Effect of Creatine and Glucose on Formation of Heterocyclic Amines in Grilled Chicken Breasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
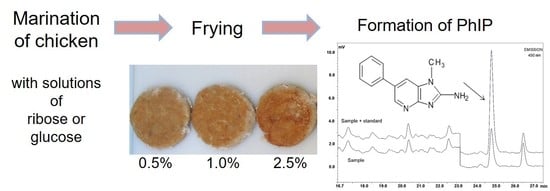
2.2. Preparation and Heating of Chicken Breasts
2.3. Preparation and Coating of Chicken Patties
2.4. Determination of Principal Components
2.5. Determination of Heterocyclic Amines
2.6. Determination of Creatine, Creatinine, and Glucose
2.7. Color Measurements and Visual Sensory Test
2.8. Statistical Analysis
3. Results and Discussions
3.1. Determination of Proximate Composition of the Raw Material
3.2. Occurrence of Heterocyclic Amines in Chicken Breasts
3.3. Influence of Precursors on Formation of Heterocyclic Amines
3.4. Influence of Coating on Formation of Heterocyclic Amines in Chicken Patties
3.5. Color Measurement and Visual Sensory Perception of Marinated and Grilled Chicken Patties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Has | Heterocyclic Aromatic Amines; |
| SD | standard deviation; |
| CV | coefficient of variation, |
| r | Pearson correlation coefficient, vs. versus, n = number of samples |
| IQ | 2-Amino-3-methylimidazo[4,5-f]quinoline (CAS No. 76180-96-6); |
| IQx | 2-Amino-3-methylimidazo[4,5-f]quinoxaline (CAS No. 108354-47-8); |
| MeIQ | 2-Amino-3,4-dimethylimidazo[4,5-f]quinoline (CAS No. 77094-11-2); |
| MeIQx | 2-Amino-3,8-dimethylimidazo[4,5-f]quinoxaline (CAS No. 77500-04-0); |
| 4,8-DiMeIQx | 2-Amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (CAS No. 95896-78-9); |
| 7,8-DiMeIQx | 2-Amino-3,7,8-trimethylimidazo[4,5-f]quinoxaline (CAS No. 92180-79-5); |
| PhIP | 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (CAS No. 105650-23-5); |
| Trp-P-1 | 3-Amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (CAS No. 62450-06-0); |
| Trp-P-2 | 3-Amino-1-methyl-5H-pyrido[4,3-b]indole (CAS No. 62450-07-1); |
| Glu-P-1 | 2-Amino-6-methyldipyrido[1,2-a:3’,2’-d]imidazole (CAS No. 67730-11-4); |
| Glu-P-2 | 2-Aminodipyrido [1,2-a:3’,2’-d]imidazole (CAS No. 67730-10-3); |
| AαC | 2-Amino-9H-pyrido[2,3-b]indole (CAS No. 26148-68-5); |
| MeAαC | 2-Amino-3-methyl-9H-pyrido[2,3-b]indole (CAS No. 68006-83-7); |
| Harman | 1-Methyl-9H-pyrido[3,4-b]indole (CAS No. 486-84-0); |
| Norharman | 9H-pyrido[3,4-b]indole (CAS No. 244-63-3); |
| n.d. | not detected |
References
- WHO-IARC. IARC Monographs Evaluate Consumption of Red Meat and Processed Meat. Available online: http://www.iarc.fr/en/media-centre/pr/2015/pdfs/pr240_E.pdf (accessed on 9 November 2015).
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Gibis, M. Heterocyclic aromatic amines in cooked meat products: Causes, formation, occurrence, and risk assessment. Compr. Rev. Food Sci. Food Saf. 2016, 15, 269–303. [Google Scholar] [CrossRef]
- Alaejos, M.S.; Afonso, A.M. Factors that affect the content of heterocyclic aromatic amines in foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 52–108. [Google Scholar] [CrossRef]
- Adamson, R.H.; Thorgeirsson, U.P.; Sugimura, T. Extrapolation of heterocyclic amine carcinogenesis data from rodents and nonhuman primates to humans. Arch. Toxicol. 1996, 18, 303–318. [Google Scholar]
- Gu, D.; Turesky, R.J.; Tao, Y.; Langouet, S.A.; Nauwelaers, G.C.; Yuan, J.-M.; Yee, D.; Yu, M.C. DNA adducts of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 4-aminobiphenyl are infrequently detected in human mammary tissue by liquid chromatography/tandem mass spectrometry. Carcinogenesis 2012, 33, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Turesky, R.J. Formation and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicol. Lett. 2007, 168, 219–227. [Google Scholar] [CrossRef]
- OEHHA. Proposition 65, Status Report, Safe Harbor Levels: No Significant Risk Levels for Carcinogens and Maximum Allowable Dose Levels for Chemicals Causing Reproductive Toxicity. Proposition 65 list; OEHHA (California Environmental Protection Agency: Office of Environmental Health Hazard Assessment): Sacramento, CA, USA, 2019; pp. 1–10. Available online: https://oehha.ca.gov/media/downloads/proposition-65//p65list062819.pdf (accessed on 27 May 2019).
