nazir. 2023. "Encapsulation Properties of Mentha piperita Leaf Extracts Prepared Using an Ultrasound-Assisted Double Emulsion Method" Foods 12, no. 9: 1838. https://doi.org/10.3390/foods12091838
APA StyleSobti, B., Kamal-Eldin, A., Rasul, S., Alnuaimi, M. S. K., Alnuaimi, K. J. J., Alhassani, A. A. K., Almheiri, M. M. A., & Nazir, A. (2023). Encapsulation Properties of Mentha piperita Leaf Extracts Prepared Using an Ultrasound-Assisted Double Emulsion Method. Foods, 12(9), 1838. https://doi.org/10.3390/foods12091838