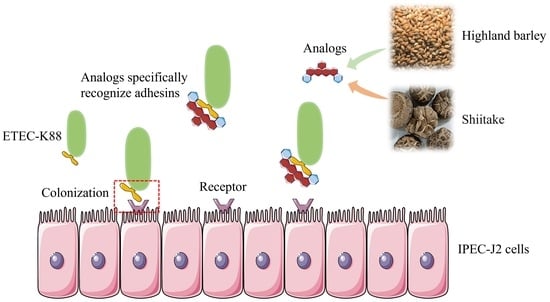
Screening Traditional Foods for the Prevention of Enterotoxigenic Escherichia coli K88ac (F4ac) Attachment to IPEC-J2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Food Ingredients and Analytical Procedures
2.2. Escherichia coli Strains
2.3. Cell Culture Growth
2.4. Adhesion Test
2.5. Miniaturised Assays with IPEC-J2 Cells
- Competition Test
- b.
- Exclusion Test
- c.
- Displacement Test
2.6. Calculations and Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly. Epidemiol. Rec.-Relev. Épidémiolog. Hebd. 2006, 81, 97–104. [Google Scholar]
- Gonzales-Siles, L.; Sjöling, Å. The different ecological niches of enterotoxigenic Escherichia coli. Environ. Microbiol. 2016, 18, 741–751. [Google Scholar] [CrossRef]
- Nagy, B.; Fekete, P.Z. Enterotoxigenic Escherichia coli in veterinary medicine. Int. J. Med. Microbiol. 2005, 295, 443–454. [Google Scholar] [CrossRef]
- Fleckenstein, J.M.; Hardwidge, P.R.; Munson, G.P.; Rasko, D.A.; Sommerfelt, H.; Steinsland, H. Molecular mechanisms of enterotoxigenic Escherichia coli infection. Microbes Infect. 2010, 12, 89–98. [Google Scholar] [CrossRef]
- de Hostos, E.L.; Choy, R.K.; Nguyen, T. Developing novel antisecretory drugs to treat infectious diarrhoea. Future Med. Chem. 2011, 3, 1317–1325. [Google Scholar] [CrossRef]
- Hermes, R.G.; Manzanilla, E.G.; Martín-Orúe, S.M.; Pérez, J.F.; Klasing, K.C. Influence of dietary ingredients on in vitro inflammatory response of intestinal porcine epithelial cells challenged by an enterotoxigenic Escherichia coli (K88). Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 479–488. [Google Scholar] [CrossRef]
- González-Ortiz, G.; Hermes, R.G.; Jiménez-Díaz, R.; Pérez, J.F.; Martín-Orúe, S.M. Screening of extracts from natural feed ingredients for their ability to reduce enterotoxigenic Escherichia coli (ETEC) K88 adhesion to porcine intestinal epithelial cell-line IPEC-J2. Vet. Microbiol. 2013, 167, 494–499. [Google Scholar] [CrossRef]
- Zhu, Y.; González-Ortiz, G.; Solà-Oriol, D.; López-Colom, P.; Martín-Orúe, S.M. Screening of the ability of natural feed ingredients commonly used in pig diets to interfere with the attachment of ETEC K88 (F4) to intestinal epithelial cells. Anim. Feed. Sci. Technol. 2018, 242, 111–119. [Google Scholar] [CrossRef]
- Nirmala Prasadi, V.P.; Joye, I.J. Dietary fibre from whole grains and their benefits on metabolic health. Nutrients 2020, 12, 3045. [Google Scholar]
- Stuyven, E.; Cox, E.; Vancaeneghem, S.; Arnouts, S.; Deprez, P.; Goddeeris, B.M. Effect of β-glucans on an ETEC infection in piglets. Vet. Immunol. Immunopathol. 2009, 128, 60–66. [Google Scholar] [CrossRef]
- Kšonžeková, P.; Bystrický, P.; Vlčková, S.; Pätoprstý, V.; Pulzová, L.; Mudroňová, D.; Kubašková, T.; Csank, T.; Tkáčiková, L. Exopolysaccharides of Lactobacillus reuteri: Their influence on adherence of E. coli to epithelial cells and inflammatory response. Carbohydr. Polym. 2016, 141, 10–19. [Google Scholar] [CrossRef]