- Skog, K.I.; Johansson, M.A.E.; Jägerstad, M.I. Carcinogenic heterocyclic amines in model systems and cooked foods: A review on formation, occurrence and intake. Food Chem. Toxicol. 1998, 36, 879–896. [Google Scholar] [CrossRef]
- Skog, K.; Steineck, G.; Augustsson, K.; Jägerstad, M. Effect of cooking temperature on the formation of heterocyclic amines in fried meat products and pan residues. Carcinogenesis 1995, 16, 861–867. [Google Scholar] [CrossRef]
- Gross, G.A.; Grueter, A. Quantitation of mutagenic/carcinogenic heterocyclic aromatic amines in food products. J. Chromatogr. A. 1992, 592, 271–278. [Google Scholar] [CrossRef]
- Skog, K.; Solyakov, A. Heterocyclic amines in poultry products: A literature review. Food Chem. Toxicol. 2002, 40, 1213–1221. [Google Scholar] [CrossRef]
- Gibis, M.; Weiss, J. Impact of precursors creatine, creatinine and glucose on the formation of heterocyclic aromatic amines in grilled patties of various animal species. J. Food Sci. 2015. [Google Scholar] [CrossRef]
- Liao, G.Z.; Wang, G.Y.; Xu, X.L.; Zhou, G.H. Effect of cooking methods on the formation of heterocyclic aromatic amines in chicken and duck breast. Meat Sci. 2010, 85, 149–154. [Google Scholar] [CrossRef]
- Zöchling, S.; Murkovic, M. Formation of the heterocyclic aromatic amine PhIP: Identification of precursors and intermediates. Food Chem. 2002, 79, 125–134. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef] [PubMed]
- Purchas, R.W.; Rutherfurd, S.M.; Pearce, P.D.; Vather, R.; Wilkinson, B.H.P. Concentrations in beef and lamb of taurine, carnosine, coenzyme Q10, and creatine. Meat Sci. 2004, 66, 629–637. [Google Scholar] [CrossRef]
- Skog, K.; Jägerstad, M. Effects of monosaccharides and disaccharides on the formation of food mutagens in model systems. Mutat. Res. 1990, 230, 263–272. [Google Scholar] [CrossRef]
- Gibis, M.; Weiss, J. Antioxidant capacity and inhibitory effect of grape seed and rosemary extract in marinades on the formation of heterocyclic amines in fried beef patties. Food Chem. 2012, 134, 766–774. [Google Scholar] [CrossRef]
- BVL. Amtliche Sammlung von Untersuchungsverfahren nach § 64 LFGB, vorläufiges Tabakgesetz, §28b GenTG—Verfahren zur Probennahme und Untersuchung von Lebensmitteln, Bundesamt für Verbraucherschutz und Lebensmittelsicherheit (BVL); Beuth Verlag: Berlin, Germany, 2011. [Google Scholar]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Gibis, M. Effect of oil marinades with garlic, onion, and lemon juice on the formation of heterocyclic aromatic amines in fried beef patties. J. Agric. Food Chem. 2007, 55, 10240–10247. [Google Scholar] [CrossRef]
- Gibis, M. Optimized HPLC method for analysis of polar and nonpolar heterocyclic amines in cooked meat products. J. AOAC Int. 2009, 92, 715–724. [Google Scholar]
- Wahlefeld, A.W.; Holz, G.; Bergmeyer, H.U. Creatinine. In Methoden der Enzymatischen Analyse, 3rd ed.; Bergmeyer, H.U., Ed.; Verlag Chemie GmbH: Weinheim, Germany, 1974; Volume 2, pp. 1834–1838. [Google Scholar]
- Muñoz, A.M.; Civille, G.V.; Carr, B.T. “In/Out” Method. In Sensory Evaluation in Quality Control; Springer: Boston, MA, USA, 1992; pp. 140–167. [Google Scholar] [CrossRef]
