Comprehensive Review on the Biocontrol of Listeria monocytogenes in Food Products
Abstract
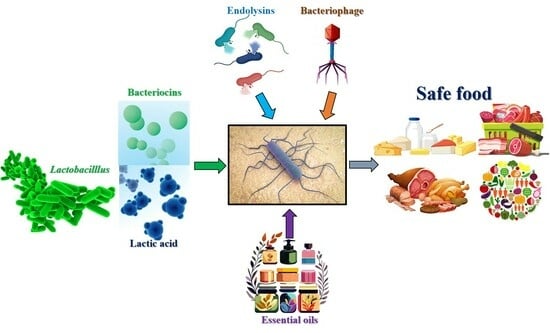
:1. Introduction
2. Biological Control of L. monocytogenes in Food
3. Biocontrol of L. monocytogenes in Meat and Meat Products
3.1. The Use of Bacteriophages in Meat Products
3.2. Endolysins in Meat Products
3.3. Lactic Acid Bacteria (LAB) in Meat Products
3.4. Bacteriocins in Meat Products
3.5. Essential Oils in Meat Products
| Meat or Meat Products | Application of EOs | Contamination Procedure | Storage Conditions and Results | References |
|---|---|---|---|---|
| Beef meatballs | Addition of O. vulgare, R. officinalis, and T. vulgaris at concentrations of 0.5%, 1%, or 2% (v/w) | Inoculation with a five-strain cocktail (L. monocytogenes HSD 2434, HSD3261, HSD 3705, HSD 3948, HSD 4210) at 10, 102, 103, and 104 CFU/g | Concentrations of 2% and 1% restricted the growth of L. monocytogenes, regardless of the initial microbial loading, during storage at 4 °C for 14 days, but affected the meatballs flavor. Concentration of 0.5% restricted the growth of L. monocytogenes at initial counts of <102, and the taste of meatballs was acceptable. | [92] |
| Italian mortadella | Addition of combined T. vulgaris and R. officinalis at concentrations of 0.025% and 0.05% during manufacturing | Contamination of mortadella slices with a three-strain cocktail (L. monocytogenes ATCC 19111, ATCC 13932, and ATCC 19117) at ~2.5 log CFU/g | Compared to the untreated contaminated mortadella, addition of combined EOs to the concentrations of 0.025% and 0.05% led to a reduction in L. monocytogenes by 2.29 log CFU/g and 2.79 log CFU/g by the end of storage at 4 °C for 30 days. | [102] |
| Ground beef | Addition of crude and commercial C. cassia and S. aromaticum EOs at concentrations of 5% and 10%, and 2.5% and 5%, respectively | Inoculation with a five-strain cocktail (L. monocytogenes ATCC 43256, ATCC 49594, JCM 7676, JCM 7672, and JCM 7671) | The ground beef was stored at 8 °C and 0 °C for 7 days and at −18 °C for 60 days. A 10% concentration of clove EO (both crude and commercial) completely inactivated L. monocytogenes within 3 days of storage, irrespective of temperature. A 5% concentration of clove EO (both crude and commercial) reduced L. monocytogenes gradually throughout storage, irrespective of temperature, without achieving complete inactivation. The 2.5% and 5% concentrations of crude and commercial cinnamon EO did not inactivate L. monocytogenes throughout storage. Consumers did not find the ground beef treated with 10% clove EO acceptable, while some of them found the meat treated with 5% clove EO acceptable. | [60,74,94] |
| Dry-cured ham-based medium | Addition of C. cassia EO in dry-cured ham-based medium with water activity of 0.93 or 0.95 at a concentration of 10% | Inoculation with a serotype 4 L. monocytogenes strain at ~4 log CFU/mL | During storage at 7 °C for 7 days, 10% cinnamon EO completely inhibited L. monocytogenes growth irrespective of the ham-based medium’s aw. | [60] |
| Fresh chicken meat | Corn starch edible coating containing Zataria multiflora EO nanoemulsion alone and fortified with cinnamaldehyde | Contamination of the meat with L. monocytogenes to a final concentration of ~104 CFU/g followed by its immersion in the corn starch solutions | The coating with fortified nanoemulsion was more effective in controlling L. monocytogenes than that with the nanoemulsion alone during storage at 4 ± 1 °C for 20 days, with a growth difference between the treatments of ~1 log CFU/g. | [96] |
| Fresh beef | Soy protein edible coatings containing 1%, 2%, or 3% thyme or oregano EOs | Contamination with L. monocytogenes at 5.59 log CFU/g followed by beef pieces immersion in the coating solutions | At the end of storage (14 days at 4 °C) period, compared to the uncoated beef pieces, coating with 1, 2, and 3% thyme and oregano EOs reduced L. monocytogenes by 1.02, 1.73, and 1.97 log CFU/g and 0.91, 1.66, and 1.90 log CFU/g, respectively. The treatments improved the color of beef, and its organoleptic properties were acceptable. | [103] |
| Spiced beef | Chitosan films incorporated with apricot (Prunus armeniaca) kernel EO at 0%, 0.125%, 0.25%, 0.5%, and 1% (v/v) | The beef slices were inoculated with L. monocytogenes to 104 CFU/g and placed in contact with the antimicrobial films | After 15 days of storage at 4 °C, compared to the control samples (film without EO addition), the chitosan films containing 0.5 and 1% apricot kernel EO reduced L. monocytogenes by 3.3 and 4.1 log CFU/g. After 24 days of storage, the sensorial attributes (taste, color, texture, and overall acceptance) of the spiced beef packed with the chitosan film containing 1% apricot kernel oil were significantly improved compared to those of the unpacked one. | [104] |
4. Biocontrol of L. monocytogenes in Milk and Dairy Products
4.1. Bacteriophages in Milk and Dairy Products
4.2. Lactic Acid Bacteria and Bacteriocins in Dairy Products
4.3. Essential Oils in Dairy Products
4.4. Endolysins in Different Antilisterial Formulae of Dairy Products
5. Biocontrol of L. monocytogenes in Vegetables and Fruits
5.1. Bacteriophages in Vegetables and Fruits
5.2. Lactic Acid Bacteria and Bacteriocins in Vegetables and Fruits
5.3. Essential Oils in Vegetables and Fruits
5.4. Endolysins
6. SWOT Analysis for Using Biological Antilisterial Agents in Food Product Formulations
7. Conclusions and Remarks for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2022 Zoonoses Report. EFSA J. 2023, 21, e8442. [Google Scholar] [CrossRef]
- Thakur, M.; Asrani, R.K.; Patial, V. Listeria monocytogenes: A Food-Borne Pathogen. In Foodborne Diseases; Elsevier: Amsterdam, The Netherlands, 2018; pp. 157–192. ISBN 978-0-12-811444-5. [Google Scholar]
- Fox, E.; Hunt, K.; O’Brien, M.; Jordan, K. Listeria monocytogenes in Irish Farmhouse Cheese Processing Environments. Int. J. Food Microbiol. 2011, 145, S39–S45. [Google Scholar] [CrossRef]
- Coughlan, L.M.; Cotter, P.D.; Hill, C.; Alvarez-Ordóñez, A. New Weapons to Fight Old Enemies: Novel Strategies for the (Bio)Control of Bacterial Biofilms in the Food Industry. Front. Microbiol. 2016, 7, 1641. [Google Scholar] [CrossRef]
