Application of Ultrasound in Food Science and Technology: A Perspective
Abstract
:Highlights
1. Introduction
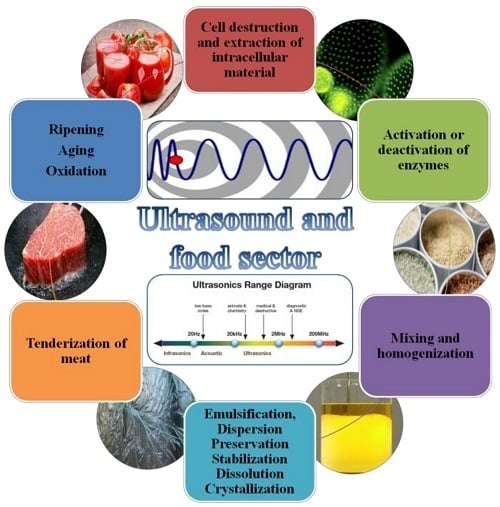
2. US in Food Science and Technology
3. US Technology in Different Processes
4. Cost Reduction and Improvements in Quality with US Technology
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheeke, J.; David, N. Fundamental and Applications of Ultrasonics, 2nd ed.; CRC Press: Boca Raton, Florida, USA, 2002. [Google Scholar]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications; Woodhead Publishing: Sawston, Cambridge, UK, 2002. [Google Scholar]
- Lempriere, B.M. Ultrasound and Elastic Waves: Frequently Asked Questions; Elsevier: San Diego, CA, USA, 2013. [Google Scholar]
- Krautkrämer, J.; Krautkrämer, H. Ultrasonic Testing of Materials; Springer Science & Business Media: Heidelberg, Germany, 2013. [Google Scholar]
- Rose, J.L. Ultrasonic Guided Waves in Solid Media; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Yasui, K. Influence of ultrasonic frequence on multibubble sonoluminescence. J. Acoust. Soc Am. 2002, 112, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Yasui, K.; Towata, A.; Tuziuti, T.; Kozuka, T.; Kato, K. Effect of static pressure on acoustic Energy radiated by cavitation bubbles in viscous liquid under ultrasound. J. Acoust. Soc. Am. 2011, 130, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Lee, H. Effect of Power Ultrasound on Food Quality. In Ultrasound Technologies for Food and Bioprocessing; Springer: New York, NY, USA, 2011. [Google Scholar]
- Ikeda, T.; Yoshizawa, S.; Koizumi, N.; Mitsuishi, M.; Matsumoto, Y. Focused ultrasound and Lithotripsy. In Therapeutic Ultrasound; Springer: Cham, Switzerland, 2016; pp. 113–129. [Google Scholar]
- Paliwal, S.; Mitragotri, S. Ultrasound-induced cavitation: Applications in drug and gene delivery. Expert Opin. Drug Deliv. 2006, 3, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Barnett, S.B.; Ter Haar, G.R.; Ziskin, M.C.; Rott, H.D.; Duck, F.A.; Maeda, K. International recommendations and guidelines for the safe use of diagnostic ultrasound in medicine. Ultrasound Med. Biol. 2000, 26, 355–366. [Google Scholar] [CrossRef]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R.S. Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gkanatsas, Y.; Palasubramaniam, J.; Hohmann, J.D.; Chen, Y.C.; Lim, B.; Hagemeyer, C.E.; Peter, K. Thrombus-targeted theranostic microbubbles: A new technology towards concurrent rapid ultrasound diagnosis and bleeding-free fibrinolytic treatment of thrombosis. Theranostics 2016, 6, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.K.; Sehgal, C.M. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med. Biol. 2015, 41, 905–928. [Google Scholar] [CrossRef] [PubMed]
- Rumack, C.M.; Levine, D. Diagnostic Ultrasound E-Book; Elsevier Health Sciences: Philadelphia, PA, USA, 2017. [Google Scholar]
- Kapoor, R.; Shome, D.; Ranjan, A. Use of a novel combined radiofrequency and ultrasound device for lipolysis, skin tightening and cellulite treatment. J. Cosmet. Laser Ther. 2017, 19, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Shirsath, S.R.; Sonawane, S.H.; Gogate, P.R. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process. Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Laborde, J.-L.; Bouyer, C.; Caltagirone, J.-P.; Gerard, A. Acoustic bubble cavitation at low frequencies. Ultrasonics 1998, 36, 589–594. [Google Scholar] [CrossRef]
- Luque de Castro, M.D.; Priego-Capote, F. Ultrasound-assisted crystallization (sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.L.K.; Hartel, R.W. Mechanisms of ice crystallization in ice cream production. Compr. Rev. Food Sci. Food Saf. 2010, 9, 213–222. [Google Scholar] [CrossRef]
- Dedhia, A.C.; Ambulgekar, P.V.; Pandit, A.B. Static foam destruction: Role of ultrasound. Ultrason. Sonochem. 2004, 11, 67–75. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Sun, D.W. Enhancement of food processes by ultrasound: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 570–594. [Google Scholar] [CrossRef] [PubMed]
