Blueberry Pruning Wastes: From an Undervalued Agricultural Residue to a Safe and Valuable Source of Antioxidant Compounds for the Food Industry
Abstract
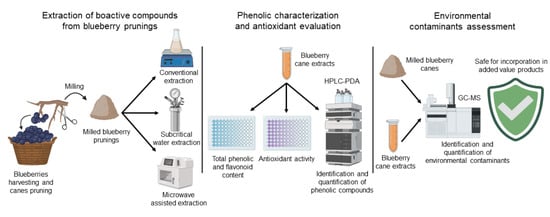
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Regents
2.2. Sample Collection and Preparation
2.3. Extraction of Bioactive Compounds
2.3.1. Conventional Extraction
2.3.2. Microwave-Assisted Extraction
2.3.3. Subcritical Water Extraction
2.4. Determination of Total Phenolic and Flavonoid Contents
2.5. Quantitative and Qualitative Polyphenol Characterization
2.6. DPPH-RSA, FRAP and ABTS Assays
2.7. Screening of Pesticides and Other Environmental Contaminants in BPWs and Their Extracts
2.7.1. OPP Compounds’ Gas Chromatography Analysis
2.7.2. OCP and PYR Gas Chromatography Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic and Flavonoid Contents
3.2. Antioxidant Activity
3.3. Identification and Quantification of Polyphenols
3.4. Screening of Pesticides and Environmental Contaminants in Blueberry Canes and Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T. Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Avram, A.M.; Morin, P.; Brownmiller, C.; Howard, L.R.; Sengupta, A.; Wickramasinghe, S.R. Concentrations of polyphenols from blueberry pomace extract using nanofiltration. Food Bioprod. Process 2017, 106, 91–101. [Google Scholar] [CrossRef]
- Miraghajani, M.; Momenyan, S.; Arab, A.; Hasanpour Dehkordi, A.; Symonds, M.E. Blueberry and cardiovascular disease risk factors: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 53, 102389. [Google Scholar] [CrossRef]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pereira, A.L.D.; Barbero, G.F.; Martínez, J. Recovery of anthocyanins from residues of Rubus fruticosus, Vaccinium myrtillus and Eugenia brasiliensis by ultrasound assisted extraction, pressurized liquid extraction and their combination. Food Chem. 2017, 231, 1–10. [Google Scholar] [CrossRef]
- Tian, Y.; Puganen, A.; Alakomi, H.L.; Uusitupa, A.; Saarela, M.; Yang, B. Antioxidative and antibacterial activities of aqueous ethanol extracts of berries, leaves, and branches of berry plants. Food Res. Int. 2018, 106, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Yang, G.; Yue, J.; Qian, B.; Liu, Z.; Wang, D.; Zhong, Y.; Zhao, Y. Influences of ripening stages and extracting solvents on the polyphenolic compounds, antimicrobial and antioxidant activities of blueberry leaf extracts. Food Control 2014, 38, 184–191. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Nakurte, I.; Klavins, M. Berry press residues as a valuable source of polyphenolics: Extraction optimisation and analysis. LWT 2018, 93, 583–591. [Google Scholar] [CrossRef]