- Colagiorgi, A.; Bruini, I.; Di Ciccio, P.A.; Zanardi, E.; Ghidini, S.; Ianieri, A. Listeria monocytogenes Biofilms in the Wonderland of Food Industry. Pathogens 2017, 6, 41. [Google Scholar] [CrossRef]
- Drew, C.A.; Clydesdale, F.M. New Food Safety Law: Effectiveness on the Ground. Crit. Rev. Food Sci. Nutr. 2015, 55, 689–700. [Google Scholar] [CrossRef]
- Tompkin, R.B. Control of Listeria monocytogenes in the Food-Processing Environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Połaska, M.; Sokołowska, B. Bacteriophages—A New Hope or a Huge Problem in the Food Industry. AIMS Microbiol. 2019, 5, 324–346. [Google Scholar] [CrossRef] [PubMed]
- European Commission. The Use and Mode of Action of Bacteriophages in Food Production-Endorsed for Public Consultation 22 January 2009-Public Consultation 30 January–6 March 2009. EFSA J. 2009, 7, 1076. [Google Scholar] [CrossRef]
- Moye, Z.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.-P.; et al. Engineered Endolysin-Based “Artilysins” to Combat Multidrug-Resistant Gram-Negative Pathogens. mBio 2014, 5, e01379-14. [Google Scholar] [CrossRef]
- Oliveira, H.; Melo, L.D.R.; Santos, S.B.; Nóbrega, F.L.; Ferreira, E.C.; Cerca, N.; Azeredo, J.; Kluskens, L.D. Molecular Aspects and Comparative Genomics of Bacteriophage Endolysins. J. Virol. 2013, 87, 4558–4570. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.U.; Wang, W.; Sun, Q.; Shah, J.A.; Li, C.; Sun, Y.; Li, Y.; Zhang, B.; Chen, W.; Wang, S. Endolysin, a Promising Solution against Antimicrobial Resistance. Antibiotics 2021, 10, 1277. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-Encoded Endolysins. Antibiotics 2021, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Bao, H.; Billington, C.; Hudson, J.A.; Wang, R. Isolation and Lytic Activity of the Listeria Bacteriophage Endolysin LysZ5 against Listeria Monocytogenes in Soya Milk. Food Microbiol. 2012, 31, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Waldherr, F.; Loessner, M.J. Listeria Bacteriophage Peptidoglycan Hydrolases Feature High Thermoresistance and Reveal Increased Activity after Divalent Metal Cation Substitution. Appl. Microbiol. Biotechnol. 2012, 93, 633–643. [Google Scholar] [CrossRef]
- Hahn-Löbmann, S.; Stephan, A.; Schulz, S.; Schneider, T.; Shaverskyi, A.; Tusé, D.; Giritch, A.; Gleba, Y. Colicins and Salmocins—New Classes of Plant-Made Non-Antibiotic Food Antibacterials. Front. Plant Sci. 2019, 10, 437. [Google Scholar] [CrossRef]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- Fidan, H.; Esatbeyoglu, T.; Simat, V.; Trif, M.; Tabanelli, G.; Kostka, T.; Montanari, C.; Ibrahim, S.A.; Özogul, F. Recent Developments of Lactic Acid Bacteria and Their Metabolites on Foodborne Pathogens and Spoilage Bacteria: Facts and Gaps. Food Biosci. 2022, 47, 101741. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; López, R.L.; Omar, N.B. Bacteriocin-Based Strategies for Food Biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.P.; Lovitt, R.W. Bacteriocins Produced by Lactic Acid Bacteria a Review Article. APCBEE Procedia 2012, 2, 50–56. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, Synthesis, Mechanism of Action and Resistance Development in Food Spoilage Causing Bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, O.; Ross, R.P.; Hill, C. Lantibiotics: Structure, Biosynthesis and Mode of Action. FEMS Microbiol. Rev. 2001, 25, 285–308. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.M.; Hill, C.; Cotter, P.D.; Ross, R.P. Bacteriocins: Structure and Function. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, Netherlands, 2022; pp. 55–64. ISBN 978-0-12-818767-8. [Google Scholar]
- Nissen-Meyer, J.; Oppegård, C.; Rogne, P.; Haugen, H.S.; Kristiansen, P.E. Structure and Mode-of-Action of the Two-Peptide (Class-IIb) Bacteriocins. Probiotics Antimicrob. Proteins 2010, 2, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Martin-Visscher, L.A.; Van Belkum, M.J.; Vederas, J.C. Class IIc or Circular Bacteriocins. In Prokaryotic Antimicrobial Peptides; Drider, D., Rebuffat, S., Eds.; Springer: New York, NY, USA, 2011; pp. 213–236. ISBN 978-1-4419-7691-8. [Google Scholar]
- Maldonado-Barragán, A.; Alegría-Carrasco, E.; Blanco, M.D.M.; Vela, A.I.; Fernández-Garayzábal, J.F.; Rodríguez, J.M.; Gibello, A. Garvicins AG1 and AG2: Two Novel Class IId Bacteriocins of Lactococcus Garvieae Lg-Granada. Int. J. Mol. Sci. 2022, 23, 4685. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, D.; Nag, M.; Dutta, B.; Sarkar, T.; Pati, S.; Basu, D.; Abdul Kari, Z.; Wei, L.S.; Smaoui, S.; Wen Goh, K.; et al. Bacteriocin: A Natural Approach for Food Safety and Food Security. Front. Bioeng. Biotechnol. 2022, 10, 1005918. [Google Scholar] [CrossRef] [PubMed]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- Özel, B.; Şimşek, Ö.; Akçelik, M.; Saris, P.E.J. Innovative Approaches to Nisin Production. Appl. Microbiol. Biotechnol. 2018, 102, 6299–6307. [Google Scholar] [CrossRef]
- Wu, L.; Li, F.; Ran, L.; Gao, Y.; Xie, P.; Yang, J.; Ke, F.; Liu, L.; Wang, Q.; Gao, X. Insight Into the Effects of Nisin and Cecropin on the Oral Microbial Community of Rats by High-Throughput Sequencing. Front. Microbiol. 2020, 11, 1082. [Google Scholar] [CrossRef]
- Liu, G.; Nie, R.; Liu, Y.; Mehmood, A. Combined Antimicrobial Effect of Bacteriocins with Other Hurdles of Physicochemic and Microbiome to Prolong Shelf Life of Food: A Review. Sci. Total Environ. 2022, 825, 154058. [Google Scholar] [CrossRef]
- Benkerroum, N.; Sandine, W.E. Inhibitory Action of Nisin against Listeria monocytogenes. J. Dairy Sci. 1988, 71, 3237–3245. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of Bacteriocins and Protective Cultures in Dairy Food Preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.K.; Kumar, A. Essential Oils as Natural Food Antimicrobial Agents: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef]
- Reis, D.R.; Ambrosi, A.; Luccio, M.D. Encapsulated Essential Oils: A Perspective in Food Preservation. Future Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
- European Commission (EC) Regulation. Official Journal of the European Union. No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on Flavourings and Certain Food Ingredients with Flavouring Properties for Use in and on Foods and Amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC; EUR-Lex; European Commission: Brussels, Belgium, 2008.
