Residue Degradation and Risk Assessment of Difenoconazole and Its Metabolite during Tea Growing, Processing and Brewing by Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments and Conditions
2.3. Field Experiments and Sampling
2.4. Sample Preparation, Extraction, and Purification
2.5. Method Validation
2.6. Data Analysis
3. Results and Discussion
3.1. Method Validation of Difenoconazole and Difenoconazole-Alcohol in Different Tea Samples
| Compound | Sample Matrix | Concentration Range (mg/L) | Regression Equation | r | MEs % | LOD (µg/L) |
|---|---|---|---|---|---|---|
| Difenoconazole | ACN | 0.0010~5.0 | y = 2,489,640x + 10,259 | 0.9957 | --- | 0.3 |
| Fresh tea leaves | y = 1,161,115x + 23,405 | 0.9990 | −53 | 0.3 | ||
| Green tea | y = 699,291x + 20,874 | 0.9997 | −72 | 0.3 | ||
| Black tea | y = 1,211,447x − 63,080 | 0.9913 | −51 | 0.3 | ||
| Green tea infusion | y = 861,093x − 6849 | 0.9981 | −65 | 0.07 | ||
| Black tea infusion | y = 954,850x + 68,794 | 0.9984 | −62 | 0.07 | ||
| Difenoconazole-alcohol | ACN | y = 1,197,384x − 10,872 | 0.9945 | --- | 0.3 | |
| Fresh tea leaves | y = 443,584x + 9557 | 0.9982 | −63 | 0.3 | ||
| Green tea | y = 350,102x + 14,624 | 0.9995 | −71 | 0.3 | ||
| Black tea | y = 481,152x − 29,396 | 0.9902 | −60 | 0.3 | ||
| Green tea infusion | y = 348,690x + 6386 | 0.9991 | −71 | 0.07 | ||
| Black tea infusion | y = 315,092x + 28,779 | 0.9968 | −74 | 0.07 |
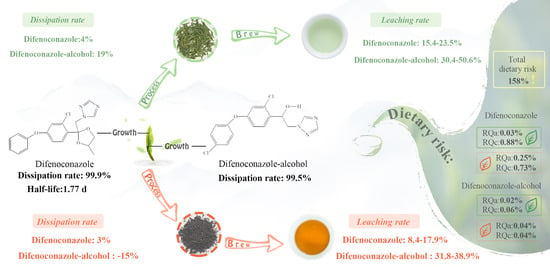
3.2. Residue Dissipation of Difenoconazole in Fresh Tea Leaves
3.3. Residue Transmission of Difenoconazole during Different Tea Processing
3.3.1. Green Tea
3.3.2. Black Tea
3.4. Leaching Rates of Difenoconazole and Difenoconazole-Alcohol from Tea
3.4.1. Black Tea
3.4.2. Green Tea
3.5. Dietary Risk Assessment of Difenoconazole and Difenoconazole-Alcohol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.Y.; Wang, D.L.; Zhang, C.Z.; Wu, C.F.; Liu, X.J. Difenoconazole residues in rice and paddy system. Chin. J. Rice Sci. 2011, 25, 339–342. [Google Scholar] [CrossRef]
- Dong, F.S.; Li, J.; Chankvetadze, B.; Cheng, Y.P.; Xu, J.; Liu, X.G.; Li, Y.B.; Chen, X.; Bertucci, C.; Tedesco, D.; et al. Chiral triazole fungicide difenoconazole: Absolute stereochemistry, stereoselective bioactivity, aquatic toxicity, and environmental behavior in vegetables and soil. Environ. Sci. Technol. 2013, 47, 3386–3394. [Google Scholar] [CrossRef] [PubMed]
- Madeira, S.A.L.; Horvath, V.B.; Vanderlei, R.M.; Aparecida, M.R.; Cristina, F.E.; Evaldo, L.G.E.; Michiel, A.D. Single and mixture toxicity of abamectin and difenoconazole to adult zebrafish (Danio rerio). Chemosphere 2017, 188, 582–587. [Google Scholar] [CrossRef]
- Wang, C.A.; Wu, R.L.; Li, Y.Y.; Qin, Y.F.; Li, Y.L.; Meng, F.Q.; Wang, L.G.; Xu, F.L. Effects of pesticide residues on bacterial community diversity and structure in typical greenhouse soils with increasing cultivation years in Northern China. Sci. Total Environ. 2020, 710, 136321. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, T.T.; Tang, J.Y.; Liu, X.L.; Liu, Y.H.; Zhong, X. The profiles of chiral pesticides in peri-urban areas near Yangtze River: Enantioselective distribution characteristics and correlations with surface sediments. J. Environ. Sci. 2022, 121, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.L.; Stenrød, M.; Wu, C.; Almvik, M.; Holten, R.; Liu, C.J.H.; Yuan, S.K.; Wu, X.H.; Xu, J.; Dong, F.S.; et al. Degradation of difenoconazole in water and soil-kinetics degradation pathways, transformation products identification and ecotoxicity assessment. J. Hazard. Mater. 