- Dorosh, O.; Moreira, M.M.; Pinto, D.; Peixoto, A.F.; Freire, C.; Costa, P.; Rodrigues, F.; Delerue-Matos, C. Evaluation of the Extraction Temperature Influence on Polyphenolic Profiles of Vine-Canes (Vitis vinifera) Subcritical Water Extracts. Foods 2020, 9, 872. [Google Scholar] [CrossRef]
- Conde, E.; Cara, C.; Moure, A.; Ruiz, E.; Castro, E.; Domínguez, H. Antioxidant activity of the phenolic compounds released by hydrothermal treatments of olive tree pruning. Food Chem. 2009, 114, 806–812. [Google Scholar] [CrossRef]
- Moreira, M.M.; Barroso, M.F.; Boeykens, A.; Withouck, H.; Morais, S.; Delerue-Matos, C. Valorization of apple tree wood residues by polyphenols extraction: Comparison between conventional and microwave-assisted extraction. Ind. Crops Prod. 2017, 104, 210–220. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro—Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018, 376, 3. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Volf, I.; Popa, V.I. A Comparative Analysis of the “Green” Techniques Applied for Polyphenols Extraction from Bioresources. Chem. Biodivers. 2015, 12, 1635–1651. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC—Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Belwal, T.; Chemat, F.; Venskutonis, P.R.; Cravotto, G.; Jaishwal, D.K.; Bhatt, I.D.; Devkota, H.P.; Luo, Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC—Trends Anal. Chem. 2020, 127, 115895. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC—Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Nair, S.V.G.; Ziaullah; Vasantha Rupasinghe, H.P. Fatty acid esters of phloridzin induce apoptosis of human liver cancer cells through altered gene expression. PLoS ONE 2014, 9, e107149. [Google Scholar] [CrossRef]
- Fernando, W.; Coyle, K.; Marcato, P.; Vasantha Rupasinghe, H.P.; Hoskin, D.W. Phloridzin docosahexaenoate, a novel fatty acid ester of a plant polyphenol, inhibits mammary carcinoma cell metastasis. Cancer Lett. 2019, 465, 68–81. [Google Scholar] [CrossRef]
- Dorosh, O.; Fernandes, V.C.; Moreira, M.M.; Delerue-Matos, C. Occurrence of pesticides and environmental contaminants in vineyards: Case study of Portuguese grapevine canes. Sci. Total Environ. 2021, 791, 148395. [Google Scholar] [CrossRef] [PubMed]
- Santana-Mayor, A.; Rodríguez-Ramos, R.; Herrera-Herrera, A.V.; Socas-Rodríguez, B.; Rodríguez-Delgado, M.A. Updated overview of QuEChERS applications in food, environmental and biological analysis (2020–2023). TrAC—Trends Anal. Chem. 2023, 169, 117375. [Google Scholar] [CrossRef]