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of Essential Oils on Pathogenic Bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential Oils: Sources of Antimicrobials and Food Preservatives. Front. Microbiol. 2017, 7, 2161. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential Oils as Additives in Active Food Packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Maćkiw, E.; Stasiak, M.; Kowalska, J.; Kucharek, K.; Korsak, D.; Postupolski, J. Occurrence and Characteristics of Listeria monocytogenes in Ready-to-Eat Meat Products in Poland. J. Food Prot. 2020, 83, 1002–1009. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, W.; Sun, T.; Gorris, L.G.M.; Wang, X.; Liu, B.; Dong, Q. The Prevalence of Listeria monocytogenes in Meat Products in China: A Systematic Literature Review and Novel Meta-Analysis Approach. Int. J. Food Microbiol. 2020, 312, 108358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Que, F.; Xu, B.; Sun, L.; Zhu, Y.; Chen, W.; Ye, Y.; Dong, Q.; Liu, H.; Zhang, X. Identification of Listeria monocytogenes Contamination in a Ready-to-Eat Meat Processing Plant in China. Front. Microbiol. 2021, 12, 628204. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Molina, A.; Cobo-Díaz, J.F.; López, M.; Prieto, M.; De Toro, M.; Alvarez-Ordóñez, A. Unraveling the Emergence and Population Diversity of Listeria monocytogenes in a Newly Built Meat Facility through Whole Genome Sequencing. Int. J. Food Microbiol. 2021, 340, 109043. [Google Scholar] [CrossRef] [PubMed]
- Zwirzitz, B.; Wetzels, S.U.; Dixon, E.D.; Fleischmann, S.; Selberherr, E.; Thalguter, S.; Quijada, N.M.; Dzieciol, M.; Wagner, M.; Stessl, B. Co-Occurrence of Listeria Spp. and Spoilage Associated Microbiota during Meat Processing due to Cross-Contamination Events. Front. Microbiol. 2021, 12, 632935. [Google Scholar] [CrossRef] [PubMed]
- Lakicevic, B.; Nastasijevic, I. Listeria monocytogenes in Retail Establishments: Contamination Routes and Control Strategies. Food Rev. Int. 2017, 33, 247–269. [Google Scholar] [CrossRef]
- Oloketuyi, S.F.; Khan, F. Inhibition Strategies of Listeria monocytogenes Biofilms—Current Knowledge and Future Outlooks. J. Basic Microbiol. 2017, 57, 728–743. [Google Scholar] [CrossRef]
- Fagerlund, A.; Langsrud, S.; Møretrø, T. Microbial Diversity and Ecology of Biofilms in Food Industry Environments Associated with Listeria monocytogenes Persistence. Curr. Opin. Food Sci. 2021, 37, 171–178. [Google Scholar] [CrossRef]
- Lang, L.H. FDA Approves Use of Bacteriophages to Be Added to Meat and Poultry Products. Gastroenterology 2006, 131, 1370. [Google Scholar] [CrossRef]
- Kawacka, I.; Olejnik-Schmidt, A.; Schmidt, M.; Sip, A. Effectiveness of Phage-Based Inhibition of Listeria monocytogenes in Food Products and Food Processing Environments. Microorganisms 2020, 8, 1764. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Fernández, L.; Martínez, B.; Rodríguez, A.; García, P. Applicability of Commercial Phage-Based Products against Listeria monocytogenes for Improvement of Food Safety in Spanish Dry-Cured Ham and Food Contact Surfaces. Food Control 2017, 73, 1474–1482. [Google Scholar] [CrossRef]
- Ahmadi, H.; Barbut, S.; Lim, L.-T.; Balamurugan, S. Examination of the Use of Bacteriophage as an Additive and Determining Its Best Application Method to Control Listeria monocytogenes in a Cooked-Meat Model System. Front. Microbiol. 2020, 11, 779. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, A.; Ebner, P.D.; Syed, Q.A.; Ubaid Ur Rahman, H. Employing List-Shield Bacteriophage as a Bio-Control Intervention for Listeria monocytogenes from Raw Beef Surface and Maintain Meat Quality during Refrigeration Storage. LWT 2020, 132, 109784. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Amaral, R.A.; Fernandes, R.; Castro, S.M.; Saraiva, J.A.; Teixeira, P. Innovative Hurdle System towards Listeria monocytogenes Inactivation in a Fermented Meat Sausage Model—High Pressure Processing Assisted by Bacteriophage P100 and Bacteriocinogenic Pediococcus Acidilactici. Food Res. Int. 2021, 148, 110628. [Google Scholar] [CrossRef] [PubMed]
- Chibeu, A.; Agius, L.; Gao, A.; Sabour, P.M.; Kropinski, A.M.; Balamurugan, S. Efficacy of Bacteriophage LISTEXTMP100 Combined with Chemical Antimicrobials in Reducing Listeria monocytogenes in Cooked Turkey and Roast Beef. Int. J. Food Microbiol. 2013, 167, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Hwang, C.-A.; Liu, Y.; Renye, J.; Jia, Z. Growth Competition between Lactic Acid Bacteria and Listeria monocytogenes during Simultaneous Fermentation and Drying of Meat Sausages—A Mathematical Modeling. Food Res. Int. 2022, 158, 111553. [Google Scholar] [CrossRef]
- Dos Santos, L.R.; Alía, A.; Martin, I.; Gottardo, F.M.; Rodrigues, L.B.; Borges, K.A.; Furian, T.Q.; Córdoba, J.J. Antimicrobial Activity of Essential Oils and Natural Plant Extracts against Listeria monocytogenes in a Dry-cured Ham-based Model. J. Sci. Food Agric. 2022, 102, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.L.; Almeida, R.C.C. Antibacterial Efficacy of Nisin, Bacteriophage P100 and Sodium Lactate against Listeria monocytogenes in Ready-to-Eat Sliced Pork Ham. Braz. J. Microbiol. 2017, 48, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Van Nassau, T.J.; Lenz, C.A.; Scherzinger, A.S.; Vogel, R.F. Combination of Endolysins and High Pressure to Inactivate Listeria monocytogenes. Food Microbiol. 2017, 68, 81–88. [Google Scholar] [CrossRef]
- Misiou, O.; Van Nassau, T.J.; Lenz, C.A.; Vogel, R.F. The Preservation of Listeria-Critical Foods by a Combination of Endolysin and High Hydrostatic Pressure. Int. J. Food Microbiol. 2018, 266, 355–362. [Google Scholar] [CrossRef]
- Giello, M.; La Storia, A.; De Filippis, F.; Ercolini, D.; Villani, F. Impact of Lactobacillus curvatus 54M16 on Microbiota Composition and Growth of Listeria monocytogenes in Fermented Sausages. Food Microbiol. 2018, 72, 1–15. [Google Scholar] [CrossRef]
- Kamiloğlu, A.; Kaban, G.; Kaya, M. Effects of Autochthonous Lactobacillus plantarum Strains on Listeria monocytogenes in Sucuk during Ripening. J. Food Saf. 2019, 39, e12618. [Google Scholar] [CrossRef]
- Beristain-Bauza, S.D.C.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial Activity of Whey Protein Films Supplemented with Lactobacillus sakei Cell-Free Supernatant on Fresh Beef. Food Microbiol. 2017, 62, 207–211. [Google Scholar] [CrossRef]
