Modeling and Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Allium sativum Leaves Using Response Surface Methodology and Artificial Neural Network Coupled with Genetic Algorithm
Abstract
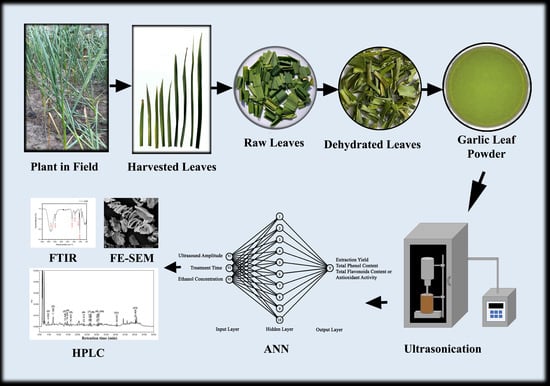
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Raw Material
2.3. Ultrasound-Assisted Extraction (UAE)
2.4. One Factor at a Time
2.5. Determination of Proximate Composition
2.6. Determination of Total Phenol Content
2.7. Determination of Total Flavonoid Content
2.8. DPPH Assay
2.9. Estimation of Organosulfur and Phenolic Compounds Using HPLC
2.10. Experimental Design
2.11. Fourier Transform Infrared Spectroscopy Analysis
2.12. Field Emission Scanning Electron Microscopy (FE-SEM)
2.13. Response Surface Methodology (RSM)
2.14. Artificial Neural Network (ANN) Modeling Coupled with Genetic Algorithm (GA)
2.15. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis
3.2. One Factor at a Time (OFAT)
3.3. RSM Analysis
3.3.1. Model Fitting
3.3.2. Effect of Ultrasound Treatment on Total Phenol Content
3.3.3. Effect of Ultrasound Treatment on Total Flavonoid Content (TFC)
3.3.4. Effect of Ultrasound Treatment on Antioxidant Activity
3.3.5. Effect of Ultrasound Treatment on Extraction Yield
3.4. Artificial Neural Network Analysis
Model Fitting
3.5. Optimization Using Genetic Algorithm (GA)
3.6. Comparison between RSM and ANN Models
3.7. FE-SEM Analysis
3.8. FTIR, Polyphenol, and Sulfur Compound Analysis
3.9. Identification and Quantification of Organosulfur Compounds (OSCs)
3.10. Identification and Quantification of Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations (FAO) 2021. Available online: http://www.fao.org/faostat/en/#data (accessed on 3 December 2022).
- Charron, C.S.; Milner, J.A.; Novotny, J.A. Garlic. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; pp. 184–190. [Google Scholar]
- Singh, R.; Prasad, K.K.; Siddiqui, M.W.; Prasad, K. Medicinal Plants in Preventive and Curative Role for Various Ailments; Apple Academic Press: New York, NY, USA, 2017; pp. 63–100. [Google Scholar]
- Kaur, R.; Shekhar, S.; Prasad, K. Secondary Metabolites of Fruits and Vegetables with Antioxidant Potential. Second. Metab. Trends Rev. 2022, 141. [Google Scholar] [CrossRef]
- Fukaya, M.; Nakamura, S.; Hayashida, H.; Noguchi, D.; Nakashima, S.; Yoneda, T.; Matsuda, H. Structures of Cyclic Organosulfur Compounds From Garlic (Allium sativum L.) Leaves. Front. Chem. 2020, 8, 282. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Kaushik, V.; Prasad, K.K. Garlic: A potential source of herbal medicine. Beverage Food World 2002, 29, 24–26. [Google Scholar]
- Prakash, P.; Prasad, K. Comparative elucidation of garlic peeling methods and positioning of quality characteristics using principal component analysis. Acta Sci. Pol. Technol. Aliment. 2023, 22, 119–131, in press. [Google Scholar]
- JĘDrszczyk, E.; KopeĆ, A.; Bucki, P.; Ambroszczyk, A.M.; Skowera, B. The Enhancing Effect of Plants Growth Biostimulants in Garlic Cultivation on the Chemical Composition and Level of Bioactive Compounds in the Garlic Leaves, Stems and Bulbs. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 81–91. [Google Scholar] [CrossRef]
