Heat- and Ultrasound-Assisted Aqueous Extraction of Soluble Carbohydrates and Phenolics from Carob Kibbles of Variable Size and Source Material
Abstract
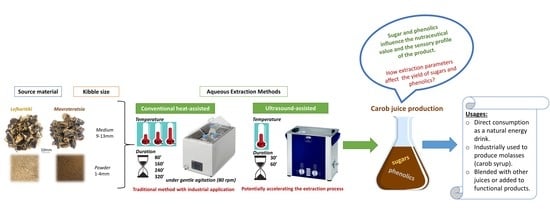
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Carob Juice Extraction
2.3. Total Phenolics Content
2.4. Soluble Carbohydrates Content
2.5. Reagents and Standards
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nasar-Abbas, S.M.; Huma, Z.; Vu, T.-H.; Khan, M.K.; Esbenshade, H.; Jayasena, V. Carob Kibble: A Bioactive-Rich Food Ingredient. Compr. Rev. Food Sci. Food Saf. 2016, 15, 63–72. [Google Scholar] [CrossRef] [Green Version]
- FAOSTAT Food and Agriculture Organization of the United Nations, Final 2018 Data. Available online: http://faostat.fao.org/site/567/DesktopDefault.aspx#ancor (accessed on 6 May 2020).
- Wursch, P.; Del Vedovo, S.; Rosset, J.; Smiley, M. The tannin granules from ripe carob pod. Lebensm. Wiss. Technol. 1984, 17, 351–354. [Google Scholar]
- Bouzouita, N.; Khaldi, A.; Zgoulli, S.; Chebil, L.; Chekki, R.; Chaabouni, M.; Thonart, P. The analysis of crude and purified locust bean gum: A comparison of samples from different carob tree populations in Tunisia. Food Chem. 2007, 101, 1508–1515. [Google Scholar] [CrossRef]
- Kotrotsios, N.; Christaki, E.; Bonos, E.; Florou-Paneri, P. Carobs in productive animal nutrition. J. Hellen. Vet. Med. Soc. 2017, 62, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Yousif, A.K.; Alghzawi, H. Processing and characterization of carob powder. Food Chem. 2000, 69, 283–287. [Google Scholar] [CrossRef]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional Components of Carob Fruit: Linking the Chemical and Biological Space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef]
- Youssef, M.; Kamal, E.; El-Manfaloty, A.M.; Moshera, M.; Ali, H.M. Assessment of proximate chemical composition, nutritional status, fatty acid composition and phenolic compounds of carob (Ceratonia siliqua L.). Food Pub. Health 2013, 3, 304–308. [Google Scholar]
- Zunft, H.J.F.; Lüder, W.; Harde, A.; Haber, B.; Graubaum, H.J.; Koebnick, C.; Grünwald, J. Carob pulp preparation rich in insoluble fibre lowers total and LDL cholesterol in hypercholesterolemic patients. Eur. J. Nutr. 2003, 42, 235–242. [Google Scholar] [CrossRef]
- Theophilou, I.C.; Neophytou, C.M.; Constantinou, A.I. Carob and its Components in the Management of Gastrointestinal Disorders. J. Hepatol Gastroenterol 2017, 1, 005. [Google Scholar]
- Khlifa, M.; Bahloul, A.; Kitane, S. Determination of chemical composition of carob pod (Ceratonia siliqua L.) and its morphological study. J. Mater. Environ. Sci. 2013, 4, 348–353. [Google Scholar]
- Roseiro, J.C.; Gírio, F.M.; Collaço, M.A. Yield improvements in carob sugar extraction. Process. Biochem. 1991, 26, 179–182. [Google Scholar] [CrossRef]
- Rababah, T.M.; Al-u’datt, M.; Ereifej, K.; Almajwal, A.; Al-Mahasneh, M.; Brewer, S.; Alsheyab, F.; Yang, W. Chemical, functional and sensory properties of carob juice. J. Food Qual. 2013, 36, 238–244. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.W.; Haubner, R.; Hull, W.E.; Erben, G.; Spiegelhalder, B.; Bartsch, H.; Haber, B. Isolation and structure elucidation of the major individual polyphenols in carob fibre. Food Chem.Toxicol. 2003, 41, 1727–1738. [Google Scholar] [CrossRef]
- Tounsi, L.; Karra, S.; Kechaou, H.; Kechaou, N. Processing, physico-chemical and functional properties of carob molasses and powders. J. Food Meas. Charact. 2017, 11, 1440–1448. [Google Scholar] [CrossRef]
- Tounsi, L.; Ghazala, I.; Kechaou, N. Physicochemical and phytochemical properties of Tunisian carob molasses. J. Food Meas. Charact. 2019, 14, 20–30. [Google Scholar] [CrossRef]