- Shafipour Yordshahi, A.; Moradi, M.; Tajik, H.; Molaei, R. Design and Preparation of Antimicrobial Meat Wrapping Nanopaper with Bacterial Cellulose and Postbiotics of Lactic Acid Bacteria. Int. J. Food Microbiol. 2020, 321, 108561. [Google Scholar] [CrossRef]
- Ruiz, A.; Williams, S.K.; Djeri, N.; Hinton, A.; Rodrick, G.E. Nisin Affects the Growth of Listeria monocytogenes on Ready-to-Eat Turkey Ham Stored at Four Degrees Celsius for Sixty-Three Days. Poult. Sci. 2010, 89, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kara, R.; Yaman, H.; Gök, V.; Akkaya, L. The Effect of Nisin on Listeria monocytogenes in Chicken Burgers. Indian J. Anim. Res. 2014, 48, 171. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Delgado, J.; Córdoba, J.J. Strategies for Biocontrol of Listeria monocytogenes Using Lactic Acid Bacteria and Their Metabolites in Ready-to-Eat Meat- and Dairy-Ripened Products. Foods 2022, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fang, J.; Tian, Y.; Lu, X.Y. Mechanisms of Nisin Resistance in Gram-Positive Bacteria. Ann. Microbiol. 2014, 64, 413–420. [Google Scholar] [CrossRef]
- Wongchai, M.; Churklam, W.; Klubthawee, N.; Aunpad, R. Efficacy of a Combination of Nisin and Citric Acid against Listeria monocytogenes 10403S In Vitro and in Model Food Systems. Sci. Technol. Asia 2018, 23, 5359. [Google Scholar] [CrossRef]
- Hammou, F.N.; Skali, S.N.; Idaomar, M.; Abrini, J. Combinations of Nisin with Salt (NaCl) to Control Listeria monocytogenes on Sheep Natural Sausage Casings Stored at 6C. Afr. J. Biotechnol. 2010, 9, 1190–1195. [Google Scholar] [CrossRef]
- Raeisi, M.; Tabaraei, A.; Hashemi, M.; Behnampour, N. Effect of Sodium Alginate Coating Incorporated with Nisin, Cinnamomum Zeylanicum, and Rosemary Essential Oils on Microbial Quality of Chicken Meat and Fate of Listeria monocytogenes during Refrigeration. Int. J. Food Microbiol. 2016, 238, 139–145. [Google Scholar] [CrossRef]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The Mechanisms of Action of Carvacrol and Its Synergism with Nisin against Listeria monocytogenes on Sliced Bologna Sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Aras, S.; Kabir, M.N.; Chowdhury, S.; Fouladkhah, A.C. Augmenting the Pressure-Based Pasteurization of Listeria monocytogenes by Synergism with Nisin and Mild Heat. Int. J. Environ. Res. Public. Health 2020, 17, 563. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Llera-Oyola, J.; Gil, M.V.; Ayuso-Yuste, M.C.; García-Parra, J.; Delgado-Adámez, J. Control of Listeria monocytogenes in Sliced Dry-Cured Iberian Ham by High Pressure Processing in Combination with an Eco-Friendly Packaging Based on Chitosan, Nisin and Phytochemicals from Rice Bran. Food Control 2021, 124, 107933. [Google Scholar] [CrossRef]
- Teixeira, J.S.; Repková, L.; Gänzle, M.G.; McMullen, L.M. Effect of Pressure, Reconstituted RTE Meat Microbiota, and Antimicrobials on Survival and Post-Pressure Growth of Listeria monocytogenes on Ham. Front. Microbiol. 2018, 9, 1979. [Google Scholar] [CrossRef]
- Jin, T.; Liu, L.; Sommers, C.H.; Boyd, G.; Zhang, H. Radiation Sensitization and Postirradiation Proliferation of Listeria monocytogenes on Ready-to-Eat Deli Meat in the Presence of Pectin-Nisin Films. J. Food Prot. 2009, 72, 644–649. [Google Scholar] [CrossRef]
- Turgis, M.; Stotz, V.; Dupont, C.; Salmieri, S.; Khan, R.A.; Lacroix, M. Elimination of Listeria monocytogenes in Sausage Meat by Combination Treatment: Radiation and Radiation-Resistant Bacteriocins. Radiat. Phys. Chem. 2012, 81, 1185–1188. [Google Scholar] [CrossRef]
- Huq, T.; Vu, K.D.; Riedl, B.; Bouchard, J.; Lacroix, M. Synergistic Effect of Gamma (γ)-Irradiation and Microencapsulated Antimicrobials against Listeria Monocytogenes on Ready-to-Eat (RTE) Meat. Food Microbiol. 2015, 46, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.M.H.; Elnawawi, F.A.; Yousef, A.E. Nisin Treatment to Enhance the Efficacy of Gamma Radiation against Listeria monocytogenes on Meat. J. Food Prot. 2011, 74, 193–199. [Google Scholar] [CrossRef]
- Foegeding, P.M.; Thomas, A.B.; Pilkington, D.H.; Klaenhammer, T.R. Enhanced Control of Listeria monocytogenes by in Situ-Produced Pediocin during Dry Fermented Sausage Production. Appl. Environ. Microbiol. 1992, 58, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Papagianni, M.; Anastasiadou, S. Pediocins: The Bacteriocins of Pediococci. Sources, Production, Properties and Applications. Microb. Cell Factories 2009, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Schlyter, J.H.; Glass, K.A.; Loeffelholz, J.; Degnan, A.J.; Luchansky, J.B. The Effects of Diacetate with Nitrite, Lactate, or Pediocin on the Viability of Listeria monocytegenes in Turkey Slurries. Int. J. Food Microbiol. 1993, 19, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Goff, J.H.; Bhunia, A.K.; Johnson, M.G. Complete Inhibition of Low Levels of Listeria monocytogenes on Refrigerated Chicken Meat with Pediocin AcH Bound to Heat-Killed Pediococcus acidilactici Cells. J. Food Prot. 1996, 59, 1187–1192. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sebranek, J.G.; Dickson, J.S.; Mendonca, A.F. Use of Pediocin (Alta Tm 2341) For Control of Listeria monocytogenes on Frankfurters. J. Muscle Foods 2004, 15, 35–56. [Google Scholar] [CrossRef]
- Santiago-Silva, P.; Soares, N.F.F.; Nóbrega, J.E.; Júnior, M.A.W.; Barbosa, K.B.F.; Volp, A.C.P.; Zerdas, E.R.M.A.; Würlitzer, N.J. Antimicrobial Efficiency of Film Incorporated with Pediocin (ALTA® 2351) on Preservation of Sliced Ham. Food Control 2009, 20, 85–89. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Otoni, C.G.; Soares, N.F.F. Pediocin Applications in Antimicrobial Food Packaging Systems. In Antimicrobial Food Packaging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 445–454. ISBN 978-0-12-800723-5. [Google Scholar]
- Woraprayote, W.; Kingcha, Y.; Amonphanpokin, P.; Kruenate, J.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Anti-Listeria Activity of Poly(Lactic Acid)/Sawdust Particle Biocomposite Film Impregnated with Pediocin PA-1/AcH and Its Use in Raw Sliced Pork. Int. J. Food Microbiol. 2013, 167, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Castro, S.M.; Silva, J.; Casquete, R.; Queirós, R.; Saraiva, J.A.; Teixeira, P. Combined Effect of Pediocin bacHA-6111-2 and High Hydrostatic Pressure to Control Listeria innocua in Fermented Meat Sausage. Int. Food Res. J. 2018, 25, 553–560. [Google Scholar]