- Capasso, A. Antioxidant Action and Therapeutic Efficacy of Allium sativum L. Molecules 2013, 18, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef]
- Liu, J.; Ji, F.; Chen, F.; Guo, W.; Yang, M.; Huang, S.; Zhang, F.; Liu, Y. Determination of garlic phenolic compounds using supercritical fluid extraction coupled to supercritical fluid chromatography/tandem mass spectrometry. J. Pharm. Biomed. Anal. 2018, 159, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Nagella, P.; Thiruvengadam, M.; Ahmad, A.; Yoon, J.-Y.; Chung, I.-M. Composition of Polyphenols and Antioxidant Activity of Garlic Bulbs Collected from Different Locations of Korea. Asian J. Chem. 2014, 26, 897–902. [Google Scholar] [CrossRef]
- Fernández-López, J.; Botella-Martínez, C.; Navarro-Rodríguez de Vera, C.; Sayas-Barberá, M.E.; Viuda-Martos, M.; Sánchez-Zapata, E.; Pérez-Álvarez, J.A. Vegetable Soups and Creams: Raw Materials, Processing, Health Benefits, and Innovation Trends. Plants 2020, 9, 1769. [Google Scholar] [CrossRef] [PubMed]
- Barbhuiya, R.I.; Singha, P.; Asaithambi, N.; Singh, S.K. Ultrasound-assisted rapid biological synthesis and characterization of silver nanoparticles using pomelo peel waste. Food Chem. 2022, 385, 132602. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Prasad, K.; Kumar, V.; Prasad, K. Biosynthesis of silver nanoparticles using eclipta leaf. Biotechnol. Prog. 2009, 25, 1476–1479. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Prasad, K.; Prasad, K. A green low-cost biosynthesis of Sb2O3 nanoparticles. Biochem. Eng. J. 2009, 43, 303–306. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K. Biosynthesis of Sb2O3 nanoparticles: A low-cost green approach. Biotechnol. J. 2009, 4, 1582–1585. [Google Scholar] [CrossRef]
- Jha, A.K.; Prasad, K.; Prasad, K.; Kulkarni, A.R. Plant system: Nature’s nanofactory. Colloids Surf. B Biointerfaces 2009, 73, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary metabolites in the green synthesis of metallic nanoparticles. Materials 2018, 11, 940. [Google Scholar] [CrossRef]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Barbhuiya, R.I.; Singha, P.; Singh, S.K. A comprehensive review on impact of non-thermal processing on the structural changes of food components. Food Res. Int. 2021, 149, 110647. [Google Scholar] [CrossRef] [PubMed]
- Asaithambi, N.; Singh, S.K.; Singha, P. Current status of non-thermal processing of probiotic foods: A review. J. Food Eng. 2021, 303, 110567. [Google Scholar] [CrossRef]
- Dash, D.R.; Singh, S.K.; Singha, P. Recent advances on the impact of novel non-thermal technologies on structure and functionality of plant proteins: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 1–16. [Google Scholar] [CrossRef]
- Prasad, K.; Nath, N.; Prasad, K. Estimation of sugar content in commercially available beverages using ultrasonic velocity measurement. Indian J. Phys. 2000, 74A, 387–389. [Google Scholar]
- Prasad, K. Advances in Nondestructive Quality Measurement of Fruits and Vegetables. In Postharvest Biology and Technology of Horticultural Crops: Principles and Practices for Quality Maintenance; Apple Academic Press: New York, NY, USA, 2015; pp. 51–87. [Google Scholar]