- Gašperlin, L.; Lukan, B.; Žlender, B.; Polak, T. Effects of skin and grilling method on formation of heterocyclic amines in chicken pectoralis superficialis muscle. LWT Food Sci. Technol. 2009, 42, 1313–1319. [Google Scholar] [CrossRef]
- Liu, A.; Nishimura, T.; Takahashi, K. Relationship between structural properties of intramuscular connective tissue and toughness of various chicken skeletal muscles. Meat Sci. 1996, 43, 43–49. [Google Scholar] [CrossRef]
- Pfau, W.; Skog, K. Exposure to b-carbolines norharman and harman. J. Chromatogr. B 2004, 802, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Borgen, E.; Solyakov, A.; Skog, K. Effects of precursor composition and water on the formation of heterocyclic amines in meat model systems. Food Chem. 2001, 74, 11–19. [Google Scholar] [CrossRef]
- Skog, K.; Jägerstad, M. Effects of glucose on the formation of PhIP in a model system. Carcinogenesis 1991, 12, 2297–2300. [Google Scholar] [CrossRef] [PubMed]
- Jägerstad, M.; Skog, K.; Grivas, S.; Olsson, K. Formation of heterocyclic amines using model systems. Mutat. Res. Genet. Toxicol. 1991, 259, 219–233. [Google Scholar] [CrossRef]
- Arvidsson, P.; Van Boekel, M.A.J.S.; Skog, K.; Jagerstad, M. Kinetics of formation of polar heterocyclic amines in a meat model system. J. Food Sci. 1997, 62, 911–916. [Google Scholar] [CrossRef]
- Zamora, R.; Alcón, E.; Hidalgo, F.J. Effect of lipid oxidation products on the formation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in model systems. Food Chem. 2012, 135, 2569–2574. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Yahagi, T.; Nagao, M.; Sugimura, T. Comutagenic effect of norharman with aminopyridine derivatives. Mutat. Res. 1982, 105, 205–210. [Google Scholar] [CrossRef]
- Ziegenhagen, R.; Boczek, P.; Viell, B. Formation of the comutagenic beta-carboline norharman in a simple tryptophan-containing model system at low temperature (40 DegC-80 DegC). Adv. Exp. Med. Biol. 1999, 467, 693–696. [Google Scholar]
- Aliani, M.; Farmer, L.J.; Kennedy, J.T.; Moss, B.W.; Gordon, A. Post-slaughter changes in ATP metabolites, reducing and phosphorylated sugars in chicken meat. Meat Sci. 2013, 94, 55–62. [Google Scholar] [CrossRef]
- Aliani, M.; Farmer, L.J. Postcolumn derivatization method for determination of reducing and phosphorylated sugars in chicken by high performance liquid chromatography. J. Agric. Food Chem. 2002, 50, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Hasnol, N.D.S.; Jinap, S.; Sanny, M. Effect of different types of sugars in a marinating formulation on the formation of heterocyclic amines in grilled chicken. Food Chem. 2014, 145, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Manabe, S.; Kurihara, N.; Wada, O.; Tohyama, K.; Aramaki, T. Formation of PhIP in a mixture of creatinine, phenylalanine and sugar or aldehyde by aqueous heating. Carcinogenesis 1992, 13, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.-E.; Shin, H.-S. Inhibition of mutagenic 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) formation using various food ingredients in a model systems. Food Sci. Biotechnol. 2013, 22, 323–329. [Google Scholar] [CrossRef]
- Shin, H.S.; Strasburg, G.M.; Ustunol, Z. Influence of different unifloral honeys on heterocyclic aromatic amine formation and overall mutagenicity in fried ground-beef patties. J. Food Sci. 2003, 68, 810–815. [Google Scholar] [CrossRef]