- Pesavento, G.; Calonico, C.; Bilia, A.R.; Barnabei, M.; Calesini, F.; Addona, R.; Mencarelli, L.; Carmagnini, L.; Di Martino, M.C.; Lo Nostro, A. Antibacterial Activity of Oregano, Rosmarinus and Thymus Essential Oils against Staphylococcus aureus and Listeria monocytogenes in Beef Meatballs. Food Control 2015, 54, 188–199. [Google Scholar] [CrossRef]
- Gouveia, A.R.; Alves, M.; Silva, J.A.; Saraiva, C. The Antimicrobial Effect of Rosemary and Thyme Essential Oils against Listeria monocytogenes in Sous Vide Cook-Chill Beef During Storage. Procedia Food Sci. 2016, 7, 173–176. [Google Scholar] [CrossRef]
- Khaleque, M.A.; Keya, C.A.; Hasan, K.N.; Hoque, M.M.; Inatsu, Y.; Bari, M.L. Use of Cloves and Cinnamon Essential Oil to Inactivate Listeria monocytogenes in Ground Beef at Freezing and Refrigeration Temperatures. LWT 2016, 74, 219–223. [Google Scholar] [CrossRef]
- Radünz, M.; Dos Santos Hackbart, H.C.; Camargo, T.M.; Nunes, C.F.P.; De Barros, F.A.P.; Dal Magro, J.; Filho, P.J.S.; Gandra, E.A.; Radünz, A.L.; Da Rosa Zavareze, E. Antimicrobial Potential of Spray Drying Encapsulated Thyme (Thymus vulgaris) Essential Oil on the Conservation of Hamburger-like Meat Products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef]
- Abbasi, Z.; Aminzare, M.; Hassanzad Azar, H.; Rostamizadeh, K. Effect of Corn Starch Coating Incorporated with Nanoemulsion of Zataria Multiflora Essential Oil Fortified with Cinnamaldehyde on Microbial Quality of Fresh Chicken Meat and Fate of Inoculated Listeria monocytogenes. J. Food Sci. Technol. 2021, 58, 2677–2687. [Google Scholar] [CrossRef]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of Essential Oil as a Sustained Release Preparation in Food Packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Solomakos, N.; Govaris, A.; Koidis, P.; Botsoglou, N. The Antimicrobial Effect of Thyme Essential Oil, Nisin, and Their Combination against Listeria monocytogenes in Minced Beef during Refrigerated Storage. Food Microbiol. 2008, 25, 120–127. [Google Scholar] [CrossRef]
- Wagh, A.M.; Jaiswal, S.G.; Bornare, D.T. A Review: Extraction of Essential Oil from Lemon Grass as a Preservative for Animal Products. J. Pharmacogn. Phytochem. 2021, 10, 26–31. [Google Scholar] [CrossRef]
- Kazemeini, H.; Azizian, A.; Adib, H. Inhibition of Listeria monocytogenes Growth in Turkey Fillets by Alginate Edible Coating with Trachyspermum Ammi Essential Oil Nano-Emulsion. Int. J. Food Microbiol. 2021, 344, 109104. [Google Scholar] [CrossRef] [PubMed]
- Dini, H.; Fallah, A.A.; Bonyadian, M.; Abbasvali, M.; Soleimani, M. Effect of Edible Composite Film Based on Chitosan and Cumin Essential Oil-Loaded Nanoemulsion Combined with Low-Dose Gamma Irradiation on Microbiological Safety and Quality of Beef Loins during Refrigerated Storage. Int. J. Biol. Macromol. 2020, 164, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, F.; Muscolino, D.; Ragonese, C.; Beninati, C.; Sciarrone, D.; Ziino, G.; Mondello, L.; Giuffrida, A.; Panebianco, A. Antimicrobial Activity of Combined Thyme and Rosemary Essential Oils against Listeria monocytogenes in Italian Mortadella Packaged in Modified Atmosphere: Thyme & Rosemary EOs vs L. monocytogenes. J. Essent. Oil Res. 2016, 28, 467–474. [Google Scholar] [CrossRef]
- Yemiş, G.P.; Candoğan, K. Antibacterial Activity of Soy Edible Coatings Incorporated with Thyme and Oregano Essential Oils on Beef against Pathogenic Bacteria. Food Sci. Biotechnol. 2017, 26, 1113–1121. [Google Scholar] [CrossRef]
- Wang, D.; Dong, Y.; Chen, X.; Liu, Y.; Wang, J.; Wang, X.; Wang, C.; Song, H. Incorporation of Apricot (Prunus Armeniaca) Kernel Essential Oil into Chitosan Films Displaying Antimicrobial Effect against Listeria monocytogenes and Improving Quality Indices of Spiced Beef. Int. J. Biol. Macromol. 2020, 162, 838–844. [Google Scholar] [CrossRef]
- EC. 2005 Commission Regulation (EC) No 2073/2005. Available online: https://www.legislation.gov.uk/eur/2005/2073/data.pdf (accessed on 22 February 2024).
- Melo, J.; Andrew, P.W.; Faleiro, M.L. Listeria monocytogenes in Cheese and the Dairy Environment Remains a Food Safety Challenge: The Role of Stress Responses. Food Res. Int. 2015, 67, 75–90. [Google Scholar] [CrossRef]
- Chow, J.T.H.; Gall, A.R.; Johnson, A.K.; Huynh, T.N. Characterization of Listeria monocytogenes Isolates from Lactating Dairy Cows in a Wisconsin Farm: Antibiotic Resistance, Mammalian Cell Infection, and Effects on the Fecal Microbiota. J. Dairy Sci. 2021, 104, 4561–4574. [Google Scholar] [CrossRef]
- Rodriguez, C.; Taminiau, B.; García-Fuentes, E.; Daube, G.; Korsak, N. Listeria monocytogenes Dissemination in Farming and Primary Production: Sources, Shedding and Control Measures. Food Control 2021, 120, 107540. [Google Scholar] [CrossRef]
- Possas, A.; Hernández, M.; Esteban-Carbonero, Ó.; Valero, A.; Rodríguez-Lázaro, D. Listeria monocytogenes Survives Better at Lower Storage Temperatures in Regular and Low-Salt Soft and Cured Cheeses. Food Microbiol. 2022, 104, 103979. [Google Scholar] [CrossRef]
- Guenther, S.; Huwyler, D.; Richard, S.; Loessner, M.J. Virulent Bacteriophage for Efficient Biocontrol of Listeria monocytogenes in Ready-To-Eat Foods. Appl. Environ. Microbiol. 2009, 75, 93–100. [Google Scholar] [CrossRef] [PubMed]
- El-Bakry, M.; Sanchez, A.; Mehta, B.M.; El-Bakry, M.; Sanchez, A.; Mehta, B.M. Microstructure of Dairy Products, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 978-1-118-96421-7. [Google Scholar]
- Henderson, L.O.; Cabrera-Villamizar, L.A.; Skeens, J.; Kent, D.; Murphy, S.; Wiedmann, M.; Guariglia-Oropeza, V. Environmental Conditions and Serotype Affect Listeria monocytogenes Susceptibility to Phage Treatment in a Laboratory Cheese Model. J. Dairy Sci. 2019, 102, 9674–9688. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.A.; Desai, M.; Oladunjoye, A.; Skrobot, F.; Nannapaneni, R. Reduction of Listeria monocytogenes in Queso Fresco Cheese by a Combination of Listericidal and Listeriostatic GRAS Antimicrobials. Int. J. Food Microbiol. 2012, 155, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.G.; Figueiredo, A.C.L.; Miranda, F.A.; Almeida, R.C.D.C. Control of Listeria monocytogenes Growth in Soft Cheeses by Bacteriophage P100. Braz. J. Microbiol. 2014, 45, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.C.; Almeida, F.A.D.; Medeiros, M.M.; Miranda, B.R.; Pinto, U.M.; Alves, V.F. Listeria monocytogenes: An Inconvenient Hurdle for the Dairy Industry. Dairy 2023, 4, 316–344. [Google Scholar] [CrossRef]