- Prasad, K. Non-destructive Quality Analysis of Fruits. In Postharvest Quality Assurance of Fruits: Practical Approaches for Developing Countries; Springer International Publishing: Cham, Switzerland, 2015; pp. 239–258. [Google Scholar]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A 2003, 1012, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Ramić, M.; Brindza, J.; Vidović, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar] [CrossRef]
- Kaur, R.; Shekhar, S.; Chaudhary, S.; Singh, B.; Prasad, K. Non-thermal Food Preservation Technologies. In Smart and Sustainable Food Technologies; Springer Nature: Basel, Switzerland, 2022; p. 157. [Google Scholar]
- Patra, A.; Abdullah, S.; Pradhan, R.C. Optimization of ultrasound-assisted extraction of ascorbic acid, protein and total antioxidants from cashew apple bagasse using artificial neural network-genetic algorithm and response surface methodology. J. Food Process. Preserv. 2022, 46, e16317. [Google Scholar] [CrossRef]
- Nelofer, R.; Ramanan, R.N.; Rahman, R.N.Z.R.A.; Basri, M.; Ariff, A.B. Comparison of the estimation capabilities of response surface methodology and artificial neural network for the optimization of recombinant lipase production by E. coli BL21. J. Ind. Microbiol. Biotechnol. 2012, 39, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Baş, D.; Boyacı, İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Kukreja, T.R.; Kumar, D.; Prasad, K.; Chauhan, R.C.; Choe, S.; Kundu, P.P. Optimisation of physical and mechanical properties of rubber compounds by response surface methodology––Two component modelling using vegetable oil and carbon black. Eur. Polym. J. 2002, 38, 1417–1422. [Google Scholar] [CrossRef]
- Kumar, S.; Baniwal, P.; Nayik, G.A.; Prasad, K.; Khan, K.A.; Ghramh, H.A.; Kumar, H.; Karabagias, I.K. Optimization and Development of Ready to Eat Chocolate Coated Roasted Flaked Rice as Instant Breakfast Food. Foods 2021, 10, 1658. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Ciric, A.; Krajnc, B.; Heath, D.; Ogrinc, N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef]
- Desai, K.M.; Survase, S.A.; Saudagar, P.S.; Lele, S.S.; Singhal, R.S. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in fermentation media optimization: Case study of fermentative production of scleroglucan. Biochem. Eng. J. 2008, 41, 266–273. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Yang, L.; Zhang, S.; Jiang, H. Optimization of Ultrasonic-Assisted Extraction Conditions for Bioactive Components and Antioxidant Activity of Poria cocos (Schw.) Wolf by an RSM-ANN-GA Hybrid Approach. Foods 2023, 12, 619. [Google Scholar] [CrossRef]
- Simoncini, D.; Zhang, K.Y.J. Population-Based Sampling and Fragment-Based De Novo Protein Structure Prediction. In Encyclopedia of Bioinformatics and Computational Biology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 774–784. [Google Scholar]
- Albadr, M.A.; Tiun, S.; Ayob, M.; Al-Dhief, F. Genetic Algorithm Based on Natural Selection Theory for Optimization Problems. Symmetry 2020, 12, 1758. [Google Scholar] [CrossRef]
