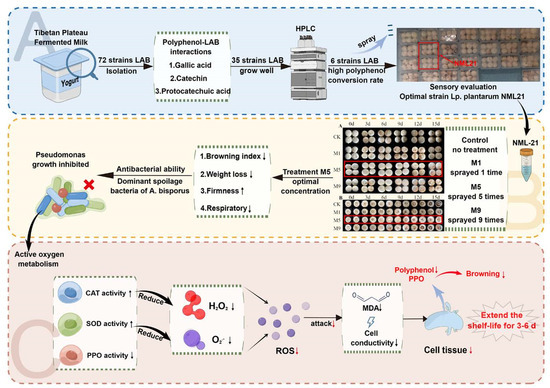
Screening of Lactiplantibacillus plantarum NML21 and Its Maintenance on Postharvest Quality of Agaricus bisporus through Anti-Browning and Mitigation of Oxidative Damage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. LAB Sensitivity to Phenolic Compounds
2.3. Screening of Optimal Strains by Sensory Evaluation
2.4. Screening of Optimal Strains
2.5. Concentration of Lp. plantarum NML21 Used and Packaging Scheme for A. bisporus Samples
2.6. Determination of Weight Loss, Firmness, Browning Index (BI), and Respiratory Rate
2.7. Counting Method of Pseudomonas spp.
2.8. Activities of PPO, Superoxide Dismutase (SOD), Catalase (CAT), and Levels of Hydrogen Peroxide (H2O2) and Superoxide Anion (O2.-) in Mushrooms
2.9. Determination of Cell Conductivity and Malondialdehyde Content (MDA)
2.10. Statistical Analysis
3. Results
3.1. Interaction of Phenolic Compounds with LAB
3.2. Determination of Phenolic Conversion Ability of LAB by HPLC
3.3. Screening for the Optimal Strain to Use in the Treatment
3.4. Effects of NML21 Treatments on the Overall Acceptability, BI, and Weight Loss of A. bisporus during Storage
3.5. Effects of NML21 Treatment on Firmness and Respiration Rate of A. bisporus
3.6. Effects of NML21 Treatment on the Number of Pseudomonas spp. in A. bisporus
3.7. Effects of NML21 Treatments on the Cellular Integrity of A. bisporus
3.8. Effects of NML21 Treatment on the O2.- Production Rate and H2O2 Content of A. bisporus
3.9. Effects of NML21 Treatment on PPO, SOD, and CAT in A. bisporus
3.10. Correlation Analysis of the Effects of NML21 Treatment on Storage Quality of A. bisporus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gholami, R.; Ahmadi, E.; Farris, S. Shelf life extension of white mushrooms (Agaricus bisporus) by low temperatures conditioning, modified atmosphere, and nanocomposite packaging material. Food Packag. Shelf Life 2017, 14, 88–95. [Google Scholar] [CrossRef]
- Xia, R.; Hou, Z.; Xu, H.; Li, Y.; Sun, Y.; Wang, Y.; Zhu, J.; Wang, Z.; Pan, S.; Xin, G. Emerging technologies for preservation and quality evaluation of postharvest edible mushrooms: A review. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.W.; Zhu, D.; Zhao, L.G.; Liu, Y.; Wang, C.P.; Farid, M.S.; Gu, Y.Y.; Li, J.; Li, T.H.; Sun, Y.A.; et al. L-Cysteine Treatment Delayed the Quality Deterioration of Fresh-Cut Button Mushrooms by Regulating Oxygen Metabolism, Inhibiting Water Loss, and Stimulating Endogenous H2S Production. J. Agric. Food Chem. 2023, 71, 974–984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pu, Y.-Y.; Sun, D.-W. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Nxumalo, K.A.; Aremu, A.O.; Fawole, O.A. Potentials of Medicinal Plant Extracts as an Alternative to Synthetic Chemicals in Postharvest Protection and Preservation of Horticultural Crops: A Review. Sustainability 2021, 13, 5897. [Google Scholar] [CrossRef]