- Romero-Calle, D.X.; De Santana, V.P.; Benevides, R.G.; Aliaga, M.T.A.; Billington, C.; Góes-Neto, A. Systematic Review and Meta-Analysis: The Efficiency of Bacteriophages Previously Patented against Pathogenic Bacteria on Food. Syst. Rev. 2023, 12, 201. [Google Scholar] [CrossRef]
- Rodríguez-Rubio, L.; García, P.; Rodríguez, A.; Billington, C.; Hudson, J.A.; Martínez, B. Listeriaphages and Coagulin C23 Act Synergistically to Kill Listeria monocytogenes in Milk under Refrigeration Conditions. Int. J. Food Microbiol. 2015, 205, 68–72. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Elkenany, R.M.; Zakari, A.I.; Badawy, B.M. Isolation and Characterization of Bacteriophages for Combating Multidrug-Resistant Listeria monocytogenes from Dairy Cattle Farms in Conjugation with Silver Nanoparticles. BMC Microbiol. 2023, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Margalho, L.P.; Feliciano, M.D.; Silva, C.E.; Abreu, J.S.; Piran, M.V.F.; Sant’Ana, A.S. Brazilian Artisanal Cheeses Are Rich and Diverse Sources of Nonstarter Lactic Acid Bacteria Regarding Technological, Biopreservative, and Safety Properties—Insights through Multivariate Analysis. J. Dairy Sci. 2020, 103, 7908–7926. [Google Scholar] [CrossRef] [PubMed]
- Margalho, L.P.; Jorge, G.P.; Noleto, D.A.P.; Silva, C.E.; Abreu, J.S.; Piran, M.V.F.; Brocchi, M.; Sant’Ana, A.S. Biopreservation and Probiotic Potential of a Large Set of Lactic Acid Bacteria Isolated from Brazilian Artisanal Cheeses: From Screening to in Product Approach. Microbiol. Res. 2021, 242, 126622. [Google Scholar] [CrossRef] [PubMed]
- Mirkovic, N.; Kulas, J.; Miloradovic, Z.; Miljkovic, M.; Tucovic, D.; Miocinovic, J.; Jovcic, B.; Mirkov, I.; Kojic, M. Lactolisterin BU-Producer Lactococcus lactis Subsp. Lactis BGBU1-4: Bio-Control of Listeria monocytogenes and Staphylocococcus aureus in Fresh Soft Cheese and Effect on Immunological Response of Rats. Food Control 2020, 111, 107076. [Google Scholar] [CrossRef]
- Kondrotiene, K.; Kasnauskyte, N.; Serniene, L.; Gölz, G.; Alter, T.; Kaskoniene, V.; Maruska, A.S.; Malakauskas, M. Characterization and Application of Newly Isolated Nisin Producing Lactococcus lactis Strains for Control of Listeria monocytogenes Growth in Fresh Cheese. LWT 2018, 87, 507–514. [Google Scholar] [CrossRef]
- Coelho, M.C.; Silva, C.C.G.; Ribeiro, S.C.; Dapkevicius, M.L.N.E.; Rosa, H.J.D. Control of Listeria monocytogenes in Fresh Cheese Using Protective Lactic Acid Bacteria. Int. J. Food Microbiol. 2014, 191, 53–59. [Google Scholar] [CrossRef]
- Dal Bello, B.; Cocolin, L.; Zeppa, G.; Field, D.; Cotter, P.D.; Hill, C. Technological Characterization of Bacteriocin Producing Lactococcus Lactis Strains Employed to Control Listeria monocytogenes in Cottage Cheese. Int. J. Food Microbiol. 2012, 153, 58–65. [Google Scholar] [CrossRef]
- Khay, E.O.; Idaomar, M.; El Moussaoui, N.; Abrini, J. Application of a Bacteriocin-like Inhibitory Substance Producing Enterococcus durans E204 Strain, Isolated from Camel Milk, to Control Listeria monocytogenes CECT 4032 in Goat Jben. Ann. Microbiol. 2014, 64, 313–319. [Google Scholar] [CrossRef]
- Guitián, M.V.; Ibarguren, C.; Soria, M.C.; Hovanyecz, P.; Banchio, C.; Audisio, M.C. Anti- Listeria monocytogenes Effect of Bacteriocin-Incorporated Agar Edible Coatings Applied on Cheese. Int. Dairy J. 2019, 97, 92–98. [Google Scholar] [CrossRef]
- Silva, S.P.M.; Teixeira, J.A.; Silva, C.C.G. Application of Enterocin-Whey Films to Reduce Listeria monocytogenes Contamination on Ripened Cheese. Food Microbiol. 2023, 109, 104134. [Google Scholar] [CrossRef] [PubMed]
- Jutinico-Shubach, A.; Gutiérrez-Cortés, C.; Suarez, H. Antilisterial Activity of Chitosan-based Edible Coating Incorporating Cell-free Supernatant from Pediococcus pentosaceus 147 on the Preservation of Fresh Cheese. J. Food Process. Preserv. 2020, 44, e14715. [Google Scholar] [CrossRef]
- Silva, S.P.M.; Ribeiro, S.C.; Teixeira, J.A.; Silva, C.C.G. Application of an Alginate-Based Edible Coating with Bacteriocin-Producing Lactococcus Strains in Fresh Cheese Preservation. LWT 2022, 153, 112486. [Google Scholar] [CrossRef]
- Gouvea, F.D.S.; Rosenthal, A.; Ferreira, E.H.D.R. Plant Extract and Essential Oils Added as Antimicrobials to Cheeses: A Review. Ciênc. Rural 2017, 47, e20160908. [Google Scholar] [CrossRef]
- Da Silva Dannenberg, G.; Funck, G.D.; Mattei, F.J.; Da Silva, W.P.; Fiorentini, Â.M. Antimicrobial and Antioxidant Activity of Essential Oil from Pink Pepper Tree (Schinus Terebinthifolius Raddi) in vitro and in Cheese Experimentally Contaminated with Listeria monocytogenes. Innov. Food Sci. Emerg. Technol. 2016, 36, 120–127. [Google Scholar] [CrossRef]
- Elsherif, W.M.; Talaat Al Shrief, L.M. Effects of Three Essential Oils and Their Nano-Emulsions on Listeria monocytogenes and Shigella flexneri in Egyptian Talaga Cheese. Int. J. Food Microbiol. 2021, 355, 109334. [Google Scholar] [CrossRef]
- Ibarra-Sánchez, L.A.; Van Tassell, M.L.; Miller, M.J. Antimicrobial Behavior of Phage Endolysin PlyP100 and Its Synergy with Nisin to Control Listeria monocytogenes in Queso Fresco. Food Microbiol. 2018, 72, 128–134. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Pinto, C.A.; Ferreira, V.; Brandão, T.R.S.; Saraiva, J.M.A.; Castro, S.M.; Teixeira, P. Non-Thermal Approach to Listeria monocytogenes Inactivation in Milk: The Combined Effect of High Pressure, Pediocin PA-1 and Bacteriophage P100. Food Microbiol. 2020, 86, 103315. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Sánchez, L.A.; Kong, W.; Lu, T.; Miller, M.J. Efficacy of Nisin Derivatives with Improved Biochemical Characteristics, Alone and in Combination with Endolysin PlyP100 to Control Listeria monocytogenes in Laboratory-Scale Queso Fresco. Food Microbiol. 2021, 94, 103668. [Google Scholar] [CrossRef]
- Smith, A.; Moorhouse, E.; Monaghan, J.; Taylor, C.; Singleton, I. Sources and Survival of Listeria monocytogenes on Fresh, Leafy Produce. J. Appl. Microbiol. 2018, 125, 930–942. [Google Scholar] [CrossRef]