- Ding, S.; Xu, L.; Su, C.; Zhu, H. Using Genetic Algorithms to Optimize Artificial Neural Networks. J. Converg. Inf. Technol. 2010, 5, 54–62. [Google Scholar] [CrossRef]
- Kikuchi, T.; Uchida, T. Calorimetric method for measuring high ultrasonic power using water as a heating material. J. Phys. Conf. Ser. 2011, 279, 012012. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. In Standard Methods for the Examination; Association of Official Analytical Chemists: Washington, DC, USA, 2006. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Gorinstein, S.; Vargas, O.J.M.; Jaramillo, N.O.; Salas, I.A.; Ayala, A.L.M.; Arancibia-Avila, P.; Toledo, F.; Katrich, E.; Trakhtenberg, S. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur. Food Res. Technol. 2007, 225, 321–328. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Li, M.; Huang, X.; Liu, H.; Liu, B.; Wu, Y.; Xiong, A.; Dong, T. Prediction of gas solubility in polymers by back propagation artificial neural network based on self-adaptive particle swarm optimization algorithm and chaos theory. Fluid Phase Equilibria 2013, 356, 11–17. [Google Scholar] [CrossRef]
- Gupta, S.; Gowri, B.S.; Lakshmi, A.J.; Prakash, J. Retention of nutrients in green leafy vegetables on dehydration. J. Food Sci. Technol. 2013, 50, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Arasaretnam, S.; Kiruthika, A.; Mahendran, T. Nutritional and mineral composition of selected green leafy vegetables. Ceylon J. Sci. 2018, 47, 35. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M.; Bordbar, S.; Serjouie, A. Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: Oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem. 2015, 172, 7–17. [Google Scholar] [CrossRef]
- Yahya, N.A.; Wahab, R.A.; Xine, T.L.S.; Hamid, M.A. Ultrasound-assisted extraction of polyphenols from pineapple skin. In Proceedings of the 2nd International Conference on Biosciences And Medical Engineering (Icbme2019): Towards Innovative Research and Cross-Disciplinary Collaborations, Bali, Indonesia, 11–12 April 2019. [Google Scholar]
- Shirzad, H.; Niknam, V.; Taheri, M.; Ebrahimzadeh, H. Ultrasound-assisted extraction process of phenolic antioxidants from Olive leaves: A nutraceutical study using RSM and LC–ESI–DAD–MS. J. Food Sci. Technol. 2017, 54, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Gadioli Tarone, A.; Keven Silva, E.; Dias de Freitas Queiroz Barros, H.; Baú Betim Cazarin, C.; Roberto Marostica Junior, M. High-intensity ultrasound-assisted recovery of anthocyanins from jabuticaba by-products using green solvents: Effects of ultrasound intensity and solvent composition on the extraction of phenolic compounds. Food Res. Int. 2021, 140, 110048. [Google Scholar] [CrossRef]
