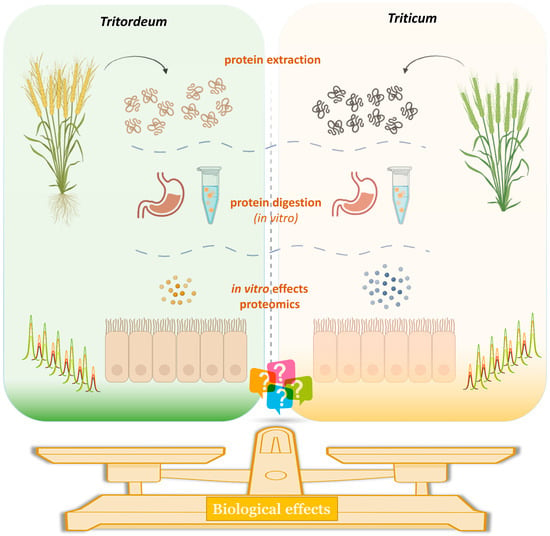
Tritordeum: Promising Cultivars to Improve Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Extraction
2.3. In Vitro Simulated Gastrointestinal Digestion
2.4. Cell Cultures
2.5. Effects of DPs on Transepithelial Electrical Resistance (TEER)
2.6. Effects of DPs on Cell Viability
2.7. Effects of DPs on the Structural Organization of F-Actin
2.8. Effects of DPs on ER Stress
2.9. Proteomic Analysis
2.10. Statistical Analysis
3. Results
3.1. Caco-2 Cells
3.2. Effect of DPs on Epithelial Permeability
3.3. Effects of DPs on Cell Viability
3.4. Structural Organization of F-Actin
3.5. Endoplasmic Reticulum Stress
3.6. Protein Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- MacEvilly, C. CEREALS|Contribution to the Diet; Academic Press: Cambridge, MA, USA, 2003; pp. 1008–1014. [Google Scholar] [CrossRef]
- Serna Saldivar, S.O. CEREALS|Dietary Importance; Academic Press: Cambridge, MA, USA, 2003; pp. 1027–1033. [Google Scholar] [CrossRef]
- Belobrajdic, D.P.; Bird, A.R. The potential role of phytochemicals in wholegrain cereals for the prevention of type-2 diabetes. Nutr. J. 2013, 12, 62. [Google Scholar] [CrossRef]
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Luthria, D.L.; Lu, Y.; John, K.M.M. Bioactive phytochemicals in wheat: Extraction, analysis, processing, and functional properties. J. Funct. Foods 2015, 18, 910–925. [Google Scholar] [CrossRef]
- Araus, J.L. Plant Breeding and Drought in C3 Cereals: What Should We Breed For? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Barcelo, P.; Cabrera, A.; Hagel, C.; Lorz, H. Production of doubled-haploid plants from tritordeum anther culture. Theor. Appl. Genet. 1994, 87, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.; Martínez-Araque, C.; Rubiales, D.; Ballesteros, J. Tritordeum: Triticale’s New Brother Cereal. In Triticale: Today and Tomorrow; Springer: Dordrecht, The Netherlands, 1996; Volume 5, pp. 57–72. [Google Scholar] [CrossRef]
- Lima-Brito, J.; Guedes-Pinto, H.; Harrison, G.E.; Heslop-Harrison, J.S. Molecular cytogenetic analysis of durum wheat × tritordeum hybrids. Genome 1997, 40, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, L.; Comino, I.; Vivas, S.; Rodríguez-Martín, L.; Giménez, M.J.; Pastor, J.; Sousa, C.; Barro, F. Tritordeum: A novel cereal for food processing with good acceptability and significant reduction in gluten immunogenic peptides in comparison with wheat. J. Sci. Food Agric. 2018, 98, 2201–2209. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.; Reyneri, A.; Locatelli, M.; Coïsson, J.D.; Blandino, M. Distribution of bioactive compounds in pearled fractions of tritordeum. Food Chem. 2019, 301, 125228. [Google Scholar] [CrossRef] [PubMed]
- Suchowilska, E.; Radawiec, W.; Wiwart, M. Tritordeum—The content of basic nutrients in grain and the morphological and anatomical features of kernels. Int. Agrophys. 2021, 35, 343–355. [Google Scholar] [CrossRef]
- Paznocht, L.; Kotikova, Z.; Sulc, M.; Lachman, J.; Orsak, M.; Eliasova, M.; Martinek, P. Free and esterified carotenoids in pigmented wheat, tritordeum and barley grains. Food Chem. 2018, 240, 670–678. [Google Scholar] [CrossRef]