2021, 418, 126303. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Y.; Bai, X.M.; Pang, X.P. Intercity variability and local factors influencing the level of pesticide residues in marketed fruits and vegetables of China. Sci. Total Environ. 2020, 700, 134481. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Fernández, D.; Moreno-González, D.; Gilbert-López, B.; García-Reyes, J.F.; Molina-Díaz, A. Worldwide survey of pesticide residues in citrus-flavored soft drinks. Food Chem. 2021, 365, 130486. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.C.; Hu, J.M.; Shi, L.; Zhang, Z.Y.; Liang, Y. Dynamics and residues of difenoconazole and chlorothalonil in leafy vegetables grown in open-field and greenhouse. J. Food Compos. Anal. 2022, 110, 104544. [Google Scholar] [CrossRef]
- Mohapatra, S. Dynamics of difenoconazole and propiconazole residues on pomegranate over 2 years under field conditions. Environ. Sci. Pollut. R 2015, 23, 5795–5806. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, B.; Dasharath, P.O.; Sangram, H.P.; Soma, D.; Pandurang, G.A. Degradation kinetics and safety evaluation of tetraconazole and difenoconazole residues in grape. Pest Manag. Sci. 2008, 64, 283–289. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Luo, F.J.; Chen, Z.M.; Liu, G.M.; Lou, Z.Y.; Wang, F.; Wu, L.C. Residue determination of epoxiconazole, indoxacarb and difenoconazole in tea, tea infusion and soil using ultra high-performance liquid chromatography coupled with tandem mass spectrometry. Chin. J. Anal. Chem. 2013, 41, 215–222. [Google Scholar] [CrossRef]
- Kong, Z.Q.; Dong, F.S.; Xu, J.; Liu, X.G.; Zhang, C.P.; Li, J.; Li, Y.B.; Chen, X.; Shan, W.L.; Zheng, Y.Q. Determination of difenoconazole residue in tomato during home canning by UPLC-MS/MS. Food Control. 2012, 23, 542–546. [Google Scholar] [CrossRef]
- Sood, C.; Jaggi, S.; Kumar, V.; Ravindranath, S.D.; Shanker, A. How manufacturing processes affect the level of pesticide residues in tea. J. Sci. Food Agric. 2014, 84, 2123–2127. [Google Scholar] [CrossRef]
- Wang, J.; Liu, F.M.; Wen, J.J.; Bian, X.L. Study on residue of difenoconazole in tea and its transfer from made tea to infusion. Chin. J. Pest. Sci. 2010, 12, 299–302. [Google Scholar]
- Wang, J.; Gong, Y.; Wen, J.J. Factors affecting infusing concentration and dissolution ratio of difenoconazole in tea infusion. Acta Agric. Boreali-Occifentalis Sin. 2011, 20, 202–206. [Google Scholar]
- Li, L. Detection on difenoconazole residue in wuyi tea. J. Shandong Agric. Univ. 2017, 48, 897–900+905. [Google Scholar] [CrossRef]
- Ma, C.; Liu, X.G.; Wu, X.H.; Dong, F.S.; Xu, J.; Zheng, Y.Q. Kinetics, mechanisms and toxicity of the degradation of imidaclothiz in soil and water. J. Hazard. Mater. 2020, 403, 124033. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, U.; Singh, S.K. Effect of handling and processing on pesticide residues in food—A review. J. Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef] [PubMed]
- NY/T 788−2018; Guideline for the Testing of Pesticide Residues in Crops. Standards of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2018.
- GB/T 23776-2018; Methodology for Sensory Evaluation of Tea. Standards of the People’s Republic of China: Beijing, China, 2018.
- SANTE/11312/2021 Version 2. (Implemented by 01.01.2024) Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. Available online: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 18 January 2024).