- Zimare, S.B.; Mankar, G.D.; Barmukh, R.B. Optimization of ultrasound-assisted extraction of total phenolics and flavonoids from the leaves of Lobelia nicotianifolia and their radical scavenging potential. Curr. Res. Green Sustain. Chem. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Rios-Romero, E.A.; Ochoa-Martínez, L.A.; Bello-Pérez, L.A.; Morales-Castro, J.; Quintero-Ramos, A.; Gallegos-Infante, J.A. Effect of ultrasound and steam treatments on bioaccessibility of β-carotene and physicochemical parameters in orange-fleshed sweet potato juice. Heliyon 2021, 7, e06632. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, D.; Xi, P.; Cai, T.; Cao, X.; Liu, H.; Li, J. Effects of temperature-controlled ultrasound treatment on sensory properties, physical characteristics and antioxidant activity of cloudy apple juice. Lwt 2021, 142, 111030. [Google Scholar] [CrossRef]
- Um, M.; Han, T.-H.; Lee, J.-W. Ultrasound-assisted extraction and antioxidant activity of phenolic and flavonoid compounds and ascorbic acid from rugosa rose (Rosa rugosa Thunb.) fruit. Food Sci. Biotechnol. 2017, 27, 375–382. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef] [PubMed]
- AL-Bukhaiti, W.Q.; Noman, A.; Mahdi, A.A.; Ali, A.H.; Abed, S.M.; Rashed, M.M.; Wang, H. Profiling of phenolic compounds and antioxidant activities of Cissus rotundifolia (Forssk.) as influenced by ultrasonic-assisted extraction conditions. J. Food Meas. Charact. 2019, 13, 634–647. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Hari, N.; Nair, V.P. FTIR spectroscopic analysis of leaf extract in hexane in Jasminum azoricum L. Recent Res. Sci. Technol. 2018, 4, 170–172. [Google Scholar]
- Alotibi, F.; Rizwana, H. Chemical composition, FTIR studies, morphological alterations, and antifungal activity of leaf extracts of Artemisia sieberi from Saudi Arabia. Int. J. Agric. Biol 2019, 21, 1241–1248. [Google Scholar]
- Ghitescu, R.-E.; Volf, I.; Carausu, C.; Bühlmann, A.-M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef]
- Medina-Jaramillo, C.; Gomez-Delgado, E.; López-Córdoba, A. Improvement of the Ultrasound-Assisted Extraction of Polyphenols from Welsh Onion (Allium fistulosum) Leaves Using Response Surface Methodology. Foods 2022, 11, 2425. [Google Scholar] [CrossRef]
- Itakura, Y.; Ichikawa, M.; Mori, Y.; Okino, R.; Udayama, M.; Morita, T. How to Distinguish Garlic from the Other Allium Vegetables. J. Nutr. 2001, 131, 963S–967S. [Google Scholar] [CrossRef]
- Szychowski, K.; Rybczyńska-Tkaczyk, K.; Gaweł-Bęben, K.; Świeca, M.; Karaś, M.; Jakubczyk, A.; Matysiak, M.; Binduga, U.; Gmiński, J. Characterization of Active Compounds of Different Garlic (Allium sativum L.) Cultivars. Pol. J. Food Nutr. Sci. 2018, 68, 73–81. [Google Scholar] [CrossRef]









| Coded Level | ||||||
|---|---|---|---|---|---|---|
| Independent Variable | Units | −α* | −1 | 0 | 1 | α* |
| Ultrasound Amplitude (X1) | % | 19.77 (~20) | 30 | 45 | 60 | 70.23 (~70) |
| Treatment Time (X2) | min | 1.60 (~2) | 5 | 10 | 15 | 18.40 (~18) |
| Ethanol Conc. (X3) | % | 33.18 (~33) | 40 | 50 | 60 | 66.82 (~67) |
| Run | Space Type | Ultrasound Amplitude (X1) | Treatment Time (X2) | Ethanol Conc. (X3) | Applied Energy (J) * | Calorimetric Energy (J) ** | Yield (%) | TPC (mg GAE/g) | TFC (mg QE/g) | Antioxidant (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental | RSM Pred. | ANN Pred. | Experimental | RSM Pred. | ANN Pred. | Experimental | RSM Pred. | ANN Pred. | Experimental | RSM Pred. | ANN Pred. | |||||||