- Arora, K.; Carafa, I.; Fava, F.; Tuohy, K.M.; Nikoloudaki, O.; Gobbetti, M.; Cagno, R.D. Sourdough performances of the golden cereal Tritordeum: Dynamics of microbial ecology, biochemical and nutritional features. Int. J. Food Microbiol. 2022, 374, 109725. [Google Scholar] [CrossRef]
- Gallardo, M.; Fereres, E. Growth, grain yield and water use efficiency of tritordeum in relation to wheat. Eur. J. Agron. 1993, 2, 83–91. [Google Scholar] [CrossRef]
- Ávila, C.M.; Rodríguez-Suárez, C.; Atienza, S.G. Tritordeum: Creating a New Crop Species-The Successful Use of Plant Genetic Resources. Plants 2021, 10, 1029. [Google Scholar] [CrossRef]
- Kakabouki, I.; Beslemes, D.F.; Tigka, E.L.; Folina, A.; Karydogianni, S.; Zisi, C.; Papastylianou, P. Performance of Six Genotypes of Tritordeum Compare to Bread Wheat under East Mediterranean Condition. Sustainability 2020, 12, 9700. [Google Scholar] [CrossRef]
- Mamone, G.; Iacomino, G. Comparison of the in vitro toxicity of ancient Triticum monococcum varieties ID331 and Monlis. Int. J. Food Sci. Nutr. 2018, 69, 954–962. [Google Scholar] [CrossRef]
- Iacomino, G.; Di Stasio, L.; Fierro, O.; Picariello, G.; Venezia, A.; Gazza, L.; Ferranti, P.; Mamone, G. Protective effects of ID331 Triticum monococcum gliadin on in vitro models of the intestinal epithelium. Food Chem. 2016, 212, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P.R.; Hey, S.J. Do we need to worry about eating wheat? Nutr. Bull. 2016, 41, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Sheats, D.B. Consumer Trends in Grain Consumption. In Encyclopedia of Food Grains, 2nd ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 2, pp. 29–34. [Google Scholar] [CrossRef]
- Dinu, M.; Whittaker, A.; Pagliai, G.; Benedettelli, S.; Sofi, F. Ancient wheat species and human health: Biochemical and clinical implications. J. Nutr. Biochem. 2018, 52, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Visioli, G.; Lauro, M.; Vamerali, T.; Dal Cortivo, C.; Panozzo, A.; Folloni, S.; Piazza, C.; Ranieri, R. A Comparative Study of Organic and Conventional Management on the Rhizosphere Microbiome, Growth and Grain Quality Traits of Tritordeum. Agronomy 2020, 10, 1717. [Google Scholar] [CrossRef]
- Nitride, C.; D’Auria, G.; Dente, A.; Landolfi, V.; Picariello, G.; Mamone, G.; Blandino, M.; Romano, R.; Ferranti, P. Tritordeum as an Innovative Alternative to Wheat: A Comparative Digestion Study on Bread. Molecules 2022, 27, 1308. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-León, S.; Haro, C.; Villatoro, M.; Vaquero, L.; Comino, I.; González-Amigo, A.B.; Vivas, S.; Pastor, J.; Sousa, C.; Landa, B.B.; et al. Tritordeum breads are well tolerated with preference over gluten-free breads in non-celiac wheat-sensitive patients and its consumption induce changes in gut bacteria. J. Sci. Food Agric. 2021, 101, 3508–3517. [Google Scholar] [CrossRef]
- Haro, C.; Guzman-Lopez, M.H.; Marin-Sanz, M.; Sanchez-Leon, S.; Vaquero, L.; Pastor, J.; Comino, I.; Sousa, C.; Vivas, S.; Landa, B.B.; et al. Consumption of Tritordeum Bread Reduces Immunogenic Gluten Intake without Altering the Gut Microbiota. Foods 2022, 11, 1439. [Google Scholar] [CrossRef]