- Wang, M.; Li, Y.J.; Wang, X.R.; Wang, Z.H.; Luo, F.J.; Zhou, L.; Chen, Z.M.; Li, H.X.; Zhang, X.Z. Enantioselective residue dissipation of oxathiapiprolin during tea growing, processing, and brewing. ACS Agric. Sci. Technol. 2022, 2, 969–979. [Google Scholar] [CrossRef]
- Li, Y.J.; Yu, X.R.; Yu, T.; Ning, B.; Feng, G.L.; Zhao, X.P.; Zhang, C.P. Deposition and residue of difenoconazole in greenhouse celery-soil. Mod. Agrochem. 2020, 19, 37–40. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yang, T.; Qin, D.M.; Gong, Y.; Ji, Y. Determination and dynamics of difenoconazole residues in Chinese cabbage and soil. Chin. Chem. Let. 2008, 19, 969–997. [Google Scholar] [CrossRef]
- Xia, H.L.; Chen, Z.M. Studies on the quantitative evaluation of environmental factors in the degradation of pesticide on tea plant. Acta Laser Biol. Sin. 1989, 16, 125–130. [Google Scholar] [CrossRef]
- Gao, G.W.; Chen, H.P.; Liu, P.X.; Hao, Z.X.; Ma, G.C.; Chai, Y.F.; Wang, C.; Lu, C.Y. Residue pattern of polycyclic aromatic hydrocarbons during green tea manufacturing and their transfer rates during tea brewing. Food Addit. Contam. A 2017, 34, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; Chen, H.P.; Zhang, M.L.; Wang, Q.H.; Jiang, Y.; Liu, X. Dissipation pattern, processing factors, and safety evaluation for dimethoate and its metabolite (Omethoate) in tea (Camellia Sinensis). PLoS ONE 2015, 10, e138309. [Google Scholar] [CrossRef] [PubMed]
- Bonnechère, A.; Hanot, V.; Jolie, R.; Hendrickx, M.; Bragard, C.; Bedoret, T.; Loco, V.J. Processing Factors of Several Pesticides and Degradation Products in Carrots by Household and Industrial Processing. J. Food Res. 2012, 1, 68–83. [Google Scholar] [CrossRef]
- Li, H.X.; Zhong, Q.; Wang, X.R.; Luo, F.J.; Zhou, L.; Sun, H.Z.; Yang, M.; Lou, Z.Y.; Chen, Z.M.; Zhang, X.Z. The degradation and metabolism of chlorfluazuron and flonicamid in tea: A risk assessment from tea garden to cup. Sci. Total Environ. 2021, 754, 142070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, X.Z.; Chen, Z.M.; Luo, F.J.; Lou, Z.Y.; Sun, W.J. Study on the brewing behavior of two kinds of different solubility pesticide from made tea to tea infusion. J. Tea Sci. 2013, 33, 482–490. [Google Scholar]
- Wu, X.Y.; Yue, Y.D.; Huang, G.S.; Lin, W.; Tang, F.; Fan, W. Dissipation behavior of fenvalerate residue in made tea during brewing process and its maximum residues levels evaluation. Acta Laser Biol. Sin. 2007, 16, 469–474. [Google Scholar]
- Wu, X.Y.; Sheng, X.; Fan, W.; Tang, F.; Yue, Y.D. Extracting rate of three kinds of pesticide in made tea during brewing process and risk assessment on human health. J. Tea Sci. 2007, 27, 141–146. [Google Scholar]





| Matrix | Spiked Level (mg/kg) | Difenoconazole | Difenoconazole-Alcohol | ||||
|---|---|---|---|---|---|---|---|
| A.R ± SDs %, n = 6 | RSDs % | LOQs (mg/kg) | A.R ± SDs %, n = 6 | RSDs % | LOQs (mg/kg) | ||
| Fresh tea leaves | 1 | 101.9 ± 4.5 | 4.4 | 0.001 | 104.3 ± 4.9 | 4.7 | 0.001 |
| 0.1 | 102.3 ± 12.0 | 11.7 | 93.4 ± 8.5 | 9.1 | |||
| 0.01 | 92.6 ± 6.0 | 6.5 | 90.5 ± 9.4 | 10.4 | |||
| 0.001 | 92.1 ± 12.2 | 13.2 | 84.4 ± 7.3 | 8.6 | |||
| Green tea | 1 | 81.0 ± 11.5 | 14.2 | 0.001 | 75.8 ± 12.0 | 15.9 | 0.001 |
| 0.1 | 92.4 ± 7.1 | 7.7 | 83.7 ± 4.8 | 5.7 | |||
| 0.01 | 73.7 ± 6.5 | 8.8 | 71.2 ± 7.2 | 10.1 | |||
| 0.001 | 81.4 ± 5.0 | 6.2 | 84.3 ± 4.3 | 5.1 | |||