- Mesías, F.J.; Martín, A.; Hernández, A. Consumers’ growing appetite for natural foods: Perceptions towards the use of natural preservatives in fresh fruit. Food Res. Int. 2021, 150, 110749. [Google Scholar] [CrossRef]
- Bazzani, C.; Maesano, G.; Begalli, D.; Capitello, R. Exploring the effect of naturalness on consumer wine choices: Evidence from a survey in Italy. Food Qual. Prefer. 2024, 113, 105062. [Google Scholar] [CrossRef]
- Moreira, M.M.; Dorosh, O.; Silva, S.; Silva, A.M.; Grosso, C.; Vieira, E.F.; Rodrigues, F.; Fernandes, V.C.; Peixoto, A.F.; Freire, C.; et al. Subcritical Water Extraction of Phenolic Compounds from Vineyard Pruning Residues: Evaluation of Chemical Composition and Bioactive Properties. Biol. Life Sci. Forum 2021, 6, 27. [Google Scholar]
- Moreira, M.M.; Barroso, M.F.; Porto, J.V.; Ramalhosa, M.J.; Švarc-Gajić, J.; Estevinho, L.; Morais, S.; Delerue-Matos, C. Potential of Portuguese vine shoot wastes as natural resources of bioactive compounds. Sci. Total Environ. 2018, 634, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Gomes, I.; Rodrigues, H.; Rodrigues, C.; Marques, M.; Paíga, P.; Paiva, A.; Simões, P.; Fernandes, V.C.; Vieira, M.; Delerue-Matos, C.; et al. Evaluation of the Biological Potential of Himanthalia elongata (L.) S.F.Gray and Eisenia bicyclis (Kjellman) Setchell Subcritical Water Extracts. Foods 2022, 11, 746. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Freitas, M.; Pacheco, J.G.; Oliveira, J.M.; Domingues, V.F.; Delerue-Matos, C. Magnetic dispersive micro solid-phase extraction and gas chromatography determination of organophosphorus pesticides in strawberries. J. Chromatogr. A 2018, 1566, 1–12. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Khanal, R.C.; Howard, L.R.; Prior, R.L. Effect of heating on the stability of grape and blueberry pomace procyanidins and total anthocyanins. Food Res. Int. 2010, 43, 1464–1469. [Google Scholar] [CrossRef]
- Aliaño-gonzález, M.J.; Richard, T.; Cantos-villar, E. Grapevine cane extracts: Raw plant material, extraction methods, quantification, and applications. Biomolecules 2020, 10, 1195. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, A.; Celeiro, M.; Jozinović, A.; Jelinić, J.; Kovač, T.; Jokić, S.; Babić, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green extraction methods for extraction of polyphenolic compounds from blueberry pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Das, Q.; Islam, M.R.; Marcone, M.F.; Warriner, K.; Diarra, M.S. Potential of Berry Extracts to Control Foodborne Pathogens; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 73, ISBN 2262178070. [Google Scholar]