- Kiani, H.; Zhang, Z.; Delgado, A.; Sun, D.W. Ultrasound assisted nucleation of some liquid and solid model foods during freezing. Food Res. Int. 2011, 44, 2915–2921. [Google Scholar] [CrossRef]
- Mason, T.J.; Riera, E.; Vercet, A.; Lopez-Buesa, P. Application of ultrasound. Emerg. Technol. Food Process. 2005, 323–351. [Google Scholar] [CrossRef]
- Riera, E.; Gallego-Juárez, J.A.; Mason, T.J. Airborne ultrasound for the precipitation of smokes and powders and the destruction of foams. Ultrason. Sonochem. 2006, 13, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Jayasooriya, S.D.; Bhandari, B.R.; Torley, P.; D’arcy, B.R. Effect of high power ultrasound waves on properties of meat: A review. Int. J. Food Prop. 2004, 7, 301–319. [Google Scholar] [CrossRef]
- Alarcon-Rojo, A.D.; Janacua, H.; Rodriguez, J.C.; Paniwnyk, L.; Mason, T.J. Power ultrasound in meat processing. Meat Sci. 2015, 107, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Chen, S.; Cao, P. Synergistic bactericidal effects and mechanisms of low intensity ultrasound and antibiotics against bacteria: A review. Ultrason. Sonochem. 2012, 19, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Oturan, M.A.; Aaron, J.J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Guo, F.; Li, P.; French, J.B.; Mao, Z.; Zhao, H.; Li, S.; Nama, N.; Fick, J.R.; Benkovic, S.J.; Huang, T.J. Controlling cell–cell interactions using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2015, 112, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Chen, S.; Dai, C.; Sun, L.; Sun, W.; Tang, Y.; Xiong, F.; He, R.; Ma, H. Effects of ultrasound on microbial growth and enzyme activity. Ultrason. Sonochem. 2017, 37, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Koubaa, M.; Mhemdi, H.; Fages, J. Recovery of valuable components and inactivating microorganisms in the agro-food industry with ultrasound-assisted supercritical fluid technology. J. Supercrit. Fluids 2018, 134, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Patist, A. Industrial applications of high power ultrasonics in the food, beverage and wine industry. In Case Studies in Novel Food Processing Technologies; Woodhead Publishing Series in Food Science; Technology and Nutrition: San Diego, CA, USA, 2010; pp. 119–138. [Google Scholar]
- Arvanitoyannis, I.S.; Kotsanopoulos, K.V.; Savva, A.G. Use of ultrasounds in the food industry–Methods and effects on quality, safety, and organoleptic characteristics of foods: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 109–128. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Paniwnyk, L. Applications of ultrasound in processing of liquid foods: A review. Ultrason. Sonochem. 2017, 38, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Kentish, S.; Feng, H. Applications of power ultrasound in food processing. Annu. Rev. Food Sci. Technol. 2014, 5, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Dolatowski, Z.J.; Stadnik, J.; Stasiak, D. Applications of ultrasound in food technology. Acta Sci. Polonorum Technol. Aliment. 2007, 6, 89–99. [Google Scholar]
- Clark, J.P. An update on ultrasonics. Food Technol. 2008, 26, 75–77. [Google Scholar]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Leong, T.S.; Martin, G.J.; Ashokkumar, M. Ultrasonic food processing. In Alternatives to Conventional Food Processing; Royal Society of Chemistry: Washington DC, USA, 2018; pp. 316–354. [Google Scholar]
- Cinelli, G.; Avino, P.; Notardonato, I.; Russo, M.V. Ultrasound-vortex-assisted dispersive liquid–liquid microextraction coupled with gas chromatography with a nitrogen–phosphorus detector for simultaneous and rapid determination of organophosphorus pesticides and triazines in wine. Anal. Methods 2014, 6, 782–790. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Perugini, L.; Notardonato, I. Extraction and GC-MS analysis of phthalate esters in food matrices: A review. RSC Adv. 2015, 5, 37023–37043. [Google Scholar] [CrossRef]