| Black tea | 1 | 98.3 ± 5.9 | 6.0 | 0.001 | 114.9 ± 17.9 | 15.6 | 0.001 |
| 0.1 | 96.7 ± 5.1 | 5.3 | 81.1 ± 8.8 | 10.9 | |||
| 0.01 | 77.6 ± 3.1 | 3.9 | 80.6 ± 5.5 | 6.8 | |||
| 0.001 | 118.6 ± 8.0 | 6.7 | 82.8 ± 6.3 | 7.1 | |||
| Green tea infusion (mg/L) | 0.2 | 87.4 ± 3.6 | 4.1 | 0.0002 | 100.5 ± 8.0 | 7.9 | 0.0002 |
| 0.02 | 83.3 ± 4.3 | 5.2 | 95.0 ± 4.1 | 4.3 | |||
| 0.002 | 96.5 ± 8.1 | 8.4 | 89.8 ± 4.5 | 5.1 | |||
| 0.0002 | 73.4 ± 8.9 | 12.2 | 88.5 ± 12.7 | 14.4 | |||
| Black tea infusion (mg/L) | 0.2 | 101.1 ± 8.7 | 8.6 | 0.0002 | 82.3 ± 12.1 | 14.7 | 0.0002 |
| 0.02 | 80.7 ± 6.7 | 8.3 | 81.2 ± 8.0 | 9.9 | |||
| 0.002 | 91.3 ± 10.1 | 11.0 | 97.5 ± 7.4 | 7.6 | |||
| 0.0002 | 88.3 ± 3.0 | 3.4 | 87.3 ± 2.5 | 2.8 | |||
| Compound | Item | Difenoconazole | Difenoconazole-Alcohol |
|---|---|---|---|
| Black tea | FI (kg) | 0.013 | 0.013 |
| STMR (mg/kg) | 2.049 | 0.055 | |
| LR (%) | 17.9 | 38.9 | |
| bw (kg) | 65 | 65 | |
| ADI (mg/kg b.w.) | 0.010 | 0.010 | |
| RQc (%) | 0.734 | 0.043 | |
| HR (mg/kg) | 20.983 | 1.455 | |
| ARfD (mg/kg b.w.) | 0.300 | 0.300 | |
| RQa (%) | 0.250 | 0.038 | |
| Green tea | FI (kg) | 0.013 | 0.013 |
| STMR (mg/kg) | 1.876 | 0.054 | |
| LR (%) | 23.5 | 50.6 | |
| bw (kg) | 65 | 65 | |
| ADI (mg/kg b.w.) | 0.010 | 0.010 | |
| RQc (%) | 0.882 | 0.055 | |
| HR (mg/kg) | 19.196 | 0.698 | |
| ARfD (mg/kg b.w.) | 0.300 | 0.300 | |
| RQa (%) | 0.300 | 0.024 |
| Food Category | Food Intake /kg | Reference MRLs mg/kg | Commodity | NEDI /mg | Acceptable Daily Intake /mg | Risk Probability /% |
|---|---|---|---|---|---|---|
| Rice and its products | 0.240 | 0.5 | Paddy | 0.120 | ADI×63 | - |
| Wheat flour and its products | 0.139 | 0.1 | Wheat | 0.014 | ||
| Dark color vegetables | 0.092 | 15 | Celery | 1.373 | ||
| Light color vegetables | 0.184 | 2 | Scallion | 0.367 | ||
| Fruit | 0.046 | 5 | Strawberry | 0.229 | ||
| Vegetable oil | 0.033 | 2 | Sesame | 0.065 | ||
| Drinks | 0.013 | 10 | Tea | 0.130 | ||
| SUM | - | - | - | 0.995 | 0.63 | 158 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Ning, Y.; Hu, Y.; Cui, X.; Luo, F.; Zhou, L.; Yu, M.; Zhang, X. Residue Degradation and Risk Assessment of Difenoconazole and Its Metabolite during Tea Growing, Processing and Brewing by Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Determination. Foods 2024, 13, 1123. https://doi.org/10.3390/foods13071123
Wang M, Ning Y, Hu Y, Cui X, Luo F, Zhou L, Yu M, Zhang X. Residue Degradation and Risk Assessment of Difenoconazole and Its Metabolite during Tea Growing, Processing and Brewing by Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Determination. Foods. 2024; 13(7):1123. https://doi.org/10.3390/foods13071123
Chicago/Turabian StyleWang, Min, Yating Ning, Yue Hu, Xinyi Cui, Fengjian Luo, Li Zhou, Miao Yu, and Xinzhong Zhang. 2024. "Residue Degradation and Risk Assessment of Difenoconazole and Its Metabolite during Tea Growing, Processing and Brewing by Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Determination" Foods 13, no. 7: 1123. https://doi.org/10.3390/foods13071123
APA StyleWang, M., Ning, Y., Hu, Y., Cui, X., Luo, F., Zhou, L., Yu, M., & Zhang, X. (2024). Residue Degradation and Risk Assessment of Difenoconazole and Its Metabolite during Tea Growing, Processing and Brewing by Ultra-Performance Liquid Chromatography–Tandem Mass Spectrometry Determination. Foods, 13(7), 1123. https://doi.org/10.3390/foods13071123