- Atasoy, A.F. The effects of carob juice concentrates on the properties of yoghurt. Int. J. Dairy Technol. 2009, 62, 228–233. [Google Scholar] [CrossRef]
- Kumazawa, S.; Taniguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, M.-S.; Nakayama, T. Antioxidant activity of polyphenols in carob pods. J. Agric. Food Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef]
- El Bouzdoudi, B.; El Ansari, Z.N.; Mangalagiu, I.; Mantu, D.; Badoc, A.; Lamarti, A. Determination of Polyphenols Content in Carob Pulp from Wild and Domesticated Moroccan Trees. Am. J. Plant Sci. 2016, 7, 1937–1951. [Google Scholar] [CrossRef] [Green Version]
- Rtibi, K.; Selmi, S.; Grami, D.; Amri, M.; Eto, B.; El-Benna, J.; Sebai, H.; Marzouki, L. Chemical constituents and pharmacological actions of carob pods and leaves (Ceratonia siliqua L.) on the gastrointestinal tract: A review. Biomed. Pharmacother. 2017, 93, 522–528. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Almanasrah, M.; Roseiro, L.B.; Bogel-Lukasik, R.; Carvalheiro, F.; Brazinha, C.; Crespo, J.; Kallioinen, M.; Mänttäri, M.; Duarte, L.C. Selective recovery of phenolic compounds and carbohydrates from carob kibbles using water-based extraction. Ind. Crops Prod. 2015, 70, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Kyratzis, A.; Antoniou, C.; Papayiannis, L.C.; Graziani, G.; Rouphael, Y.; Kyriacou, M.C. Carob pod morphology and physicochemical composition of indigenous Cyprus germplasm are modulated by genotype, agro-environmental zone and growing season. 2020; to be submitted. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Kyriacou, M.C.; Emmanouilidou, M.G.; Soteriou, G.A. Asynchronous ripening behavior of cactus pear (Opuntia ficus-indica) cultivars with respect to physicochemical and physiological attributes. Food Chem. 2016, 211, 598–607. [Google Scholar] [CrossRef]
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Papagiannopoulos, M.; Wollseifen, H.R.; Mellenthin, A.; Haber, B.; Galensa, R. Identification and quantification of polyphenols in Carob Fruits (Ceratonia siliqua L.) and derived products by HPLC-UV-ESI/MS. J. Agric. Food Chem. 2004, 52, 3784–3791. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Fidan, H.; Petkova, N.; Sapoundzhieva, T.; Abanoz, E.I. Carbohydrate Content in Bulgarian and Turkish Carob Pods and Their Products. CBU Int. Conf. Proc. 2016, 4, 796–802. [Google Scholar] [CrossRef] [Green Version]
- Custodio, L.; Fernandes, E.; Escapa, A.L.; Fajardo, A.; Aligue, R.; Albericio, F.; Neng, N.R.; Nogueira, J.M.; Romano, A. Antioxidant and cytotoxic activities of carob tree fruit pulps are strongly influenced by gender and cultivar. J. Agric. Food Chem. 2011, 59, 7005–7012. [Google Scholar] [CrossRef]
- Biner, B.; Gubbuk, H.; Karhan, M.; Aksu, M.; Pekmezci, M. Sugar profiles of the pods of cultivated and wild types of carob bean (Ceratonia siliqua L.) in Turkey. Food Chem. 2007, 100, 1453–1455. [Google Scholar] [CrossRef]
- Nasrabadi, M.; Ramezanian, A.; Eshghi, S.; Kamgar-Haghighi, A.A.; Vazifeshenas, M.R.; Valero, D. Biochemical changes and winter hardiness in pomegranate (Punica granatum L.) trees grown under deficit irrigation. Sci. Hortic. 2019, 251, 39–47. [Google Scholar] [CrossRef]
- Tavarini, S.; Gil, M.I.; Tomas-Barberan, F.A.; Buendia, B.; Remorini, D.; Massai, R.; Degl’Innocenti, E.; Guidi, L. Effects of water stress and rootstocks on fruit phenolic composition and physical/chemical quality in Suncrest peach. Ann. Appl. Biol. 2011, 158, 226–233. [Google Scholar] [CrossRef]