- Fernandes, A.; Barreira, J.C.M.; Gunaydi, T.; Alkan, H.; Antonio, A.L.; Oliveira, M.; Martins, A.; Ferreira, I. Effect of gamma irradiation and extended storage on selected chemical constituents and antioxidant activities of sliced mushroom. Food Control 2017, 72, 328–337. [Google Scholar] [CrossRef]
- Barbosa, A.A.T.; Mantovani, H.C.; Jain, S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit. Rev. Biotechnol. 2017, 37, 852–864. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, S.; Fang, X.; Wang, Z.; Jiang, Y.; Guo, X.; Zhu, J.; Zhang, Y. The Effect of Lactiplantibacillus plantarum BX62 Alone or in Combination with Chitosan on the Qualitative Characteristics of Fresh-Cut Apples during Cold Storage. Microorganisms 2021, 9, 2404. [Google Scholar] [CrossRef]
- Ren, B.J.; Wu, W.; Soladoye, O.P.; Bak, K.H.; Fu, Y.; Zhang, Y.H. Application of biopreservatives in meat preservation: A review. Int. J. Food Sci. Technol. 2021, 56, 6124–6141. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Guo, W.; Ke, W.; Bi, S.; Guo, X.; Zhang, Y. Lactobacillus delbrueckii subsp. bulgaricus F17 and Leuconostoc lactis H52 supernatants delay the decay of strawberry fruits: A microbiome perspective. Food Funct. 2019, 10, 7767–7781. [Google Scholar] [CrossRef]
- Martínez-Castellanos, G.; Pelayo-Zaldívar, C.; Pérez-Flores, L.J.; López-Luna, A.; Gimeno, M.; Bárzana, E.; Shirai, K. Postharvest litchi (Litchi chinensis Sonn.) quality preservation by Lactobacillus plantarum. Postharvest Biol. Technol. 2011, 59, 172–178. [Google Scholar] [CrossRef]
- Li, F.H.; Ding, Z.T.; Chen, X.Z.; Zhang, Y.X.; Ke, W.C.; Zhang, X.; Li, Z.Q.; Usman, S.; Guo, X.S. The effects of Lactobacillus plantarum with feruloyl esterase-producing ability or high antioxidant activity on the fermentation, chemical composition, and antioxidant status of alfalfa silage. Anim. Feed Sci. Technol. 2021, 273, 114835. [Google Scholar] [CrossRef]
- Landete, J.M.; Curiel, J.A.; Rodríguez, H.; de las Rivas, B.; Muñoz, R. Study of the inhibitory activity of phenolic compounds found in olive products and their degradation by Lactobacillus plantarum strains. Food Chem. 2008, 107, 320–326. [Google Scholar] [CrossRef]
- Liu, J.; Jia, L.; Kan, J.; Jin, C.H. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem. Toxicol. 2013, 51, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Yang, X.L.; Gao, H.A.; Shi, C.R.; Wang, L.L.; Lu, D.Y.; Li, Y.H.; Zhang, J.L.; Zhang, W.B.; Wen, P.C. Research on bacterial community characteristics of traditional fermented yak milk in the Tibetan Plateau based on high-throughput sequencing. PeerJ 2023, 11, 20. [Google Scholar] [CrossRef]
- Wu, J.; Gao, J.; Chen, H.; Liu, X.; Cheng, W.; Ma, X.; Tong, P. Purification and Characterization of Polyphenol Oxidase from Agaricus bisporus. Int. J. Food Prop. 2013, 16, 1483–1493. [Google Scholar] [CrossRef]
- Tao, L.; Long, H.; Zhang, J.; Qi, L.; Zhang, S.; Li, T.; Li, S. Preparation and coating application of γ-polyglutamic acid hydrogel to improve storage life and quality of shiitake mushrooms. Food Control 2021, 130, 108404. [Google Scholar] [CrossRef]