| 1 | Center | 45 | 10 | 50 | 16,744 | 294.34 | 31.65 | 31.91 | 31.90 | 9.81 | 9.85 | 9.85 | 6.80 | 6.71 | 6.69 | 57.04 | 57.84 | 57.85 |
| 2 | Center | 45 | 10 | 50 | 16,744 | 277.73 | 32.14 | 31.91 | 31.90 | 9.89 | 9.85 | 9.85 | 6.78 | 6.71 | 6.69 | 57.74 | 57.84 | 57.85 |
| 3 | Factorial | 60 | 5 | 40 | 11,187 | 302.98 | 30.65 | 30.51 | 30.65 | 9.49 | 9.48 | 9.49 | 6.32 | 6.09 | 6.32 | 55.23 | 54.70 | 55.23 |
| 4 | Center | 45 | 10 | 50 | 16,744 | 837.19 | 31.74 | 31.91 | 31.90 | 9.82 | 9.85 | 9.85 | 6.85 | 6.71 | 6.69 | 57.87 | 57.84 | 57.85 |
| 5 | Factorial | 30 | 15 | 60 | 18,366 | 97.68 | 31.16 | 31.29 | 31.16 | 9.63 | 9.60 | 9.63 | 5.97 | 6.14 | 5.97 | 56.55 | 56.71 | 56.55 |
| 6 | Factorial | 30 | 5 | 60 | 6261 | 188.38 | 31.26 | 31.11 | 31.26 | 9.57 | 9.56 | 9.57 | 5.72 | 5.70 | 5.72 | 56.93 | 56.35 | 56.93 |
| 7 | Factorial | 30 | 5 | 40 | 7390 | 173.16 | 28.71 | 28.84 | 28.71 | 9.38 | 9.31 | 9.38 | 5.39 | 5.21 | 5.39 | 53.21 | 52.34 | 53.21 |
| 8 | Axial | 45 | 2 | 50 | 2846 | 166.50 | 30.45 | 30.42 | 30.45 | 9.47 | 9.53 | 9.47 | 5.49 | 5.78 | 5.49 | 53.86 | 54.89 | 53.86 |
| 9 | Axial | 70 | 10 | 50 | 23,389 | 336.38 | 31.73 | 31.68 | 31.73 | 9.82 | 9.82 | 9.82 | 6.88 | 7.01 | 6.88 | 57.80 | 57.55 | 57.80 |
| 10 | Axial | 20 | 10 | 50 | 10,390 | 134.66 | 30.11 | 30.17 | 30.11 | 9.38 | 9.42 | 9.38 | 5.53 | 5.49 | 5.53 | 53.39 | 54.16 | 53.39 |
| 11 | Factorial | 30 | 15 | 40 | 22,332 | 219.78 | 31.31 | 31.10 | 31.31 | 9.62 | 9.63 | 9.62 | 5.68 | 5.72 | 5.68 | 54.39 | 54.07 | 54.39 |
| 12 | Axial | 45 | 10 | 67 | 13,877 | 158.85 | 31.62 | 31.59 | 31.62 | 9.75 | 9.76 | 9.75 | 6.69 | 6.57 | 6.69 | 58.58 | 58.38 | 58.58 |
| 13 | Axial | 45 | 18 | 50 | 33,414 | 116.80 | 32.12 | 32.16 | 32.12 | 9.89 | 9.88 | 9.89 | 6.71 | 6.51 | 6.71 | 57.62 | 57.12 | 57.62 |
| 14 | Center | 45 | 10 | 50 | 16,744 | 170.09 | 32.21 | 31.91 | 31.90 | 9.90 | 9.85 | 9.85 | 6.67 | 6.71 | 6.69 | 58.59 | 57.84 | 57.85 |
| 15 | Center | 45 | 10 | 50 | 16,744 | 173.16 | 32.05 | 31.91 | 31.90 | 9.88 | 9.85 | 9.85 | 6.54 | 6.71 | 6.69 | 57.73 | 57.84 | 57.85 |
| 16 | Factorial | 60 | 15 | 40 | 30,957 | 109.41 | 32.24 | 32.39 | 32.24 | 9.88 | 9.86 | 9.88 | 6.57 | 6.52 | 6.57 | 56.79 | 56.99 | 56.79 |
| 17 | Axial | 45 | 10 | 33 | 17,820 | 166.50 | 30.46 | 30.50 | 30.46 | 9.48 | 9.52 | 9.48 | 5.48 | 5.70 | 5.48 | 53.12 | 53.84 | 53.12 |
| 18 | Factorial | 60 | 5 | 60 | 10,050 | 173.16 | 31.42 | 31.62 | 31.42 | 9.84 | 9.80 | 9.84 | 6.82 | 6.71 | 6.82 | 57.52 | 57.47 | 57.52 |
| 19 | Factorial | 60 | 15 | 60 | 28,857 | 146.52 | 31.57 | 31.43 | 31.57 | 9.88 | 9.91 | 9.88 | 6.96 | 7.07 | 6.96 | 57.89 | 58.39 | 57.89 |
| 20 | Center | 45 | 10 | 50 | 16,744 | 173.16 | 31.67 | 31.91 | 31.90 | 9.80 | 9.85 | 9.85 | 6.63 | 6.71 | 6.69 | 58.13 | 57.84 | 57.85 |
| Chemical Components | Amount (%) |
|---|---|
| Moisture | 90.04 |
| Protein | 2.23 |
| Fat | 0.18 |
| Ash | 1.56 |
| Carbohydrate | 5.99 |
| Coefficient | Yield (%) | TPC (mg GAE/g) | TFC (mg QE/g) | Antioxidant (%) |
|---|---|---|---|---|
| b0 | +31.91 | +9.85 | +6.71 | +57.84 |
| b1 | +0.4514 * | +0.1194 * | +0.4526 * | +1.01 * |
| b2 | +0.5161 * | +0.1052 * | +0.2183 * | +0.6629 * |