- Sivignon, A.; Yu, S.Y.; Ballet, N.; Vandekerckove, P.; Barnich, N.; Guerardel, Y. Heteropolysaccharides from S. cerevisiae show anti-adhesive properties against E. coli associated with Crohn’s disease. Carbohydr. Polym. 2021, 271, 118415. [Google Scholar] [CrossRef]
- Rong, Y.; Xu, N.; Xie, B.; Hao, J.; Yi, L.; Cheng, R.; Li, D.; Linhardt, R.J.; Zhang, Z. Sequencing analysis of β-glucan from highland barley with high performance anion exchange chromatography coupled to quadrupole time-of-Flight mass spectrometry. Food Hydrocoll. 2017, 73, 235–242. [Google Scholar] [CrossRef]
- Nitschke, J.; Modick, H.; Busch, E.; Von Rekowski, R.W.; Altenbach, H.J.; Mölleken, H. A new colorimetric method to quantify β-1,3-1,6-glucans in comparison with total β-1,3-glucans in edible mushrooms. Food Chem. 2011, 127, 791–796. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef]
- Miao, J.; Regenstein, J.M.; Qiu, J.; Zhang, J.; Zhang, X.; Li, H.; Wang, Z. Isolation, structural characterization and bioactivities of polysaccharides and its derivatives from Auricularia-A review. Int. J. Biol. Macromol. 2020, 150, 102–113. [Google Scholar] [CrossRef]
- Lante, A.; Canazza, E.; Tessari, P. Beta-glucans of cereals: Functional and technological properties. Nutrients 2023, 15, 2124. [Google Scholar] [CrossRef]
- Xia, X.; Li, G.; Ding, Y.; Ren, T.; Zheng, J.; Kan, J. Effect of whole grain Qingke (Tibetan Hordeum vulgare L. Zangqing 320) on the serum lipid levels and intestinal microbiota of rats under high-fat diet. J. Agric. Food Chem. 2017, 65, 2686–2693. [Google Scholar] [CrossRef]
- Morales, D.; Shetty, S.A.; López-Plaza, B.; Gomez-Candela, C.; Smidt, H.; Ramon Marin, F.; Soler-Rivas, C. Modulation of human intestinal microbiota in a clinical trial by consumption of a β-D-glucan-enriched extract obtained from Lentinula edodes. Eur. J. Nutr. 2021, 60, 3249–3265. [Google Scholar] [CrossRef]
- AOAC (Official Methods of Analysis). Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; and Lewis, B.A. Methods for dietary fibre, neutral detergent fibre, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Blanco, M.; Blanco, J.E.; Gonzalez, E.A.; Mora, A.; Jansen, W.; Gomes, T.A.; Zerbini, L.F.; Yano, T.; de Castro, A.F.; Blanco, J. Genes coding for enterotoxins and verotoxins in porcine Escherichia coli strains belonging to different O:K:H serotypes: Relationship with toxic phenotypes. J. Clin. Microbiol. 1997, 35, 2958–2963. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.M.; Galletti, S.; Roubos-van den Hil, P.J.; Van Wikselaar, P.G. Validation of growth as measurand for bacterial adhesion to food and feed ingredients. J. Appl. Microbiol. 2007, 103, 2686–2696. [Google Scholar] [CrossRef]
- Zhu, Y.; González-Ortiz, G.; Jiménez-Díaz, R.; Pérez-Trujillo, M.; Parella, T.; López-Colom, P.; Martín-Orúe, S.M. Exopolysaccharides from olive brines could reduce the adhesion of ETEC K88 to intestinal epithelial cells. Food Funct. 2018, 9, 3884–3894. [Google Scholar] [CrossRef]
- Becker, P.M.; Galletti, S. Food and feed components for gut health-promoting adhesion of E. coli and Salmonella enterica. J. Sci. Food Agric. 2008, 88, 2026–2035. [Google Scholar] [CrossRef]