- Siroli, L.; Patrignani, F.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of bio-control agents to improve the safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 302–310. [Google Scholar] [CrossRef]
- Palou, E.; López-Malo, A.; Barbosa-Cánovas, G.V.; Welti-Chanes, J.; Swanson, B.G. Polyphenoloxidase Activity and Color of Blanched and High Hydrostatic Pressure Treated Banana Puree. J. Food Sci. 1999, 64, 42–45. [Google Scholar] [CrossRef]
- Nasiri, M.; Barzegar, M.; Sahari, M.A.; Niakousari, M. Application of Tragacanth gum impregnated with Satureja khuzistanica essential oil as a natural coating for enhancement of postharvest quality and shelf life of button mushroom (Agaricus bisporus). Int J Biol Macromol 2018, 106, 218–226. [Google Scholar] [CrossRef]
- Augustin, M.A.; Ghazali, H.M.; Hashim, H. Polyphenoloxidase from guava (Psidium guajava L.). J. Sci. Food Agric. 1985, 36, 1259–1265. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhang, X.; Kan, J.; Jin, C. Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 147, 39–47. [Google Scholar] [CrossRef]
- Dong, S.; Guo, J.; Yu, J.; Bai, J.; Xu, H.; Li, M. Effects of electron-beam generated X-ray irradiation on the postharvest storage quality of Agaricus bisporus. Innov. Food Sci. Emerg. Technol. 2022, 80, 103079. [Google Scholar] [CrossRef]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Fang, D.L.; Yu, K.L.; Deng, Z.L.; Hu, Q.H.; Zhao, L.Y. Storage quality and flavor evaluation of Volvariella volvacea packaged with nanocomposite-based packaging material during commercial storage condition. Food Packag. Shelf Life 2019, 22, 100412. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Tian, Y.; Ma, R.N.; Liu, Q.H.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Jo, I.H.; Kim, J.; An, H.; Lee, H.Y.; So, Y.S.; Ryu, H.; Sung, G.H.; Shim, D.; Chung, J.W. Pseudo-Chromosomal Genome Assembly in Combination with Comprehensive Transcriptome Analysis in Agaricus bisporus Strain KMCC00540 Reveals Mechanical Stimulus Responsive Genes Associated with Browning Effect. J. Fungi 2022, 8, 886. [Google Scholar] [CrossRef]
- Mohapatra, D.; Frias, J.M.; Oliveira, F.; Bira, Z.M.; Kerry, J. Development and validation of a model to predict enzymatic activity during storage of cultivated mushrooms (Agaricus bisporus spp.). J. Food Eng. 2008, 86, 39–48. [Google Scholar] [CrossRef]
- Huang, G.; Lai, M.; Xu, C.; He, S.; Dong, L.; Huang, F.; Zhang, R.; Young, D.J.; Liu, H.; Su, D. Novel Catabolic Pathway of Quercetin-3-O-Rutinose-7-O-α-L-Rhamnoside by Lactobacillus plantarum GDMCC 1.140: The Direct Fission of C-Ring. Front. Nutr. 2022, 9, 849439. [Google Scholar] [CrossRef]
- Filannino, P.; Gobbetti, M.; De Angelis, M.; Di Cagno, R. Hydroxycinnamic Acids Used as External Acceptors of Electrons: An Energetic Advantage for Strictly Heterofermentative Lactic Acid Bacteria. Appl. Environ. Microbiol. 2014, 80, 7574–7582. [Google Scholar] [CrossRef]