- Wellman, A.; Bazaco, M.C.; Blessington, T.; Pightling, A.; Dwarka, A.; Hintz, L.; Wise, M.E.; Gieraltowski, L.; Conrad, A.; Nguyen, T.-A.; et al. An Overview of Foodborne Sample-Initiated Retrospective Outbreak Investigations and Interagency Collaboration in the United States. J. Food Prot. 2023, 86, 100089. [Google Scholar] [CrossRef]
- Sadekuzzaman, M.; Yang, S.; Mizan, F.R.; Kim, H.-S.; Ha, S.-D. Effectiveness of a Phage Cocktail as a Biocontrol Agent against Listeria monocytogenes Biofilms. Food Control 2017, 78, 256–263. [Google Scholar] [CrossRef]
- Truchado, P.; Elsser-Gravesen, A.; Gil, M.I.; Allende, A. Post-Process Treatments Are Effective Strategies to Reduce Listeria monocytogenes on the Surface of Leafy Greens: A Pilot Study. Int. J. Food Microbiol. 2020, 313, 108390. [Google Scholar] [CrossRef] [PubMed]
- Boyacioglu, O.; Sulakvelidze, A.; Sharma, M.; Goktepe, I. Effect of a Bacteriophage Cocktail in Combination with Modified Atmosphere Packaging in Controlling Listeria monocytogenes on Fresh-Cut Spinach. Ir. J. Agric. Food Res. 2016, 55, 74–79. [Google Scholar] [CrossRef]
- Oliveira, M.; Viñas, I.; Colàs, P.; Anguera, M.; Usall, J.; Abadias, M. Effectiveness of a Bacteriophage in Reducing Listeria monocytogenes on Fresh-Cut Fruits and Fruit Juices. Food Microbiol. 2014, 38, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.; Lhomet, A.; Neve, H.; Grant, I.R.; Campbell, K.; McAuliffe, O. Isolation and Characterization of Listeria monocytogenes Phage vB_LmoH_P61, a Phage with Biocontrol Potential on Different Food Matrices. Front. Sustain. Food Syst. 2020, 4, 521645. [Google Scholar] [CrossRef]
- Byun, K.-H.; Han, S.H.; Choi, M.W.; Park, S.H.; Ha, S.-D. Isolation, Characterization, and Application of Bacteriophages to Reduce and Inhibit Listeria monocytogenes in Celery and Enoki Mushroom. Food Control 2022, 135, 108826. [Google Scholar] [CrossRef]
- Oladunjoye, A.O.; Oyewole, S.A.; Singh, S.; Ijabadeniyi, O.A. Prediction of Listeria monocytogenes ATCC 7644 Growth on Fresh-Cut Produce Treated with Bacteriophage and Sucrose Monolaurate by Using Artificial Neural Network. LWT-Food Sci. Technol. 2017, 76, 9–17. [Google Scholar] [CrossRef]
- Hossain, M.I.; Mizan, M.F.R.; Ashrafudoulla, M.; Nahar, S.; Joo, H.-J.; Jahid, I.K.; Park, S.H.; Kim, K.-S.; Ha, S.-D. Inhibitory Effects of Probiotic Potential Lactic Acid Bacteria Isolated from Kimchi against Listeria monocytogenes Biofilm on Lettuce, Stainless-Steel Surfaces, and MBECTM Biofilm Device. LWT 2020, 118, 108864. [Google Scholar] [CrossRef]
- Ramos, B.; Brandão, T.R.S.; Teixeira, P.; Silva, C.L.M. Biopreservation Approaches to Reduce Listeria monocytogenes in Fresh Vegetables. Food Microbiol. 2020, 85, 103282. [Google Scholar] [CrossRef]
- Amado, I.R.; Fuciños, C.; Fajardo, P.; Guerra, N.P.; Pastrana, L. Evaluation of Two Bacteriocin-Producing Probiotic Lactic Acid Bacteria as Inoculants for Controlling Listeria monocytogenes in Grass and Maize Silages. Anim. Feed Sci. Technol. 2012, 175, 137–149. [Google Scholar] [CrossRef]
- McManamon, O.; Kaupper, T.; Scollard, J.; Schmalenberger, A. Nisin Application Delays Growth of Listeria monocytogenes on Fresh-Cut Iceberg Lettuce in Modified Atmosphere Packaging, While the Bacterial Community Structure Changes within One Week of Storage. Postharvest Biol. Technol. 2019, 147, 185–195. [Google Scholar] [CrossRef]
- Hossain, M.I.; Rahaman Mizan, M.F.; Toushik, S.H.; Roy, P.K.; Jahid, I.K.; Park, S.H.; Ha, S.-D. Antibiofilm Effect of Nisin Alone and Combined with Food-Grade Oil Components (Thymol and Eugenol) against Listeria monocytogenes Cocktail Culture on Food and Food-Contact Surfaces. Food Control 2022, 135, 108796. [Google Scholar] [CrossRef]
- Shi, D.L.; Shi, H. The Synergistic Antibacterial Effect and Inhibition of Biofilm Formation of Nisin in Combination with Terpenes against Listeria monocytogenes. Lett. Appl. Microbiol. 2022, 75, 632–642. [Google Scholar] [CrossRef]
- Ndoti-Nembe, A.; Vu, K.D.; Doucet, N.; Lacroix, M. Antimicrobial Effects of Essential Oils, Nisin, and Irradiation Treatments against Listeria monocytogenes on Ready-to-Eat Carrots. J. Food Sci. 2015, 80, M795–M799. [Google Scholar] [CrossRef]
- Oladunjoye, A.O.; Singh, S.; Ijabadeniyi, O.A. Inactivation of Listeria Monocytogenes ATCC 7644 on Fresh-Cut Tomato Using Nisin in Combinations with Organic Salts. Braz. J. Microbiol. 2016, 47, 757–763. [Google Scholar] [CrossRef]
- Cobo Molinos, A.; Abriouel, H.; Ben Omar, N.; Valdivia, E.; Lucas López, R.; Maqueda, M.; Cañamero, M.M.; Gálvez, A. Effect of Immersion Solutions Containing Enterocin AS-48 on Listeria monocytogenes in Vegetable Foods. Appl. Environ. Microbiol. 2005, 71, 7781–7787. [Google Scholar] [CrossRef]
- Molinos, A.C.; Abriouel, H.; Ben Omar, N.; Lucas, R.; Valdivia, E.; Gálvez, A. Inactivation of Listeria monocytogenes in Raw Fruits by Enterocin AS-48. J. Food Prot. 2008, 71, 2460–2467. [Google Scholar] [CrossRef]
- Cobo Molinos, A.; Abriouel, H.; López, R.L.; Omar, N.B.; Valdivia, E.; Gálvez, A. Enhanced Bactericidal Activity of Enterocin AS-48 in Combination with Essential Oils, Natural Bioactive Compounds and Chemical Preservatives against Listeria monocytogenes in Ready-to-Eat Salad. Food Chem. Toxicol. 2009, 47, 2216–2223. [Google Scholar] [CrossRef]
- López Aguayo, M.D.C.; Grande Burgos, M.J.; Pérez Pulido, R.; Gálvez, A.; Lucas López, R. Effect of Different Activated Coatings Containing Enterocin AS-48 against Listeria monocytogenes on Apple Cubes. Innov. Food Sci. Emerg. Technol. 2016, 35, 177–183. [Google Scholar] [CrossRef]
- Ortega Blázquez, I.; Grande Burgos, M.; Pérez-Pulido, R.; Gálvez, A.; Lucas, R. Inactivation of Listeria in Foods Packed in Films Activated with Enterocin AS-48 plus Thymol Singly or in Combination with High-Hydrostatic Pressure Treatment. Coatings 2017, 7, 204. [Google Scholar] [CrossRef]
- Tao, R.; Sedman, J.; Ismail, A. Antimicrobial Activity of Various Essential Oils and Their Application in Active Packaging of Frozen Vegetable Products. Food Chem. 2021, 360, 129956. [Google Scholar] [CrossRef]