- Zhang, G.; Junmei, W.; Jinxin, C. Analysis of β-glucan content in barley cultivars from different locations of China. Food Chem. 2002, 79, 251–254. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Di Criscio, T.; Marconi, E. Tocol and β-glucan levels in barley varieties and in pearling by-products. Food Chem. 2008, 107, 84–91. [Google Scholar] [CrossRef]
- Guo, F.C.; Kwakkel, R.P.; Williams, B.A.; Li, W.K.; Li, H.S.; Luo, J.Y.; Li, Y.X.; Wei, Z.T.Y.; Verstegen, M.W.A. Effects of mushroom and herb polysaccharides, as alternatives for an antibiotic, on growth performance of broilers. Br. Poult. Sci. 2004, 45, 684–694. [Google Scholar] [CrossRef]
- van Nevel, C.J.; Decuypere, J.A.; Dierick, N.; Molly, K. The influence of Lentinus edodes (Shiitake mushroom) preparations on bacteriological and morphological aspects of the small intestine in piglets. Arch. Anim. Nutr. 2003, 57, 399–412. [Google Scholar] [CrossRef]
- Grange, P.A.; Mouricout, M.A.; Levery, S.B.; Francis, D.H.; Erickson, A.K. Evaluation of receptor binding specificity of Escherichia coli K88 (F4) fimbrial adhesin variants using porcine serum transferrin and glycosphingolipids as model receptors. Infect. Immun. 2002, 70, 2336–2343. [Google Scholar] [CrossRef]
- Payne, D.; O’Reilly, M.; Williamson, D. The K88 fimbrial adhesin of enterotoxigenic Escherichia coli binds to β1-linked galactosyl residues in glycosphingolipids. Infect. Immun. 1993, 61, 3673–3677. [Google Scholar] [CrossRef] [PubMed]
- Sauvaitre, T.; Durif, C.; Sivignon, A.; Chalancon, S.; Van de Wiele, T.; Etienne-Mesmin, L.; Blanquet-Diot, S. In vitro evaluation of dietary fibre anti-infectious properties against food-borne enterotoxigenic Escherichia coli. Nutrients 2021, 13, 3188. [Google Scholar] [CrossRef]
- Wijemanne, P.; Moxley, R.A. Glucose significantly enhances enterotoxigenic Escherichia coli adherence to intestinal epithelial cells through its effects on heat-labile enterotoxin production. PLoS ONE 2014, 9, e113230. [Google Scholar] [CrossRef]
| Ingredient | DM | Ash | CP | CF | EE | NDF | ADF | ADL | NFE |
|---|---|---|---|---|---|---|---|---|---|
| Cereals | |||||||||
| Highland barley | 87.65 | 2.48 | 11.11 | 2.07 | 1.46 | 31.22 | 4.00 | 0.50 | 82.89 |
| Little millet | 87.67 | 0.81 | 11.10 | 0.25 | 1.93 | 3.10 | 1.28 | 0.10 | 85.91 |
| Black rice | 87.89 | 1.55 | 8.19 | 1.39 | 2.39 | 7.62 | 1.68 | 1.09 | 86.48 |
| Mushrooms | |||||||||
| Shiitake | 88.51 | 5.48 | 21.87 | 11.56 | 1.56 | 36.48 | 17.28 | 0.56 | 59.53 |
| Jelly ear | 86.41 | 4.59 | 14.38 | 15.54 | 0.94 | ND | 21.50 | 1.47 | 64.54 |
| Test Extracts | Concentration | Incubated Bacteria | |
|---|---|---|---|
| ETEC K88ac | NF-E. coli | ||
| PBS | 6.67 ± 0.31 e | 7.57 ± 0.09 | |
| CGMP | 1% | 7.88 ± 0.03 a | 7.79 ± 0.09 |
| EHB | 2% | 7.46 ± 0.02 bc | 7.69 ± 0.09 |
| 1% | 7.48 ± 0.03 abc | 7.40 ± 0.28 | |
| EBR | 2% | 7.38 ± 0.15 bcd | 7.59 ± 0.35 |
| 1% | 7.41 ± 0.08 bcd | 7.51 ± 0.42 | |
| ELM | 2% | 7.54 ± 0.07 ab | 7.64 ± 0.35 |
| 1% | 7.44 ± 0.15 bcd | 7.58 ± 0.33 | |
| EJE | 2% | 7.07 ± 0.12 cde | 7.60 ± 0.09 |
| 1% | 7.04 ± 0.01 de | 7.53 ± 0.12 | |