- Vera, J.; Fernandes, V.C.; Correia-Sá, L.; Mansilha, C.; Delerue-Matos, C.; Domingues, V.F. Occurrence of Selected Known or Suspected Endocrine-Disrupting Pesticides in Portuguese Surface Waters Using SPME-GC-IT/MS. Separations 2021, 8, 81. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2018/686 of 4 May 2018 as regards maximum residue levels for chlorpyrifos, chlorpyrifos-methyl and triclopyr in or on certain products. Off. J. Eur. Union 2018, 15. L 121/30–62.
- Food, E.; Authority, S. Statement on the available outcomes of the human health assessment in the context of the pesticides peer review of the active substance chlorpyrifos-methyl. EFSA J. 2019, 17, e05810. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Vojinović, U.D.; Kostić, A.; Pešić, M.B.; Špirović Trifunović, B.D.; Brkić, D.V.; Stević, M.; Kojić, M.O.; Stanisavljević, N.S. In vitro assessment of pesticide residues bioaccessibility in conventionally grown blueberries as affected by complex food matrix. Chemosphere 2020, 252, 126568. [Google Scholar] [CrossRef]
- EWG Dirty Dozen—2023 Shopper’s Guide to Pesticides in Produce. Available online: https://www.ewg.org/foodnews/full-list.php (accessed on 14 November 2023).
- Stelzer, L.; Hoberg, F.; Bach, V.; Schmidt, B.; Pfeiffer, S.; Meyer, V.; Finkbeiner, M. Life cycle assessment of fungal-based composite bricks. Sustainability 2021, 13, 11573. [Google Scholar] [CrossRef]

| BPW Variety and Year | Extraction Technique | TPC * (mg GAE/g dw) | TFC * (mg EE/g dw) | DPPH-RSA * (mg TE/g dw) | FRAP * (mg AAE/g dw) | ABTS * (mg AAE/g dw) |
|---|---|---|---|---|---|---|
| Duke 2019 | CE | 335 ± 12 a | 205 ± 17 a,b | 453 ± 21 a | 280 ± 4 b | 468 ± 17 a |
| MAE | 193 ± 16 d,e | 129 ± 11 c,d | 258 ± 3 c,d,e | 175 ± 1 d | 257 ± 19 d,e | |
| SWE | 241 ± 11 c | 123 ± 12 c,d | 226 ± 13 d,e | 345 ± 15 a | 300 ± 11 c,d | |
| Duke 2020 | CE | 286 ± 4 b | 208 ± 10 a | 354 ± 23 b | 255 ± 5 c | 412 ± 25 a,b |
| MAE | 187 ± 4 e | 128 ± 2 c,d | 269 ± 20 c,d | 154 ± 5 d | 234 ± 21 e | |
| SWE | 243 ± 11 c | 137 ± 5 c | 223 ± 22 e | 359 ± 21 a | 281 ± 26 c,d | |
| Bluecrop 2019 | CE | 333 ± 23 a | 217 ± 17 a | 452 ± 36 a | 283 ± 17 b | 457 ± 36 a |
| MAE | 199 ± 10 d,e | 134 ± 14 c | 287 ± 18 c | 159 ± 11 d | 256 ± 6 d,e | |
| SWE | 213 ± 9 d | 137 ± 12 c | 230 ± 13 d,e | 299 ± 7 b | 281 ± 20 c,d | |
| Bluecrop 2020 | CE | 277 ± 10 b | 180 ± 15 b | 385 ± 19 b | 246 ± 7 c | 427 ± 39 a,b |
| MAE | 157 ± 5 f | 105 ± 8 d | 225 ± 23 d,e | 120 ± 2 e | 224 ± 14 e | |