| b3 | +0.3259 * | +0.0735 * | +0.2596 * | +1.35 * |
| b12 | −0.0950 | +0.0163 | −0.0188 | +0.1412 |
| b13 | −0.2875 * | +0.0187 | +0.0337 | −0.3113 |
| b23 | −0.5175 * | −0.0662 * | −0.0188 | −0.3438 |
| b11 | −0.3482 * | −0.0794 * | −0.1614 * | −0.6993 * |
| b22 | −0.2192 * | −0.0511 * | −0.1985 * | −0.6481 * |
| b33 | −0.3058 * | −0.0740 * | −0.2038 * | −0.6092 * |
| R2 | 0.963 | 0.962 | 0.932 | 0.917 |
| Adj. R2 | 0.930 | 0.928 | 0.870 | 0.842 |
| Lack of fit | 0.660 | 0.336 | 0.051 | 0.109 |
| EY | TPC | TFC | Antioxidant | |||||
|---|---|---|---|---|---|---|---|---|
| RSM | ANN | RSM | ANN | RSM | ANN | RSM | ANN | |
| RMSE | 0.1630 | 0.1259 | 0.0353 | 0.0224 | 0.1442 | 0.0604 | 0.5298 | 0.2558 |
| NMSE | 0.0052 | 0.0040 | 0.0036 | 0.0023 | 0.0228 | 0.0095 | 0.0094 | 0.0045 |
| MSE | 0.0266 | 0.0158 | 0.0012 | 0.0005 | 0.0208 | 0.0036 | 0.2807 | 0.0655 |
| NRMSE | 0.0008 | 0.0005 | 0.0001 | 0.0001 | 0.0033 | 0.0006 | 0.0050 | 0.0012 |
| MPE | 0.4500 | 0.2099 | 0.3160 | 0.1218 | 2.0121 | 0.4388 | 0.7860 | 0.1799 |
| AAD | 0.1416 | 0.0670 | 0.0306 | 0.0120 | 0.1248 | 0.0295 | 0.4376 | 0.1040 |
| R2 | 0.9633 | 0.9781 | 0.9622 | 0.9848 | 0.9317 | 0.9883 | 0.9171 | 0.9807 |
| Peak Number Compounds | Concentration (ppm) | ||
|---|---|---|---|
| Organosulfur | Alliin | 90.207 | |
| S-Allyl-L-cysteine | 4.314 | ||
| Allicin | 219.536 | ||
| Phenolic | 1 | Gallic acid | 13.591 |
| 2 | 3,4-Dihydroxybenzoic acid | 34.403 | |
| 3 | Chlorogenic acid | 36.537 | |
| 4 | Catechin hydrate | 26.327 | |
| 5 | Syringic acid | 4.276 | |
| 6 | p-Coumaric acid | 1.228 | |
| 7 | Rutin | 21.741 | |
| 8 | Ellagic acid | 0.968 | |
| 9 | Benzoic acid | 15.234 | |
| 10 | Hesperidin | 9.272 | |
| 11 | Quercetin | 6.140 | |
| 12 | β-Carotene | 117.607 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shekhar, S.; Prakash, P.; Singha, P.; Prasad, K.; Singh, S.K. Modeling and Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Allium sativum Leaves Using Response Surface Methodology and Artificial Neural Network Coupled with Genetic Algorithm. Foods 2023, 12, 1925. https://doi.org/10.3390/foods12091925
Shekhar S, Prakash P, Singha P, Prasad K, Singh SK. Modeling and Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Allium sativum Leaves Using Response Surface Methodology and Artificial Neural Network Coupled with Genetic Algorithm. Foods. 2023; 12(9):1925. https://doi.org/10.3390/foods12091925
Chicago/Turabian StyleShekhar, Shubhra, Prem Prakash, Poonam Singha, Kamlesh Prasad, and Sushil Kumar Singh. 2023. "Modeling and Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Allium sativum Leaves Using Response Surface Methodology and Artificial Neural Network Coupled with Genetic Algorithm" Foods 12, no. 9: 1925. https://doi.org/10.3390/foods12091925
APA StyleShekhar, S., Prakash, P., Singha, P., Prasad, K., & Singh, S. K. (2023). Modeling and Optimization of Ultrasound-Assisted Extraction of Bioactive Compounds from Allium sativum Leaves Using Response Surface Methodology and Artificial Neural Network Coupled with Genetic Algorithm. Foods, 12(9), 1925. https://doi.org/10.3390/foods12091925