- Song, M.W.; Park, J.Y.; Lee, H.S.; Kim, K.T.; Paik, H.D. Co-Fermentation by Lactobacillus brevis B7 Improves the Antioxidant and Immunomodulatory Activities of Hydroponic Ginseng-Fortified Yogurt. Antioxidants 2021, 10, 1447. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Z.; Rao, L.; Zhao, L.; Wang, Y.T.; Liao, X.J. Multispectroscopic and computational simulation insights into the inhibition mechanism of epigallocatechin-3-gallate on polyphenol oxidase. Food Chem. 2022, 393, 133415. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Yang, Z.F.; Cui, J.X.; Zhu, S.H. Nitric Oxide Made a Major Contribution to the Improvement of Quality in Button Mushrooms (Agaricus bisporus) by the Combined Treatment of Nitric Oxide with 1-MCP. Foods 2022, 11, 3147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, X.J.; Yagoub, A.E.; Xia, G.H.; Zhou, C.S. Effect of vacuum impregnation assisted probiotics fermentation suspension on shelf life quality of freshly cut lotus root. Food Chem. 2022, 381, 132281. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.R.; Wang, Y.; Li, X.; Hao, X.; Xu, D.X.; Zhou, Y.N.; Mehmood, A.; Wang, C.T. Genetic and Biochemical Evidence That Enterococcus faecalis Gr17 Produces a Novel and Sec-Dependent Bacteriocin, Enterocin Gr17. Front. Microbiol. 2019, 10, 1806. [Google Scholar] [CrossRef] [PubMed]
- Lo Cantore, P.; Lazzaroni, S.; Coraiola, M.; Dalla Serra, M.; Cafarchia, C.; Evidente, A.; Iacobellis, N.S. Biological characterization of white line-inducing principle (WLIP) produced by Pseudomonas reactans NCPPB1311. Mol. Plant-Microbe Interact. MPMI 2006, 19, 1113–1120. [Google Scholar] [CrossRef]
- Li, J.Z.; Xiao, X.T.; Li, H.X.; Chen, X.H.; Li, A.P. Creening of lactic acid bacteria against pseudomonas aeruginsa and preliminary study on its mechanism. Food Mach. 2021, 37, 6–11+149. [Google Scholar] [CrossRef]
- Banerjee, P.; Sahoo, P.K.; Sheenu; Adhikary, A.; Ruhal, R.; Jain, D. Molecular and structural facets of c-di-GMP signalling associated with biofilm formation in Pseudomonas aeruginosa. Mol. Asp. Med. 2021, 81, 17. [Google Scholar] [CrossRef]
- Rana, S.; Bhawal, S.; Kumari, A.; Kapila, S.; Kapila, R. pH-dependent inhibition of AHL-mediated quorum sensing by cell-free supernatant of lactic acid bacteria in Pseudomonas aeruginosa PAO1. Microb. Pathog. 2020, 142, 104105. [Google Scholar] [CrossRef]
- Pellan, L.; Durand, N.; Martinez, V.; Fontana, A.; Schorr-Galindo, S.; Strub, C. Commercial Biocontrol Agents Reveal Contrasting Comportments against Two Mycotoxigenic Fungi in Cereals: Fusarium graminearum and Fusarium verticillioides. Toxins 2020, 12, 152. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Hu, Q.H.; Li, Z.X.; Pei, F.; Mariga, A.M.; Yang, W.J. Effect of nanocomposite-based packaging on microstructure and energy metabolism of Agaricus bisporus. Food Chem. 2019, 276, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.; Chen, K.S.; Wang, G.L. Combination of the biocontrol yeast Cryptococcus laurentii with UV-C treatment for control of postharvest diseases of tomato fruit. Biocontrol 2013, 58, 269–281. [Google Scholar] [CrossRef]