- Russo, M.V.; Avino, P.; Notardonato, I. Fast analysis of phthalates in freeze-dried baby foods by ultrasound-vortex-assisted liquid-liquid microextraction coupled with gas chromatography-ion trap/mass spectrometry. J. Chromatogr. A 2016, 1474, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Canselier, J.P.; Delmas, H.; Wilhelm, A.M.; Abismail, B. Ultrasound emulsification—An overview. J. Dispers. Sci. Technol. 2002, 23, 333–349. [Google Scholar] [CrossRef]
- Freitas, S.; Hielscher, G.; Merkle, H.; Gander, B. Continuous contact and contamination free ultrasonic emulsification—A useful tool for pharmaceutical development and production. Ultrason. Sonochem. 2006, 13, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hulbert, G.J.; Mount, J.R. Effects of ultrasound on milk homogenization and fermentation with yogurt starter. Innov. Food Sci. Emerg. Technol. 2000, 1, 211–218. [Google Scholar] [CrossRef]
- Virone, C.; Kramer, H.J.M.; van Rosmalen, G.M.; Stoop, A.H.; Bakker, T.W. Primary nucleation induced by ultrasonic cavitation. J. Cryst. Growth 2006, 1, 9–15. [Google Scholar] [CrossRef]
- Zheng, L.; Sun, D.W. Innovative applications of power ultrasound during food freezing processes—A review. Trends Food Sci. Technol. 2006, 17, 16–23. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Industrial applications of high power ultrasonics. In Ultrasound Technologies for Food and Bioprocessing; Springer: New York, NY, USA, 2011; pp. 599–616. [Google Scholar]
- Pangu, G.D.; Feke, D.L. Acoustically aided separation of oil droplets from aqueous emulsions. Chem. Eng. Sci. 2004, 59, 3183–3193. [Google Scholar] [CrossRef]
- Luján-Facundo, M.J.; Mendoza-Roca, J.A.; Cuartas-Uribe, B.; Álvarez-Blanco, S. Cleaning efficiency enhancement by ultrasounds for membranes used in dairy industries. Ultrason. Sonochem. 2016, 33, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Sfakianakis, P.M.; Tzia, C. Conventional and innovative processing of milk for yogurt manufacture, development of texture and flavor: A review. Foods 2014, 3, 176–193. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Candrapala, J.; Martin, G.J.O.; Zisu, B.; Kentish, S.E.; Ashokkumar, M. The effect of ultrasound on casein micelle integrity. J. Dairy Sci. 2012, 95, 6882–6890. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Juárez, J.A. Basic principles of ultrasound. In Ultrasound in Food Processing: Recent Advances; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Pitt, W.G.; Rodd, A. Ultrasound increases the rate of bacterial growth. Biotechnol. Progress 2003, 19, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound technology for food fermentation applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Benedito, J.; Carcel, J.A.; Sanjuan, N.; Mulet, A. Use of ultrasound to assess Cheddar cheese characteristics. Ultrasonics 2000, 38, 727–730. [Google Scholar] [CrossRef]
- Contreras, N.I.; Fairley, P.; McClements, D.J.; Povey, M.J. Analysis of the sugar content of fruit juices and drinks using ultrasonic velocity measurements. Int. J. Food Sci. Technol. 1992, 27, 515–529. [Google Scholar] [CrossRef]
- Delgado-Povedano, M.M.; de Castro, M.L. A review on enzyme and ultrasound: A controversial but fruitful relationship. Anal. Chim. Acta 2015, 889, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Villamiel, M.; de Jong, P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins and native enzymes of milk. Agric. Food Chem. 2000, 48, 472–478. [Google Scholar] [CrossRef]
- Tsukamoto, I.; Yim, B.; Stavarache, C.E.; Furuta, M.; Hashiba, K.; Maeda, Y. Inactivation of Saccharomyces cerevisiae by ultrasonic irradiation. Ultrason. Sonochem. 2004, 11, 61–65. [Google Scholar] [CrossRef]