- Russo, F.; Riezzo, G.; Linsalata, M.; Orlando, A.; Tutino, V.; Prospero, L.; D’Attoma, B.; Giannelli, G. Managing Symptom Profile of IBS-D Patients With Tritordeum-Based Foods: Results From a Pilot Study. Front. Nutr. 2022, 9, 797192. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, G.; Prospero, L.; Orlando, A.; Linsalata, M.; D’Attoma, B.; Ignazzi, A.; Giannelli, G.; Russo, F. A Tritordeum-Based Diet for Female Patients with Diarrhea-Predominant Irritable Bowel Syndrome: Effects on Abdominal Bloating and Psychological Symptoms. Nutrients 2023, 15, 1361. [Google Scholar] [CrossRef] [PubMed]
- Gianfrani, C.; Camarca, A.; Mazzarella, G.; Di Stasio, L.; Giardullo, N.; Ferranti, P.; Picariello, G.; Rotondi Aufiero, V.; Picascia, S.; Troncone, R.; et al. Extensive in vitro gastrointestinal digestion markedly reduces the immune-toxicity of Triticum monococcum wheat: Implication for celiac disease. Mol. Nutr. Food Res. 2015, 59, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, G.; Marano, A.; Stillitano, I.; Aufiero, V.R.; Iaquinto, G.; Schettino, M.; Masucci, A.; Troncone, R.; Auricchio, S.; Mazzarella, G. Celiac disease: Role of intestinal compartments in the mucosal immune response. Mol. Cell. Biochem. 2016, 411, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. In Genotoxicity—A Predictable Risk to Our Actual World; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Iacomino, G.; Rotondi Aufiero, V.; Iannaccone, N.; Melina, R.; Giardullo, N.; De Chiara, G.; Venezia, A.; Taccone, F.S.; Iaquinto, G.; Mazzarella, G. IBD: Role of intestinal compartments in the mucosal immune response. Immunobiology 2019, 225, 151849. [Google Scholar] [CrossRef]
- Cawley, K.; Deegan, S.; Samali, A.; Gupta, S. Assays for Detecting the Unfolded Protein Response. Methods Enzymol. 2011, 490, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Sander, G.R.; Cummins, A.G.; Henshall, T.; Powell, B.C. Rapid disruption of intestinal barrier function by gliadin involves altered expression of apical junctional proteins. FEBS Lett. 2005, 579, 4851–4855. [Google Scholar] [CrossRef]
- Madara, J.L.; Stafford, J.; Barenberg, D.; Carlson, S. Functional coupling of tight junctions and microfilaments in T84 monolayers. Am. J. Physiol. 1988, 254, G416–G423. [Google Scholar] [CrossRef]
- Ensari, A.; Marsh, M.N.; Morgan, S.; Lobley, R.; Unsworth, D.J.; Kounali, D.; Crowe, P.T.; Paisley, J.; Moriarty, K.J.; Lowry, J. Diagnosing coeliac disease by rectal gluten challenge: A prospective study based on immunopathology, computerized image analysis and logistic regression analysis. Clin. Sci. 2001, 101, 199–207. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, G.; Holm, P.B.; Brinch-Pedersen, H. Wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) multiple inositol polyphosphate phosphatases (MINPPs) are phytases expressed during grain filling and germination. Plant Biotechnol. J. 2007, 5, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Cunsolo, V.; Muccilli, V.; Saletti, R.; Foti, S. Mass spectrometry in the proteome analysis of mature cereal kernels. Mass. Spectrom. Rev. 2012, 31, 448–465. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Arora, K.; Gaudioso, G.; Solovyev, P.; Tuohy, K.; Di Cagno, R.; Gobbetti, M.; Fava, F. In vitro faecal fermentation of Tritordeum breads and its effect on the human gut health. Curr. Res. Microb. Sci. 2024, 6, 100214. [Google Scholar] [CrossRef] [PubMed]