- De Moreno de LeBlanc, A.; LeBlanc, J.G.; Perdigón, G.; Miyoshi, A.; Langella, P.; Azevedo, V.; Sesma, F. Oral administration of a catalase-producing Lactococcus lactis can prevent a chemically induced colon cancer in mice. J. Med. Microbiol. 2008, 57, 100–105. [Google Scholar] [CrossRef]
- Rani, A.; Saini, K.; Bast, F.; Mehariya, S.; Bhatia, S.; Lavecchia, R.; Zuorro, A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules 2021, 26, 1142. [Google Scholar] [CrossRef]
- Shekari, A.; Hassani, R.N.; Aghdam, M.S. Exogenous application of GABA retards cap browning in Agaricus bisporus and its possible mechanism. Postharvest Biol. Technol. 2021, 174, 7. [Google Scholar] [CrossRef]








| Score | Browning Degree (S1) | Cap Morphology (S2) | Smell (S3) | Softness of Fruiting Body (S4) | Consumer Acceptance (S5) | Sensory Score |
|---|---|---|---|---|---|---|
| 20–16 | White and lustrous | Closed/No dent | Clear aroma of A. bisporus/No LAB flavor | Stretchy | Very Satisfied | |
| 15–11 | Slight browning | Slightly open/Few dents | A. bisporus fragrance/Slight LAB odor | Slightly soft | Satisfied | |
| 10–7 | Mild browning | Half open/More dents | No distinctive fragrance of A. bisporus/LAB odor is obvious | Mildly soft | Grudging acceptance | |
| <7 | Seriously browning | Totally open/Severe denting | Severely Off-odor/LAB odor is strong | Severely soft | Unacceptable |
| Strains Number | Control | Gallic Acid | Catechin | Protocatechuic Acid | |||
|---|---|---|---|---|---|---|---|
| 0.4 g L−1 | 0.8 g L−1 | 0.4 g L−1 | 0.8 g L−1 | 0.4 g L−1 | 0.8 g L−1 | ||
| 1-MN3 | 1.48 ± 0.13 | 1.42 ± 0.05 | 1.59 ± 0.11 | 1.68 ± 0.07 | 1.72 ± 0.05 | 1.49 ± 0.04 | 1.49 ± 0.10 |
| 2-NML21 | 1.53 ± 0.09 | 1.65 ± 0.10 | 1.71 ± 0.14 | 1.55 ± 0.09 | 1.58 ± 0.13 | 1.64 ± 0.10 | 1.59 ± 0.06 |
| 3-HG26 | 0.87 ± 0.01 | 1.18 ± 0.04 | 0.77 ± 0.13 | 1.28 ± 0.10 | 1.23 ± 0.02 | 1.22 ± 0.07 | 1.30 ± 0.06 |
| 4-TG4 | 0.67 ± 0.14 | 1.05 ± 0.05 | 1.41 ± 0.03 | 1.23 ± 0.04 | 1.53 ± 0.02 | 1.46 ± 0.09 | 1.41 ± 0.12 |
| 5-NX3 | 1.54 ± 0.08 | 1.66 ± 0.03 | 1.43 ± 0.13 | 1.60 ± 0.15 | 1.64 ± 0.07 | 1.52 ± 0.03 | 1.65 ± 0.08 |
| 6-HG23 | 1.60 ± 0.12 | 1.87 ± 0.01 | 1.81 ± 0.08 | 1.58 ± 0.07 | 1.35 ± 0.01 | 1.55 ± 0.12 | 1.63 ± 0.15 |
| 7-XWW1 | 1.67 ± 0.03 | 1.34 ± 0.03 | 1.02 ± 0.05 | 1.32 ± 0.12 | 0.85 ± 0.03 | 1.42 ± 0.11 | 1.39 ± 0.09 |
| 8-Q10 | 1.66 ± 0.05 | 1.65 ± 0.01 | 1.62 ± 0.13 | 1.51 ± 0.07 | 1.63 ± 0.10 | 1.52 ± 0.02 | 1.62 ± 0.03 |
| 9-ZHG1 | 0.92 ± 0.04 | 1.64 ± 0.02 | 1.32 ± 0.07 | 1.25 ± 0.07 | 1.33 ± 0.06 | 0.92 ± 0.07 | 1.34 ± 0.07 |
| 10-NML22 | 1.69 ± 0.08 | 1.67 ± 0.15 | 1.69 ± 0.11 | 1.55 ± 0.09 | 1.54 ± 0.11 | 1.76 ± 0.03 | 1.72 ± 0.01 |
| 11-HG1 | 1.43 ± 0.01 | 1.01 ± 0.05 | 0.52 ± 0.03 | 1.23 ± 0.14 | 1.31 ± 0.01 | 1.42 ± 0.09 | 1.37 ± 0.08 |
| 12-XH1 | 1.51 ± 0.02 | 1.40 ± 0.13 | 1.39 ± 0.07 | 1.50 ± 0.08 | 1.45 ± 0.11 | 1.32 ± 0.05 | 1.27 ± 0.09 |