- Takala, P.N.; Vu, K.D.; Salmieri, S.; Khan, R.A.; Lacroix, M. Antibacterial Effect of Biodegradable Active Packaging on the Growth of Escherichia Coli, Salmonella typhimurium and Listeria monocytogenes in Fresh Broccoli Stored at 4 °C. LWT-Food Sci. Technol. 2013, 53, 499–506. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Use of Essential Oils in Bioactive Edible Coatings: A Review. Food Eng. Rev. 2011, 3, 1–16. [Google Scholar] [CrossRef]
- Das, S.; Vishakha, K.; Banerjee, S.; Mondal, S.; Ganguli, A. Sodium Alginate-Based Edible Coating Containing Nanoemulsion of Citrus Sinensis Essential Oil Eradicates Planktonic and Sessile Cells of Food-Borne Pathogens and Increased Quality Attributes of Tomatoes. Int. J. Biol. Macromol. 2020, 162, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, G.D.; Klaus, A.S.; Nikšić, M.P. Antimicrobial Activity of Chitosan Coatings and Films against Listeria monocytogenes on Black Radish. Rev. Argent. Microbiol. 2016, 48, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Severino, R.; Vu, K.D.; Donsì, F.; Salmieri, S.; Ferrari, G.; Lacroix, M. Antibacterial and Physical Effects of Modified Chitosan Based-Coating Containing Nanoemulsion of Mandarin Essential Oil and Three Non-Thermal Treatments against Listeria innocua in Green Beans. Int. J. Food Microbiol. 2014, 191, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Donsì, F.; Marchese, E.; Maresca, P.; Pataro, G.; Vu, K.D.; Salmieri, S.; Lacroix, M.; Ferrari, G. Green Beans Preservation by Combination of a Modified Chitosan Based-Coating Containing Nanoemulsion of Mandarin Essential Oil with High Pressure or Pulsed Light Processing. Postharvest Biol. Technol. 2015, 106, 21–32. [Google Scholar] [CrossRef]
- Solanki, K.; Grover, N.; Downs, P.; Paskaleva, E.E.; Mehta, K.K.; Lee, L.; Schadler, L.S.; Kane, R.S.; Dordick, J.S. Enzyme-Based Listericidal Nanocomposites. Sci. Rep. 2013, 3, 1584. [Google Scholar] [CrossRef]
| Meat or Meat Products | Contamination Procedure | Antimicrobial Agent | Treatment Conditions | Storage Conditions and Results | References |
|---|---|---|---|---|---|
| Fresh beef | Surface inoculation with L. monocytogenes LM-94 at 6.2 log CFU/g | ListShieldTM | 1 × 109 PFU/mL spot inoculation followed by incubation at RT for 2.5 h | Reduction by 2.3 log CFU/g after storage at 4 ± 1 °C for 15 days | [56] |
| Spanish dry-cured ham | Surface inoculation with L. monocytogenes S2 at 105 CFU/cm2, 104 CFU/cm2, and 103 CFU/cm2 | ListShieldTM | 107 PFU/cm2 | Reduction below the detection limit (10 CFU/cm2) for lower contamination level (104 CFU/cm2 and 103 CFU/cm2) and by 3.5 log units for high contamination level (105 CFU/cm2) after storage at 4 °C for 14 days Reduction below the detection limit in low contaminated samples (103 CFU/cm2) after storage at 12 °C for 8 days | [54] |
| ListexTM | 109 PFU/cm2 | Reduction below the detection limit (10 CFU/cm2) for all contamination levels after storage at 4 and 12 °C for 24 h | |||
| Fermented meat sausage (Alheira) | Contamination with L. monocytogenes Scott A and L. monocytogenes 1942 at 105 CFU/g | ListexTM P100 | 108 PFU/g | Reduction below the detection limit of both strains after storage at 4 °C for 14 days | [57] |
| Cooked turkey and roast beef | Surface contamination with a four-strain cocktail (L. monocytogenes 08-5578, Li0512, Li0529, and ATCC19115) at 103 CFU/cm2 | ListexTM P100 | 107 PFU/cm2 | Reduction by 2.1 log10 CFU/cm2 and 1.7 log10 CFU/cm2 in cooked turkey and roast beef, respectively, compared to the control (non-treated samples) during storage at 4 °C for 28 days | [58] |
| RTE pork ham | Surface contamination with a two-strain cocktail (L. monocytogenes B7, AL48/15, and L. monocytogenes Scott A) at ~2.5 log CFU/g | ListexTM P100 | 5 × 105 PFU/g | Reduction to undetectable level after storage at 6–8 °C for 72 h | [59,60,61] |
| Strengths | Opportunities |
| Are effective if used at the right concentrations. Are generally regarded as safe. Except EOs, they do not impact the taste, texture, and nutritional quality of the food. Are highly specific. Are cost-effective. Are easy to be controlled. Have higher acceptance than other methods. Enhance microbial safety in the food industry. Some LABs are probiotics too. EOs have both antimicrobial and antioxidant activities. Applicable to various food products. | Pathogen-free meals without synthetic additives are in demand. Specialists are looking for alternatives to antibiotics since antimicrobial resistance has grown and is now a worldwide concern. Food processors are interested in preventing recalls, legal liabilities, and the loss of consumer trust. Combination of different antilisterial agents is possible. Some antilisterial agents are commercially available, including phage suspensions (e.g., ListShield™). EOs and bacteriocins may be incorporated in packaging materials. |
| Weaknesses | Threats |
| Before being used in foods, the biological antilisterial agents need regulatory approval. EOs impact the taste of food, if high doses are used. Bacteriophages have low tolerance to unfavorable environmental conditions. LAB do not produce bacteriocins, if they experience low-temperature stress. Distribution of antilisterial agents into the food matrix can be a factor that diminishes their effectiveness. | Lysogenic phages could be vehicles for horizontal gene transfer. Some bacteriocins may induce changes in the diversity of intestinal microbiota in different regions of the gastrointestinal tract. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigore-Gurgu, L.; Bucur, F.I.; Mihalache, O.A.; Nicolau, A.I. Comprehensive Review on the Biocontrol of Listeria monocytogenes in Food Products. Foods 2024, 13, 734. https://doi.org/10.3390/foods13050734
Grigore-Gurgu L, Bucur FI, Mihalache OA, Nicolau AI. Comprehensive Review on the Biocontrol of Listeria monocytogenes in Food Products. Foods. 2024; 13(5):734. https://doi.org/10.3390/foods13050734
Chicago/Turabian StyleGrigore-Gurgu, Leontina, Florentina Ionela Bucur, Octavian Augustin Mihalache, and Anca Ioana Nicolau. 2024. "Comprehensive Review on the Biocontrol of Listeria monocytogenes in Food Products" Foods 13, no. 5: 734. https://doi.org/10.3390/foods13050734
APA StyleGrigore-Gurgu, L., Bucur, F. I., Mihalache, O. A., & Nicolau, A. I. (2024). Comprehensive Review on the Biocontrol of Listeria monocytogenes in Food Products. Foods, 13(5), 734. https://doi.org/10.3390/foods13050734