| EST | 2% | 7.33 ± 0.10 bcd | 7.82 ± 0.21 |
| 1% | 7.26 ± 0.14 bcd | 7.48 ± 0.16 | |
| SEM | 0.103 | 0.199 | |
| p-Value | <0.001 | 0.332 |
| Test Extracts | Concentration | Incubated Bacteria | |
|---|---|---|---|
| ETEC K88ac | NF-E. coli | ||
| PBS | 6.96 ± 0.09 a | 6.56 ± 0.02 | |
| CGMP | 1% | 6.12 ± 0.06 e | 6.37 ± 0.09 |
| EHB | 2% | 6.42 ± 0.03 de | 6.59 ± 0.24 |
| 1% | 6.55 ± 0.05 cd | 6.48 ± 0.34 | |
| EBR | 2% | 6.68 ± 0.08 abcd | 6.52 ± 0.26 |
| 1% | 6.81 ± 0.10 abc | 6.92 ± 0.26 | |
| ELM | 2% | 6.83 ± 0.17 abc | 6.54 ± 0.19 |
| 1% | 6.95 ± 0.04 ab | 7.01 ± 0.42 | |
| EJE | 2% | 6.80 ± 0.15 abcd | 6.66 ± 0.32 |
| 1% | 6.77 ± 0.001 abcd | 6.66 ± 0.30 | |
| EST | 2% | 6.46 ± 0.11 cde | 6.22 ± 0.35 |
| 1% | 6.57 ± 0.14 bcd | 6.48 ± 0.30 | |
| SEM | 0.097 | 0.252 | |
| p-Value | <0.001 | 0.206 |
| Test Extracts | Concentration | Incubated Bacteria | |
|---|---|---|---|
| ETEC K88ac | NF-E. coli | ||
| PBS | 7.05 ± 0.02 | 6.37 ± 0.19 | |
| CGMP | 1% | 6.93 ± 0.10 | 6.26 ± 0.26 |
| EHB | 2% | 7.07 ± 0.03 | 6.25 ± 0.26 |
| 1% | 7.05 ± 0.03 | 6.33 ± 0.14 | |
| EBR | 2% | 7.00 ± 0.03 | 6.31 ± 0.09 |
| 1% | 7.07 ± 0.14 | 6.32 ± 0.07 | |
| ELM | 2% | 7.06 ± 0.09 | 6.32 ± 0.11 |
| 1% | 7.07 ± 0.06 | 6.50 ± 0.10 | |
| EJE | 2% | 6.90 ± 0.11 | 6.55 ± 0.16 |
| 1% | 6.86 ± 0.17 | 6.45 ± 0.05 | |
| EST | 2% | 7.19 ± 0.04 | 6.37 ± 0.17 |
| 1% | 7.04 ± 0.23 | 6.55 ± 0.04 | |
| SEM | 0.099 | 0.158 | |
| p-Value | 0.188 | 0.607 |
| Test Extracts | Concentration | Incubated Bacteria | |
|---|---|---|---|
| ETEC K88ac | NF-E. coli | ||
| PBS | 7.17 ± 0.01 a | 5.79 ± 0.10 b | |
| CGMP | 1% | 6.58 ± 0.04 b | 6.18 ± 0.06 ab |
| EHB | 2% | 7.33 ± 0.07 a | 6.27 ± 0.13 ab |
| 1% | 7.16 ± 0.02 a | 6.08 ± 0.29 ab | |
| EBR | 2% | 7.23 ± 0.09 a | 6.29 ± 0.30 ab |
| 1% | 7.09 ± 0.05 a | 6.28 ± 0.17 ab | |
| ELM | 2% | 7.16 ± 0.06 a | 6.35 ± 0.09 ab |
| 1% | 7.17 ± 0.06 a | 6.26 ± 0.29 ab | |
| EJE | 2% | 7.26 ± 0.32 a | 6.65 ± 0.31 a |
| 1% | 7.25 ± 0.12 a | 6.64 ± 0.05 a | |
| EST | 2% | 7.06 ± 0.13 a | 6.35 ± 0.21 ab |
| 1% | 7.11 ± 0.05 a | 6.45 ± 0.02 ab | |
| SEM | 0.104 | 0.170 | |
| p-Value | 0.002 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Shao, C.; Martín-Orúe, S.M. Screening Traditional Foods for the Prevention of Enterotoxigenic Escherichia coli K88ac (F4ac) Attachment to IPEC-J2 Cells. Foods 2024, 13, 952. https://doi.org/10.3390/foods13060952
Zhu Y, Shao C, Martín-Orúe SM. Screening Traditional Foods for the Prevention of Enterotoxigenic Escherichia coli K88ac (F4ac) Attachment to IPEC-J2 Cells. Foods. 2024; 13(6):952. https://doi.org/10.3390/foods13060952
Chicago/Turabian StyleZhu, Yanan, Changyan Shao, and Susana María Martín-Orúe. 2024. "Screening Traditional Foods for the Prevention of Enterotoxigenic Escherichia coli K88ac (F4ac) Attachment to IPEC-J2 Cells" Foods 13, no. 6: 952. https://doi.org/10.3390/foods13060952
APA StyleZhu, Y., Shao, C., & Martín-Orúe, S. M. (2024). Screening Traditional Foods for the Prevention of Enterotoxigenic Escherichia coli K88ac (F4ac) Attachment to IPEC-J2 Cells. Foods, 13(6), 952. https://doi.org/10.3390/foods13060952