- Jiranek, V.; Grbin, P.; Yap, A.; Barnes, M.; Bates, D. High power ultrasonics as a novel tool offering new opportunities for managing wine microbiology. Biotechnol. Lett. 2008, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Selma, M.V.; Suslow, T.; Jacxsens, L.; Uyttendaele, M.; Allende, A. Pre-and postharvest preventive measures and intervention strategies to control microbial food safety hazards of fresh leafy vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Banach, J.L.; Sampers, I.; Van Haute, S.; Van der Fels-Klerx, H.J. Effect of disinfectants on preventing the cross-contamination of pathogens in fresh produce washing water. Int. J. Environ. Res. Public Health 2015, 12, 8658–8677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-López, V.M.; Gil, M.I.; Allende, A.; Blancke, J.; Schouteten, L.; Selma, M.V. Disinfection capacity of High-Power Ultrasound against E. coli O157:H7 in process water of the fresh-cut industry. Food Bioprocess. Technol. 2014, 7, 3390–3397. [Google Scholar]
- Mizrach, A. Determination of avocado and mango fruit properties by ultrasonic technique. Ultrasonics 2000, 38, 717–722. [Google Scholar] [CrossRef]
- Mizrach, A. Ultrasonic technology for quality evaluation of fresh fruit and vegetables in pre- and postharvest processes. Postharvest Biol. Technol. 2008, 48, 315–330. [Google Scholar] [CrossRef]
- Valente, M.; Prades, A.; Laux, D. Potential use of physical measurements including ultrasound for a better mango fruit quality characterization. J. Food Eng. 2013, 116, 57–64. [Google Scholar] [CrossRef]
- Santos, J.G.; Fernandes, F.A.N.; de Siqueira Oliveira, L.; de Miranda, M.R.A. Influence of ultrasound on fresh-cut mango quality through evaluation of enzymatic and oxidative metabolism. Food Bioprocess. Technol. 2015, 8, 1532–1542. [Google Scholar] [CrossRef]
- Mulet, A.; Benedito, J.; Golas, Y.; Carcel, J.A. Non invasive ultrasonic measurements in the food industry. Food Rev. Int. 2002, 18, 123–133. [Google Scholar] [CrossRef]
- Hauptmann, P.; Hoppe, N.; Püttmer, A. Application of ultrasonic sensors in the process industry. Meas. Sci. Technol. 2002, 13, R73. [Google Scholar] [CrossRef]
- Khairi, M.T.M.; Ibrahim, S.; Yunus, M.A.M.; Faramarzi, M. Contact and non-contact ultrasonic measurement in the food industry: A review. Meas. Sci. Technol. 2015, 27, 012001. [Google Scholar] [CrossRef]
- Nikitenko, S.I.; Chemat, F. Ultrasound in process engineering. In Green Process Engineering: From Concepts to Industrial Applications; CRC Press: Boca Raton, Florida, USA, 2015; pp. 145–165. [Google Scholar]
- Schneider, Y.; Zahn, S.; Schindler, C.; Rohm, H. Ultrasonic excitation affects friction interactions between food materials and cutting tools. Ultrasonics 2009, 49, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Arnold, G.; Leiteritz, L.; Zahn, S.; Rohm, H. Ultrasonic cutting of cheese: Composition affects cutting work reduction and energy demand. Int. Dairy J. 2009, 19, 314–320. [Google Scholar] [CrossRef]
- Comandini, P.; Blanda, G.; Soto-Caballero, M.C.; Sala, V.; Tylewicz, U.; Mujica-Paz, H.; Fragoso, A.V.; Toschi, T.G. Effects of power ultrasound on immersion freezing parameters of potatoes. Innov. Food Sci. Emerg. Technol. 2013, 18, 120–125. [Google Scholar] [CrossRef]
- Carrillo-López, L.M.; Alarcon-Rojo, A.D.; Luna-Rodríguez, L.; Reyes-Villagrana, R. Modification of food systems by ultrasound. J. Food Qual. 2017, 2017, 5794931. [Google Scholar] [CrossRef]
- Kiani, H.; Sun, D.W.; Zhang, Z. The effect of ultrasound irradiation on the convective heat transfer rate during immersion cooling of a stationary sphere. Ultrason. Sonochem. 2012, 19, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Zhang, M.; Xu, B.; Adhikari, B.; Sun, J. The principles of ultrasound and its application in freezing related processes of food materials: A review. Ultrason. Sonochem. 2015, 27, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Leong, T.S.; Zhou, M.; Kukan, N.; Ashokkumar, M.; Martin, G.J. Preparation of water-in-oil-in-water emulsions by low frequency ultrasound using skim milk and sunflower oil. Food Hydrocol. 2017, 63, 685–695. [Google Scholar] [CrossRef]
- Musielak, G.; Mierzwa, D.; Kroehnke, J. Food drying enhancement by ultrasound—A review. Trends Food Sci. Technol. 2016, 56, 126–141. [Google Scholar] [CrossRef]