| 13-GN3 | 1.55 ± 0.03 | 1.53 ± 0.02 | 1.45 ± 0.13 | 1.32 ± 0.07 | 1.30 ± 0.08 | 1.48 ± 0.11 | 1.02 ± 0.11 |
| 14-TG1 | 1.63 ± 0.03 | 1.54 ± 0.06 | 1.37 ± 0.09 | 1.62 ± 0.03 | 1.53 ± 0.04 | 1.51 ± 0.01 | 1.42 ± 0.04 |
| 15-TG2 | 1.59 ± 0.04 | 1.41 ± 0.13 | 1.63 ± 0.08 | 1.42 ± 0.04 | 1.39 ± 0.09 | 1.41 ± 0.14 | 1.33 ± 0.10 |
| 16-XH1 | 1.62 ± 0.06 | 1.60 ± 0.03 | 1.70 ± 0.02 | 1.26 ± 0.04 | 0.68 ± 0.11 | 1.02 ± 0.12 | 1.01 ± 0.05 |
| 17-XWW2 | 1.54 ± 0.09 | 1.60 ± 0.01 | 1.47 ± 0.02 | 1.33 ± 0.09 | 0.92 ± 0.07 | 1.52 ± 0.03 | 1.43 ± 0.12 |
| 18-YLJ1 | 1.52 ± 0.09 | 1.48 ± 0.14 | 1.31 ± 0.02 | 1.48 ± 0.10 | 1.20 ± 0.01 | 1.34 ± 0.14 | 1.22 ± 0.08 |
| 19-YLJ2 | 1.10 ± 0.12 | 0.84 ± 0.04 | 0.79 ± 0.09 | 0.72 ± 0.10 | 0.33 ± 0.13 | 1.32 ± 0.06 | 1.42 ± 0.08 |
| 20-CQ5 | 1.54 ± 0.10 | 1.27 ± 0.09 | 1.38 ± 0.13 | 1.36 ± 0.06 | 1.38 ± 0.08 | 1.44 ± 0.11 | 1.40 ± 0.03 |
| Time | 0 d | 3 d | 6 d | 9 d | 12 d | |||
|---|---|---|---|---|---|---|---|---|
| Index | ||||||||
| Sensory score | CK | 100 ± 0.00 | 92.00 ± 0.23 b | 80.18 ± 0.72 c | 60.63 ± 0.47 cd | 51.43 ± 0.67 d | ||
| Strain name | MN3 | 100 ± 0.00 | 91.11 ± 0.94 b | 82.32 ± 1.03 c | 59.97 ± 0.63 d | 59.08 ± 0.44 c | ||
| NML21 | 100 ± 0.00 | 91.64 ± 0.44 b | 90.56 ± 0.47 a | 73.43 ± 1.31 a | 65.58 ± 0.49 a | |||
| HG26 | 100 ± 0.00 | 92.68 ± 0.84 b | 81.91 ± 0.53 c | 64.42 ± 0.71 b | 61.17 ± 0.14 c | |||
| TG4 | 100 ± 0.00 | 95.73 ± 0.62 a | 86.20 ± 0.51 b | 62.20 ± 0.57 bcd | 64.15 ± 0.98 ab | |||
| NX3 | 100 ± 0.00 | 89.02 ± 0.85 c | 76.89 ± 0.94 d | 62.60 ± 0.49 bc | 52.03 ± 1.74 d | |||
| HG23 | 100 ± 0.00 | 95.22 ± 0.80 a | 91.62 ± 0.96 a | 72.25 ± 0.48 a | 61.66 ± 0.44 bc | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, C.; Yang, X.; Wang, P.; Zhang, H.; Wang, Q.; Wang, B.; Oyom, W.; Zhang, W.; Wen, P. Screening of Lactiplantibacillus plantarum NML21 and Its Maintenance on Postharvest Quality of Agaricus bisporus through Anti-Browning and Mitigation of Oxidative Damage. Foods 2024, 13, 168. https://doi.org/10.3390/foods13010168
Shi C, Yang X, Wang P, Zhang H, Wang Q, Wang B, Oyom W, Zhang W, Wen P. Screening of Lactiplantibacillus plantarum NML21 and Its Maintenance on Postharvest Quality of Agaricus bisporus through Anti-Browning and Mitigation of Oxidative Damage. Foods. 2024; 13(1):168. https://doi.org/10.3390/foods13010168
Chicago/Turabian StyleShi, Chengrui, Xiaoli Yang, Pengjie Wang, Hao Zhang, Qihui Wang, Bo Wang, William Oyom, Weibing Zhang, and Pengcheng Wen. 2024. "Screening of Lactiplantibacillus plantarum NML21 and Its Maintenance on Postharvest Quality of Agaricus bisporus through Anti-Browning and Mitigation of Oxidative Damage" Foods 13, no. 1: 168. https://doi.org/10.3390/foods13010168
APA StyleShi, C., Yang, X., Wang, P., Zhang, H., Wang, Q., Wang, B., Oyom, W., Zhang, W., & Wen, P. (2024). Screening of Lactiplantibacillus plantarum NML21 and Its Maintenance on Postharvest Quality of Agaricus bisporus through Anti-Browning and Mitigation of Oxidative Damage. Foods, 13(1), 168. https://doi.org/10.3390/foods13010168