- Turhan, I.; Tetik, N.; Aksu, M.; Karhan, M.; Certel, M. Liquid–solid extraction of soluble solids and total phenolic compounds of carob bean (Ceratonia siliqua L.). J. Food Process. Eng. 2006, 29, 498–507. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Duarte, L.C.; Oliveira, D.L.; Roque, R.; Bernardo-Gil, M.G.; Martins, A.I.; Sepúlveda, C.; Almeida, J.; Meireles, M.; Gírio, F.M.; et al. Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: Effect on the phenolic profile and antiproliferative activity. Ind. Crops Prod. 2013, 47, 132–138. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Torun, H.; Ayaz, S.; Correia, P.J.; Alaiz, M.; Sanz, C.; Gruz, J.; Strnad, M. Determination of chemical composition of anatolian carob pod (Ceratonia siliqua L.): Sugars, amino and organic acids, minerals and phenolic compounds. J. Food Qual. 2007, 30, 1040–1055. [Google Scholar] [CrossRef]


| Source of Variation | Fructose | Glucose | Sucrose | Total Sugars | Phenolics |
|---|---|---|---|---|---|
| (g 100 mL−1) | (g 100 mL−1) | (g 100 mL−1) | (g 100 mL−1) | (mg L−1 GAE) | |
| Source material | Means | ||||
| LF | 2.6b | 1.7b | 11.3a | 15.6a | 2595a |
| MV | 3.4a | 2.5a | 8.2b | 14.0b | 715b |
| Kibble size | |||||
| M | 3.0a | 2.1 | 10.4a | 15.5a | 1573b |
| P | 2.9b | 2.1 | 9.1b | 14.1b | 1737a |
| Temperature | |||||
| 25 | 2.8b | 2.1ab | 9.7 | 14.5b | 830c |
| 50 | 3.1a | 2.10a | 9.8 | 15.0a | 1341b |
| 75 | 3.0a | 2.0b | 9.9 | 14.9ab | 2844a |
| Time | |||||
| 80 | 2.7b | 1.8b | 9.2c | 13.8b | 1127d |
| 160 | 2.9b | 1.9b | 10.6a | 15.4a | 1608c |
| 240 | 3.1a | 2.2a | 9.8b | 15.0a | 1840b |
| 320 | 3.2a | 2.3a | 9.5bc | 15.0a | 2148a |
| Percentage of Variance | |||||
| Source materials | 42.5 *** | 50.3 *** | 39.9 *** | 12.9 *** | 37.4 *** |
| Kibble size | 0.8 * | 0 | 6.3 *** | 9.6 *** | 0.3 ** |
| Temperature | 4.0 *** | 1.0 * | 0.1 | 0.8 | 30.0 *** |
| Time | 13.8 *** | 9.6 *** | 4.5 *** | 7.8 *** | 5.3 *** |
| Source of Variation | Fructose | Glucose | Sucrose | Total Sugars | Phenolics |
|---|---|---|---|---|---|
| (g 100 mL−1) | (g 100 mL−1) | (g 100 mL−1) | (g 100 mL−1) | (mg L−1 GAE) | |
| Source material | Means | ||||
| LF | 1.8b | 1.4b | 8.8a | 12.0 | 1338.8a |
| MV | 2.5a | 1.9a | 7.1b | 11.5 | 379.9b |
| Kibble size | |||||
| M | 1.9b | 1.6 | 6.5b | 10.0b | 523.9b |
| P | 2.5a | 1.7 | 9.3a | 13.6a | 1194.9a |
| Time | |||||
| 30 | 2.1 | 1.7 | 7.6b | 11.4 | 775.7b |
| 60 | 2.3 | 1.7 | 8.2a | 12.1 | 943.1a |
| Percentage of Variance (%) | |||||
| Source material | 40.1 *** | 52.0 *** | 18.7 *** | 1.1 | 53.8 *** |
| Kibble size | 33.2 *** | 4.6 | 48.2 *** | 59.7 *** | 26.3 *** |
| Time | 2.3 | 0.0 | 2.1 * | 2.6 | 1.6 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniou, C.; Kyratzis, A.; Rouphael, Y.; Stylianou, S.; Kyriacou, M.C. Heat- and Ultrasound-Assisted Aqueous Extraction of Soluble Carbohydrates and Phenolics from Carob Kibbles of Variable Size and Source Material. Foods 2020, 9, 1364. https://doi.org/10.3390/foods9101364
Antoniou C, Kyratzis A, Rouphael Y, Stylianou S, Kyriacou MC. Heat- and Ultrasound-Assisted Aqueous Extraction of Soluble Carbohydrates and Phenolics from Carob Kibbles of Variable Size and Source Material. Foods. 2020; 9(10):1364. https://doi.org/10.3390/foods9101364
Chicago/Turabian StyleAntoniou, Chrystalla, Angelos Kyratzis, Youssef Rouphael, Stelios Stylianou, and Marios C. Kyriacou. 2020. "Heat- and Ultrasound-Assisted Aqueous Extraction of Soluble Carbohydrates and Phenolics from Carob Kibbles of Variable Size and Source Material" Foods 9, no. 10: 1364. https://doi.org/10.3390/foods9101364
APA StyleAntoniou, C., Kyratzis, A., Rouphael, Y., Stylianou, S., & Kyriacou, M. C. (2020). Heat- and Ultrasound-Assisted Aqueous Extraction of Soluble Carbohydrates and Phenolics from Carob Kibbles of Variable Size and Source Material. Foods, 9(10), 1364. https://doi.org/10.3390/foods9101364