Non-Viable Lactobacillus johnsonii JNU3402 Protects against Diet-Induced Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of NV-LJ3402
2.2. Animals
2.3. Cell Culture and Transfection
2.4. Plasma and Tissue Analyses
2.5. Analyses of Mitochondrial DNA and Citrate Synthase Activity
2.6. RNA Isolation, Reverse Transcription, and RT-qPCR
2.7. Yogurt Fermentation
2.8. Viable Cells and pH Measurements
2.9. Statistical Analysis
3. Results
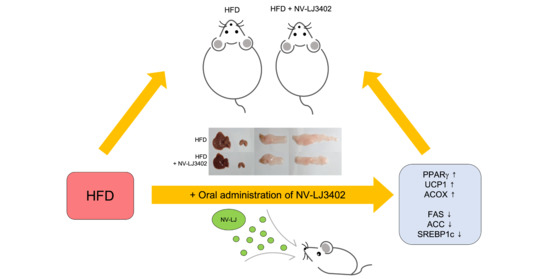
3.1. Effect of NV-LJ3402 on HFD-Induced Body Weight Gain and Adiposity in Mice
3.2. Effect of NV-LJ3402 on the Expression of Metabolic Genes in the WAT of HFD Mice and 3T3-L1 Adipocytes
3.3. NV-LJ3402 Regulates the Expression of Metabolic Genes by Promoting PPARγ Transcriptional Activity
3.4. NV-LJ3402 Administration Enhances Mitochondrial Levels and Function in WATs Along with an Increase in Body Temperature in HFD Mice
3.5. Applicability of NV-LJ3042
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, D.K.; Renuka; Puniya, M.; Shandilya, U.K.; Dhewa, T.; Kumar, N.; Kumar, S.; Puniya, A.K.; Shukla, P. Gut Microbiota Modulation and Its Relationship with Obesity Using Prebiotic Fibers and Probiotics: A Review. Front. Microbiol. 2017, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Lee, Y.J.; Song, S.; Kim, B.; Kang, H.; Oh, S.; Kim, E. Lactobacillus acidophilus NS1 attenuates diet-induced obesity and fatty liver. J. Endocrinol. 2018, 237, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Lee, Y.J.; Kang, H.; Yang, G.; Hong, E.J.; Lim, J.Y.; Oh, S.; Kim, E. Lactobacillus amylovorus KU4 ameliorates diet-induced obesity in mice by promoting adipose browning through PPARgamma signaling. Sci. Rep. 2019, 9, 20152. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food. In Proceedings of the Food and Agriculture Organization of the United Nations and World Health Organization Working Group Report, London, ON, Canada, 30 April–1 May 2002; pp. 1–11. [Google Scholar]
- Villena, J.; Barbieri, N.; Salva, S.; Herrera, M.; Alvarez, S. Enhanced immune response to pneumococcal infection in malnourished mice nasally treated with heat-killed Lactobacillus casei. Microbiol. Immunol. 2009, 53, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Pique, N.; Berlanga, M.; Minana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, R. Microbiology handbook. Dairy products; Leatherhead Pub. and Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Buhnik-Rosenblau, K.; Matsko-Efimov, V.; Jung, M.; Shin, H.; Danin-Poleg, Y.; Kashi, Y. Indication for Co-evolution of Lactobacillus johnsonii with its hosts. BMC Microbiol. 2012, 12, 149. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, W.; Lucey, K.; Jang, S.; Fujimura, K.E.; Rasky, A.; Ting, H.A.; Petersen, J.; Johnson, C.C.; Boushey, H.A.; Zoratti, E.; et al. Lactobacillus johnsonii supplementation attenuates respiratory viral infection via metabolic reprogramming and immune cell modulation. Mucosal Immunol. 2017, 10, 1569–1580. [Google Scholar] [CrossRef]