- Hudson, D.A.; Cornell, H.J.; Purdham, D.R.; Rolles, C.J. Non-specific cytotoxicity of wheat gliadin components towards cultured human cells. Lancet 1976, 1, 339–341. [Google Scholar] [CrossRef]
- Giovannini, C.; Maiuri, L.; De Vincenzi, M. Cytotoxic effect of prolamin-derived peptides on in vitro cultures of cell line Caco-2: Implications for coeliac disease. Toxicol. Vitro 1995, 9, 251–255. [Google Scholar] [CrossRef]
- Elli, L.; Dolfini, E.; Bardella, M.T. Gliadin cytotoxicity and in vitro cell cultures. Toxicol. Lett. 2003, 146, 1–8. [Google Scholar] [CrossRef]
- Sjolander, A.; Magnusson, K.E. Effects of wheat germ agglutinin on the cellular content of filamentous actin in Intestine 407 cells. Eur. J. Cell Biol. 1988, 47, 32–35. [Google Scholar] [PubMed]
- Okarter, N.; Liu, R.H. Health Benefits of Whole Grain Phytochemicals. Crit. Rev. Food Sci. Nutr. 2010, 50, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Diez-Sampedro, A.; Olenick, M.; Maltseva, T.; Flowers, M. A Gluten-Free Diet, Not an Appropriate Choice without a Medical Diagnosis. J. Nutr. Metab. 2019, 2019, 2438934. [Google Scholar] [CrossRef] [PubMed]
- Békés, F.; Schoenlechner, R.; Tömösközi, S. Ancient Wheats and Pseudocereals for Possible use in Cereal-Grain Dietary Intolerances. In Cereal Grains; Woodhead Publishing: Shaston, UK, 2017; pp. 353–389. [Google Scholar] [CrossRef]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef]
- King, J.A.; Jeong, J.; Underwood, F.E.; Quan, J.; Panaccione, N.; Windsor, J.W.; Coward, S.; de Bruyn, J.; Ronksley, P.E.; Shaheen, A.-A.; et al. Incidence of Celiac Disease Is Increasing Over Time. Am. J. Gastroenterol. 2020, 115, 507–525. [Google Scholar] [CrossRef]
- De Santis, M.A.; Giuliani, M.M.; Giuzio, L.; De Vita, P.; Lovegrove, A.; Shewry, P.R.; Flagella, Z. Differences in gluten protein composition between old and modern durum wheat genotypes in relation to 20th century breeding in Italy. Eur. J. Agron. 2017, 87, 19–29. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Caro, S.; Venezia, A.; Di Stasio, L.; Danzi, D.; Pignone, D.; Mamone, G.; Iacomino, G. Tritordeum: Promising Cultivars to Improve Health. Foods 2024, 13, 661. https://doi.org/10.3390/foods13050661
De Caro S, Venezia A, Di Stasio L, Danzi D, Pignone D, Mamone G, Iacomino G. Tritordeum: Promising Cultivars to Improve Health. Foods. 2024; 13(5):661. https://doi.org/10.3390/foods13050661
Chicago/Turabian StyleDe Caro, Salvatore, Antonella Venezia, Luigia Di Stasio, Donatella Danzi, Domenico Pignone, Gianfranco Mamone, and Giuseppe Iacomino. 2024. "Tritordeum: Promising Cultivars to Improve Health" Foods 13, no. 5: 661. https://doi.org/10.3390/foods13050661
APA StyleDe Caro, S., Venezia, A., Di Stasio, L., Danzi, D., Pignone, D., Mamone, G., & Iacomino, G. (2024). Tritordeum: Promising Cultivars to Improve Health. Foods, 13(5), 661. https://doi.org/10.3390/foods13050661