- Han, Z.; Liu, B.; Niu, Z.; Zhang, Y.; Gao, J.; Shi, L.; Wang, S.; Wang, S. Role of α-dicarbonyl compounds in the inhibition effect of reducing sugars on the formation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine. J. Agric. Food Chem. 2017, 65, 10084–10092. [Google Scholar] [CrossRef]
- Frandrup-Kuhr, O. Einfluss der Reaktionswege der Maillard-Reaktion von Pentosen auf die Bildung heterocyclischer aromatischer Amine. Ph.D. Thesis, University of Münster, Münster, Germany, 2004. [Google Scholar]
- Eskin, N.A.M.; Ho, C.-T.; Shahidi, F. Chapter 6—Browning Reactions in Foods. In Biochemistry of Foods, 3rd ed.; Eskin, N.A.M., Shahidi, F., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 245–289. [Google Scholar] [CrossRef]




| Analysis | Mean ± SD a (g/100 g) | CV b (%) |
|---|---|---|
| Protein | 23.9 ± 0.25 | 1.0 |
| Moisture | 74.2 ± 0.58 | 0.8 |
| Lipids | 1.57 ± 0.12 | 7.6 |
| Minerals (ash) | 1.18 ± 0.04 | 3.3 |
| Connective tissue protein | 0.51 ± 0.05 | 9.8 |
| Glucose | 0.035 ± 0.016 | 44.6 |
| Total creatine | 0.45 ± 0.039 | 8.6 |
| Creatinine | 0.021 ± 0.01 | 4.8 |
| HAs | Minimal Level (ng/g) | Maximal Level (ng/g) | Mean ± SD a (ng/g) | CV b (%) |
|---|---|---|---|---|
| PhIP | 1.49 | 9.05 | 3.52 ± 2.10 | 59.5 |
| MeIQx | n.d. c | 1.12 | 0.27 ± 0.23 | 83.3 |
| Norharman | 0.74 | 2.29 | 1.46 ± 0.44 | 30.3 |
| Harman | 0.55 | 2.16 | 1.33 ± 0.40 | 30.1 |
| Saccharide | Concentration (%) | PhIP a (ng/g) | MeIQx (ng/g) b | Norharman a (ng/g) | Harman a (ng/g) |
|---|---|---|---|---|---|
| Ribose | 0 | 1.74 ± 0.42 a | n.d. | 0.22 ± 0.01 a | 0.10 ± 0.01 a |
| 0.5 | 1.15 ± 0.11 a,b | n.d. | 0.22 ± 0.01 a | 0.10 ± 0.01 a | |
| 1 | 1.26 ± 0.31 a,b | n.d. | 0.23 ± 0.01 a | 0.39 ± 0.01 b | |
| 2.5 | 1.07 ± 0.25 b | n.d. | 0.32 ± 0.03 b | 2.96 ± 0.03 c | |
| 5 | 1.44 ± 0.35 a,b | n.d. | 0.39 ± 0.02 c | 2.52 ± 0.01 d | |
| 10 | 1.06 ± 0.12 b | n.d. | 0.72 ± 0.03 d | 5.48 ± 0.02 e | |
| 20 | 1.19 ± 0.08 a,b | n.d. | 4.81 ± 0.89 e | 16.76 ± 0.46 f | |
| Glucose | 0 | 1.58 ± 0.13 a | n.d. | 0.30 ± 0.01 a | 0.69 ± 0.01 a |
| 0.5 | 1.55 ± 0.17 a | n.d. | 0.22 ± 0.01 b | 1.00 ± 0.20 b | |
| 1 | 0.97 ± 0.06 b | n.d. | 0.30 ± 0.01 a | 1.04 ± 0.05 b | |
| 2.5 | 0.89 ± 0.22 b | 0.20 ± 0.01 | 0.81 ± 0.03 c | 0.66 ± 0.01 a | |
| 5 | 1.24 ± 0.12 c | 0.22 ± 0.04 | 0.81 ± 0.03 c | 0.17 ± 0.01 c | |
| 10 | 0.89 ± 0.06 b | n.d. | 1.33 ± 0.02 d | 0.20 ± 0.03 c,d | |
| 20 | 0.86 ± 0.05 b | n.d. | 2.06 ± 0.01 e | 0.22 ± 0.01 d |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gibis, M.; Loeffler, M. Effect of Creatine and Glucose on Formation of Heterocyclic Amines in Grilled Chicken Breasts. Foods 2019, 8, 616. https://doi.org/10.3390/foods8120616
Gibis M, Loeffler M. Effect of Creatine and Glucose on Formation of Heterocyclic Amines in Grilled Chicken Breasts. Foods. 2019; 8(12):616. https://doi.org/10.3390/foods8120616
Chicago/Turabian StyleGibis, Monika, and Myriam Loeffler. 2019. "Effect of Creatine and Glucose on Formation of Heterocyclic Amines in Grilled Chicken Breasts" Foods 8, no. 12: 616. https://doi.org/10.3390/foods8120616
APA StyleGibis, M., & Loeffler, M. (2019). Effect of Creatine and Glucose on Formation of Heterocyclic Amines in Grilled Chicken Breasts. Foods, 8(12), 616. https://doi.org/10.3390/foods8120616