| SWE | 248 ± 7 c | 129 ± 10 c,d | 254 ± 11 c,d,e | 355 ± 7 a | 312 ± 14 c |
| Compound | Duke 2019 | Duke 2020 | Bluecrop 2019 | Bluecrop 2020 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CE | MAE | SWE | CE | MAE | SWE | CE | MAE | SWE | CE | MAE | SWE | |
| Phenolic acids | ||||||||||||
| 3,5-di-caffeoylquinic acid | <LOQ a | <LOD b | 0.81 ± 0.04 | <LOQ | <LOD | 1.24 ± 0.06 | <LOD | <LOD | 0.68 ± 0.03 | <LOD | <LOD | 0.83 ± 0.04 |
| 4-O-caffeyolquinic acid | 1.10 ± 0.06 | 0.57 ± 0.03 | 8.13 ± 0.41 | 0.60 ± 0.03 | 0.56 ± 0.03 | 15.11 ± 0.76 | 1.63 ± 0.08 | 2.47 ± 0.12 | 10.08 ± 0.50 | <LOQ | <LOQ | 9.47 ± 0.47 |
| 4,5-di-O-caffeoylquinic acid | 1.18 ± 0.06 | 0.59 ± 0.03 | 0.87 ± 0.04 | 0.79 ± 0.04 | <LOD | 1.33 ± 0.07 | 0.75 ± 0.04 | <LOD | 0.84 ± 0.04 | 0.75 ± 0.04 | <LOQ | 0.65 ± 0.03 |
| p-Coumaric acid | 0.61 ± 0.03 | 0.84 ± 0.04 | 0.94 ± 0.05 | <LOQ | <LOQ | 1.55 ± 0.08 | 0.72 ± 0.04 | <LOD | 1.07 ± 0.05 | <LOD | <LOQ | 1.72 ± 0.09 |
| Caffeic acid | 8.79 ± 0.44 | 4.62 ± 0.23 | 1.83 ± 0.09 | 11.1 ± 0.5 | 6.25 ± 0.31 | 3.13 ± 0.16 | 14.5 ± 0.7 | 5.88 ± 0.29 | 2.14 ± 0.11 | 13.4 ± 0.7 | 6.03 ± 0.30 | 3.44 ± 0.17 |
| Caftaric acid | <LOQ | <LOD | 10.9 ± 0.5 | <LOD | <LOD | 19.7 ± 0.9 | <LOQ | <LOQ | 15.4 ± 0.8 | <LOD | <LOD | 16.1 ± 0.8 |
| Chlorogenic acid | 7.40 ± 0.37 | 4.37 ± 0.22 | 7.38 ± 0.37 | 4.40 ± 0.22 | 3.59 ± 0.18 | 14.3 ± 0.7 | 9.42 ± 0.47 | <LOQ | 8.68 ± 0.43 | 3.93 ± 0.20 | 3.58 ± 0.18 | 13.3 ± 0.7 |
| Cinnamic acid | <LOD | ND c | <LOD | <LOD | <LOD | <LOQ | ND | <LOD | <LOQ | <LOD | <LOD | 0.79 ± 0.04 |
| Ellagic acid | 13.2 ± 0.7 | 7.58 ± 0.38 | <LOQ | 11.9 ± 0.6 | 6.49 ± 0.32 | <LOD | 12.9 ± 0.6 | 6.93 ± 0.35 | 3.46 ± 0.17 | 5.21 ± 0.26 | 5.20 ± 0.26 | <LOD |
| Ferulic acid | 1.41 ± 0.07 | <LOQ | 1.19 ± 0.06 | <LOD | <LOD | 2.00 ± 0.10 | 0.61 ± 0.03 | <LOQ | 1.41 ± 0.07 | <LOD | <LOQ | 2.22 ± 0.11 |
| Gallic acid | 1.48 ± 0.07 | 1.07 ± 0.05 | 8.32 ± 0.42 | 0.68 ± 0.03 | 0.61 ± 0.03 | 8.43 ± 0.42 | 2.18 ± 0.11 | 1.04 ± 0.05 | 5.03 ± 0.25 | 0.88 ± 0.04 | 0.72 ± 0.04 | 9.07 ± 0.45 |
| Neochlorogenic acid | 31.4 ± 1.6 | 14.4 ± 0.7 | 0.77 ± 0.04 | 26.6 ± 1.3 | 12.9 ± 0.6 | 1.77 ± 0.09 | 16.7 ± 0.8 | 13.9 ± 0.7 | 1.29 ± 0.06 | 19.8 ± 0.9 | 7.49 ± 0.37 | 1.13 ± 0.06 |
| Protocatechuic acid | 30.8 ± 1.5 | 17.9 ± 0.9 | 1.26 ± 0.06 | 31.0 ± 1.5 | 14.2 ± 0.7 | <LOD | 22.1 ± 1.1 | 18.5 ± 0.9 | <LOD | 20.8 ± 1.0 | 9.18 ± 0.46 | <LOQ |
| Sinapic acid | 4.32 ± 0.22 | 0.64 ± 0.03 | 4.11 ± 0.21 | <LOD | <LOD | 6.95 ± 0.35 | <LOD | <LOQ | 5.45 ± 0.27 | <LOD | <LOD | 7.73 ± 0.39 |