- Bilek, S.E.; Turantas, F. Decontamination efficiency of high power ultrasound in the fruit and vegetable industry, a review. Int. J. Food Microbiol. 2013, 166, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops-A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Passos, C.P.; Cardoso, S.M.; Wessel, D.F.; Coimbra, M.A. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 2018, 246, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Nayak, B.; Dahmoune, F.; Moussi, K.; Remini, H.; Dairi, S.; Aoun, O.; Khodir, M. Comparison of microwave, ultrasound and accelerated-assisted solvent extraction for recovery of polyphenols from Citrus sinensis peels. Food Chem. 2015, 187, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.; Conte, E.; Naviglio, D. Analysis and comparison of the antioxidant component of Portulaca oleracea leaves obtained by different solid-liquid extraction techniques. Antioxidants 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J.; Paniwnyk, L.; Chemat, F.; Abert Vian, M. Ultrasonic Food Processing. In Alternatives to Conventional Food Processing; Proctor, A., Ed.; RSC Green Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar]
- Steele, M.; Odumenu, J. Irrigation water as source of foodborne pathogens on fruit and vegetables. J. Food Prot. 2004, 67, 2839–2849. [Google Scholar] [CrossRef]
- Mason, T.J. Ultrasonic cleaning: An historical perspective. Ultrason. Sonochem. 2016, 29, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Allen, V.M.; Whyte, R.T.; Burton, C.H.; Harris, J.A.; Lovell, R.D.; Atterbury, R.J.; Tinker, D.B. Effect of ultrasonic treatment during cleaning on the microbiological condition of poultry transport crates. Br. Poultry Sci. 2008, 49, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Coles, R.; McDowell, D.; Kirwan, M.J. (Eds.) Food Packaging Technology; CRC Press: Boca Raton, FL, USA, 2003; Volume 5. [Google Scholar]
- Ščetar, M.; Kurek, M.; Jambrak, A.R.; Debeaufort, F.; Galić, K. Effect of high power ultrasound on physical–chemical properties of polypropylene films aimed for food packaging: Structure and surface features. Polym. Bull. 2018, 1–15. [Google Scholar] [CrossRef]
- Caporaso, N.; Formisano, D. Developments, applications, and trends of molecular gastronomy among food scientists and innovative chefs. Food Rev. Int. 2016, 32, 417–435. [Google Scholar] [CrossRef]
- Chandrapala, J. Low intensity ultrasound applications on food systems. Int. Food Res. J. 2015, 22, 888–895. [Google Scholar]
- Mason, T.J.; Chemat, F.; Vinatonu, M. The extraction of natural products using ultrasound or microwaves. Curr. Org. Chem. 2011, 15, 237–247. [Google Scholar] [CrossRef]
- Afshari, K.; Sarnavati, V.; Shahidi, S.A. Ultrasonic-assisted extraction and in vitro antioxidant activity of polysaccharide from Hibiscus leaf. Int. J. Biol. Macromol. 2015, 74, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ye, X.; Hao, Y.; Xu, G.; Liu, D. Ultrasound-assisted extraction of hesperidin from Penggan (Citrus reticolata). Ultrason. Sonochem. 2008, 15, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Oroian, M. Optimizzation of ultrasound-assisted extraction of total monomeric anthocyanin (TMA) and total phenolic content (TPC) from eggplant (Solanum melanogena L.) peel. Ultrason. Sonochem. 2016, 31, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Sariburun, E.; Şahin, S.; Demir, C.; Türkben, C.; Uylaşer, V. Phenolic content and antioxidant activity of raspberry and blackberry cultivars. J. Food Sci. 2010, 75, C328–C335. [Google Scholar] [CrossRef] [PubMed]
- El Darra, N.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field, ultrasound, and thermal pretreatments for better phenolic extraction during red fermentation. Eur. Food Res. Technol. 2013, 236, 47–56. [Google Scholar] [CrossRef]
- Ferraretto, P.; Cacciola, V.; Batllò Ferran, I.; Celotti, E. Ultrasounds application in winemaking: Grape maceration and yeast lysis. Ital. J. Food Sci. 2013, 25, 160–168. [Google Scholar]
- Cacciola, V.; Ferran Batllό, I.; Ferraretto, P.; Vincenzi, S.; Celotti, E. Study of the ultrasound effects on yeast lees lysis in winemaking. Eur. Food Res. Technol. 2013, 236, 311–317. [Google Scholar] [CrossRef]
- Ferraretto, P.; Celotti, E. Preliminary study of the effects of ultrasound on red wine polyphenols. CyTa J. Food 2016, 14, 529–535. [Google Scholar] [CrossRef] [Green Version]
- Morata, A.; Suárez-Lepe, J.A. New Biotechnologies for Wine Fermentation and Ageing. Advances in Food Biotechnology; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 287. [Google Scholar]
- O’donnell, C.P.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Effect of ultrasonic processing on food enzymes of industrial importance. Trends Food Sci. Technol. 2010, 21, 358–367. [Google Scholar] [CrossRef] [Green Version]
- Raso, J.; Barbosa-Cánovas, G.V. Nonthermal preservation of foods using combined processing techniques. Crit. Rev. Food Sci. Nutr. 2003, 43, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Khandpur, P.; Gogate, P.R. Effect of novel ultrasound based processing on the nutrition quality of different fruit and vegetable juices. Ultrason. Sonochem. 2015, 27, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Yap, A.; Jiranek, V.; Grbin, P.; Barnes, M.; Bates, D. Studies on the application of high power ultrasonics for barrel and plank cleaning and disinfection. Aust. NZ Wine Ind. J. 2007, 22, 96–104. [Google Scholar]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Niakousari, M.; Gahruie, H.H.; Razmjooei, M.; Roohinejad, S.; Greiner, R. Effects of innovative processing technologies on microbial targets based on food categories: Comparing traditional and emerging technologies for food preservation. In Innovative Technologies for Food Preservation; Academic Press: Cambridge, MA, USA, 2018; pp. 133–185. [Google Scholar]


| Mechanical Effects | References |
| Crystallization of fats, sugars, etc. | Luque de Castro et al., 2007 [19]; Cook and Hartel, 2010 [20] |
| Degassing and destruction of foams | Dedhia et al., 2004 [21] |
| Extraction of aromas | Chemat et al., 2017a [22] |
| Filtration and drying | Tao and Sun, 2015 [23] |
| Freezing | Kiani et al., 2011 [24] |
| Mixing and homogenization | Mason et al., 2005 [25] |
| Precipitation of airborne powders | Riera et al., 2006 [26] |
| Meat tenderization | Jayasooriya et al., 2004 [27]; Alarcon-Rojo et al., 2015 [28] |
| Chemical and Biochemical Effects | References |
| Bactericidal action | Yu et al., 2012 [29] |
| Wastewater treatment | Oturan et al., 2014 [30] |
| Modification of the growth of living cells | Guo et al., 2015 [31] |
| Alteration of enzymatic activity | Huang et al., 2017 [32] |
| Sterilization of equipment | Chemat et al., 2017b [33]; Koubaa et al., 2018 [34] |
| Food | Purpose | Reference |
|---|---|---|
| Cheese | Reduction of product losses (cut) | Schneider et al. [79]; Arnold et al., 2009 [80] |
| Potatoes | Reduction of structural damage (freezing) | Comandini et al., 2013 [81] |
| Food systems | Time saving (marinating, filtration, and oxidation) | Carrillo-López et al., 2017 [82] |
| Food materials | Reduction of heating and cooling costs (cooking and freezing) | Kiani et al., 2012 [83]; Cheng et al., 2015 [84] |
| Skim milk and sunflower oil | Shelf-life improvement (emulsification) | Leong et al., 2017 [85] |
| Food and agricultural products | Improvement of product structure (mixing and drying) | Musielak et al., 2016 [86] |
| Fruit and vegetable | Microbiological safety (anti-foaming, degassing, and sterilization) | Bilek et al., 2013 [87] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164. https://doi.org/10.3390/foods7100164
Gallo M, Ferrara L, Naviglio D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods. 2018; 7(10):164. https://doi.org/10.3390/foods7100164
Chicago/Turabian StyleGallo, Monica, Lydia Ferrara, and Daniele Naviglio. 2018. "Application of Ultrasound in Food Science and Technology: A Perspective" Foods 7, no. 10: 164. https://doi.org/10.3390/foods7100164
APA StyleGallo, M., Ferrara, L., & Naviglio, D. (2018). Application of Ultrasound in Food Science and Technology: A Perspective. Foods, 7(10), 164. https://doi.org/10.3390/foods7100164