| Syringic acid | ND | 1.71 ± 0.09 | 1.64 ± 0.08 | 3.32 ± 0.17 | 1.51 ± 0.08 | 1.21 ± 0.06 | 4.07 ± 0.20 | 1.39 ± 0.07 | 0.77 ± 0.04 | 4.24 ± 0.21 | 1.34 ± 0.07 | 1.26 ± 0.06 |
| Vanillic acid | 8.64 ± 0.43 | 4.06 ± 0.20 | 10.4 ± 0.5 | 6.95 ± 0.35 | 4.31 ± 0.22 | 14.7 ± 0.7 | 10.9 ± 0.5 | 3.01 ± 0.15 | 9.22 ± 0.46 | 10.4 ± 0.5 | 4.74 ± 0.24 | 14.6 ± 0.7 |
| ∑Phenolic acids | 110 ± 6 | 58.5 ± 2.9 | 58.6 ± 2.9 | 97.4 ± 4.9 | 50.4 ± 2.5 | 91.4 ± 4.6 | 96.6 ± 4.8 | 53.2 ± 2.7 | 65.5 ± 3.3 | 79.4 ± 4.0 | 38.3 ± 1.9 | 82.3 ± 5.1 |
| Flavanols | ||||||||||||
| Catechin | 21.5 ± 1.1 | 3.09 ± 0.15 | 34.8 ± 1.7 | 11.7 ± 0.6 | 7.92 ± 0.40 | 62.5 ± 3.1 | 25.2 ± 1.3 | 13.1 ± 0.7 | 45.1 ± 2.2 | 16.7 ± 0.8 | 7.88 ± 0.39 | 62.9 ± 3.1 |
| Epicatechin | 20.5 ± 1.0 | 6.28 ± 0.31 | 8.33 ± 0.42 | 8.01 ± 0.40 | 3.89 ± 0.19 | 15.0 ± 0.7 | 9.96 ± 0.50 | 1.57 ± 0.08 | 10.1 ± 0.5 | 10.82 ± 0.54 | 3.76 ± 0.19 | 16.5 ± 0.8 |
| ∑Flavanols | 41.9 ± 2.1 | 9.37 ± 0.46 | 43.2 ± 2.2 | 19.7 ± 0.9 | 11.8 ± 0.6 | 77.5 ± 3.9 | 35.2 ± 1.8 | 14.7 ± 0.7 | 55.2 ± 2.7 | 27.49 ± 1.37 | 11.6 ± 0.6 | 79.4 ± 3.9 |
| Flavanones | ||||||||||||
| Naringenin | ND | <LOD | <LOD | <LOD | <LOD | <LOQ | <LOD | <LOD | <LOQ | <LOD | ND | 0.83 ± 0.04 |
| Naringin | 0.83 ± 0.04 | <LOD | 2.81 ± 0.14 | <LOD | <LOD | 5.29 ± 0.26 | <LOQ | <LOD | 4.06 ± 0.20 | <LOQ | <LOD | 7.02 ± 0.35 |
| ∑Flavanones | 0.83 ± 0.04 | 0.0 | 2.81 ± 0.14 | 0.0 | 0.0 | 5.29 ± 0.26 | 0.0 | 0.0 | 4.06 ± 0.20 | 0.0 | 0.0 | 7.85 ± 0.39 |
| Flavonols | ||||||||||||
| Isorhamnetin-3-O-rutinoside | <LOQ | <LOD | <LOD | 1.71 ± 0.09 | <LOQ | 1.04 ± 0.05 | 0.70 ± 0.03 | 1.11 ± 0.06 | <LOD | 1.46 ± 0.07 | 0.78 ± 0.04 | <LOD |
| Kaempferol-3-O-glucoside | 1.86 ± 0.09 | 0.68 ± 0.03 | <LOD | 1.38 ± 0.07 | 0.60 ± 0.03 | <LOQ | 1.05 ± 0.05 | 0.71 ± 0.04 | 1.00 ± 0.05 | 1.11 ± 0.06 | <LOQ | ND |
| Kaempferol-3-o-rutinoside | 1.27 ± 0.06 | 0.76 ± 0.04 | 0.85 ± 0.04 | 0.90 ± 0.04 | <LOQ | <LOD | 1.64 ± 0.08 | <LOQ | 0.76 ± 0.04 | 0.68 ±0.03 | <LOQ | 2.31 ± 0.12 |
| Myricetin | <LOQ | <LOD | 3.43 ± 0.17 | 0.52 ± 0.03 | 0.35 ± 0.02 | 6.29 ± 0.31 | <LOQ | <LOQ | 5.25 ± 0.26 | <LOQ | <LOQ | 8.03 ± 0.40 |
| Quercetin | 0.62 ± 0.03 | <LOQ | <LOQ | <LOQ | <LOQ | ND | 0.95 ± 0.05 | <LOQ | <LOQ | <LOQ | <LOQ | 0.73 ± 0.04 |
| Quercetin-3-O-galactoside | 67.1 ± 3.4 | 36.9 ± 1.8 | 3.70 ± 0.18 | 59.5 ± 2.9 | 32.9 ± 1.7 | 6.27 ± 0.31 | 62.4 ± 3.1 | 34.5 ± 1.7 | <LOQ | 55.7 ± 2.8 | 26.1 ± 1.3 | 8.90 ± 0.44 |
| Quercetin-3-O-glucopyranoside | <LOQ | <LOQ | <LOQ | 14.2 ± 0.7 | 8.35 ± 0.42 | 0.51 ± 0.03 | 14.6 ± 0.7 | 8.50 ± 0.42 | ND | 13.3 ± 0.7 | 6.62 ± 0.33 | 0.74 ± 0.04 |
| Rutin | 2.35 ± 0.12 | 1.41 ± 0.07 | 0.53 ± 0.03 | ND | ND | 1.15 ± 0.06 | ND | ND | <LOQ | ND | ND | 0.80 ± 0.04 |
| ∑Flavonols | 73.1 ± 3.7 | 39.8 ± 1.9 | 8.51 ± 0.42 | 78.2 ± 3.9 | 42.3 ± 2.1 | 15.3 ± 0.8 | 81.3 ± 4.1 | 44.7 ± 2.2 | 7.01 ± 0.35 | 72.3 ± 3.5 | 33.5 ± 1.7 | 21.5 ± 1.1 |
| ∑Flavonoids | 115.83 ± 5.84 | 49.17 ± 2.36 | 54.52 ± 2.76 | 97.9 ± 4.8 | 54.1 ± 2.7 | 98.09 ± 4.96 | 116.5 ± 5.9 | 59.4 ± 2.9 | 66.27 ± 3.25 | 99.79 ± 4.87 | 47.1 ± 2.3 | 108.75 ± 5.39 |
| Stilbenes | ||||||||||||
| Resveratrol | <LOQ | <LOQ | <LOQ | 2.40 ± 0.12 | 1.41 ± 0.07 | 0.69 ± 0.03 | 2.49 ± 0.12 | 1.46 ± 0.07 | <LOQ | 2.24 ± 0.11 | 1.14 ± 0.06 | <LOQ |
| trans-Polydatin | <LOD | <LOD | 0.62 ± 0.03 | <LOD | <LOD | 1.10 ± 0.06 | ND | <LOD | 0.56 ± 0.03 | <LOD | <LOQ | <LOD |
| ∑Stilbenes | 0.0 | 0.0 | 0.0 | 2.40 ± 0.12 | 1.41 ± 0.07 | 0.69 ± 0.03 | 2.49 ± 0.12 | 1.46 ± 0.07 | 0.0 | 2.24 ± 0.11 | 1.14 ± 0.06 | 0.0 |
| Others | ||||||||||||
| Caffeine | 5.82 ± 0.29 | 2.91 ± 0.15 | 2.27 ± 0.11 | 3.94 ± 0.20 | 2.68 ± 0.13 | 6.15 ± 0.31 | 7.38 ± 0.37 | 2.06 ± 0.10 | 4.13 ± 0.21 | 4.86 ± 0.24 | 2.45 ± 0.12 | 4.21 ± 0.21 |
| Phloridzin | <LOD | <LOD | 2.73 ± 0.14 | <LOD | ND | 2.85 ± 0.14 | ND | <LOD | 4.67 ± 0.23 | ND | ND | 4.12 ± 0.21 |
| ∑Others | 0.0 | 0.0 | 3.35 ± 0.17 | 0.0 | 0.0 | 3.95 ± 0.20 | 0.0 | 0.0 | 5.23 ± 0.26 | 0.0 | 0.0 | 4.12 ± 0.21 |
| ∑All phenolic compounds | 232 | 110 | 119 | 202 | 109 | 200 | 223 | 116 | 141 | 186 | 87.0 | 199 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorosh, O.; Fernandes, V.C.; Delerue-Matos, C.; Moreira, M.M. Blueberry Pruning Wastes: From an Undervalued Agricultural Residue to a Safe and Valuable Source of Antioxidant Compounds for the Food Industry. Foods 2024, 13, 317. https://doi.org/10.3390/foods13020317
Dorosh O, Fernandes VC, Delerue-Matos C, Moreira MM. Blueberry Pruning Wastes: From an Undervalued Agricultural Residue to a Safe and Valuable Source of Antioxidant Compounds for the Food Industry. Foods. 2024; 13(2):317. https://doi.org/10.3390/foods13020317
Chicago/Turabian StyleDorosh, Olena, Virgínia Cruz Fernandes, Cristina Delerue-Matos, and Manuela M. Moreira. 2024. "Blueberry Pruning Wastes: From an Undervalued Agricultural Residue to a Safe and Valuable Source of Antioxidant Compounds for the Food Industry" Foods 13, no. 2: 317. https://doi.org/10.3390/foods13020317
APA StyleDorosh, O., Fernandes, V. C., Delerue-Matos, C., & Moreira, M. M. (2024). Blueberry Pruning Wastes: From an Undervalued Agricultural Residue to a Safe and Valuable Source of Antioxidant Compounds for the Food Industry. Foods, 13(2), 317. https://doi.org/10.3390/foods13020317