- Fukushima, Y.; Miyaguchi, S.; Yamano, T.; Kaburagi, T.; Iino, H.; Ushida, K.; Sato, K. Improvement of nutritional status and incidence of infection in hospitalised, enterally fed elderly by feeding of fermented milk containing probiotic Lactobacillus johnsonii La1 (NCC533). Br. J. Nutr. 2007, 98, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Kim, S.J.; Park, S.S.; Chang, C.; Kim, E. TR4 activates FATP1 gene expression to promote lipid accumulation in 3T3-L1 adipocytes. FEBS Lett. 2011, 585, 2763–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.S.; Choi, H.; Kim, S.J.; Chang, C.; Kim, E. CREB/GSK-3beta signaling pathway regulates the expression of TR4 orphan nuclear receptor gene. Mol. Cell. Endocrinol. 2016, 423, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Choi, H.; Kim, S.J.; Kim, O.J.; Chae, K.S.; Kim, E. FXRalpha down-regulates LXRalpha signaling at the CETP promoter via a common element. Mol. Cells 2008, 26, 409–414. [Google Scholar] [PubMed]
- Soukoulis, C.; Panagiotidis, P.; Koureli, R.; Tzia, C. Industrial yogurt manufacture: Monitoring of fermentation process and improvement of final product quality. J. Dairy Sci. 2007, 90, 2641–2654. [Google Scholar] [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Carmiel-Haggai, M.; Cederbaum, A.I.; Nieto, N. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J. 2005, 19, 136–138. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Depommier, C.; Van Hul, M.; Everard, A.; Delzenne, N.M.; De Vos, W.M.; Cani, P.D. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes 2020, 11, 1231–1245. [Google Scholar] [CrossRef] [Green Version]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.C.; Liu, J.R. Effect of Lactobacillus rhamnosus GG on Energy Metabolism, Leptin Resistance, and Gut Microbiota in Mice with Diet-Induced Obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Tsai, W.H.; Jheng, Y.P.; Su, S.L.; Wang, S.Y.; Lin, C.C.; Chen, Y.H.; Chang, W.W. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: A randomized, double-blinded, placebo-controlled trial. Sci. Rep. 2018, 8, 16791. [Google Scholar] [CrossRef]




| Gene | 5′—Sense Primer—3′ | 5′—Antisense Primer—3′ |
|---|---|---|
| FAS | AGATCCTGGAACGAGAACACGAT | GAGACGTGTCACTCCTGGACTTG |
| Acc | GTATGTTCGAAGGGCTTACATTG | TGGGCAGCATGAACTGAAATT |
| SREBP1c | ACTGTGACCTCACAGGTCCA | GGCAGTTTGTCTGTGTCCACA |
| ACOX | TCGAGGCTTGGAAACCACTG | TCGAGTGATGAGCTGAGCC |
| CPT1 | ACTCCTGGAAGAAGAAGTTC | TAGGGTCCGATTGATCTTTG |
| PGC1α | GAGACTTTGGAGGCCAGCA | CGCCATCCCTTAGTTCACTGG |
| UCP1 | GGAGGTGTGGCAGTGTTC | TCTGTGGTGGCTATAACTCTG |
| PPARγ | GAAGACCACTCGCATTCCTT | GAAGGTTCTTCATGAGGCCTG |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT |
| 36B4 | AGATGCAGCAGATCCGCAT | ATATGAGGCAGCAGTTTCTCCAG |
| D-loop | AATCTACCATCCTCCGTG | GACTAATGATTCTTCACCGT |
| GAPDH | GTTGTCTCCTGCGACTTCA | GGTGGTCCAGGGTTTCTTA |
| Initial Fermentation | Final Fermentation | |
|---|---|---|
| (Means ± SD) | (Means ± SD) | |
| Control | 6.49 | 4.5 (4 h 31 min) * |
| pH | 5.23 × 106 ± 0.98 | 1.68 × 109 ± 1.06 |
| S. thermophilus L. bulgaricus subsp. bulgaricus | 4.83 × 106 ± 1.71 | 9.17 × 108 ± 0.65 |
| Non-viable L. johnsonii JNU3402 (108) pH | 6.52 | 4.5 (4 h 27 min) * |
| S. thermophilus | 5.07 × 106 ± 1.11 | 1.71 × 109 ± 0.82 |
| L. bulgaricus subsp. bulgaricus | 4.65 × 106 ± 1.62 | 9.26 × 108 ± 2.95 |
| Non-viable L. johnsonii JNU3402 (109) | 6.54 | 4.5 (4 h 24 min) * |
| pH | 5.16 × 106 ± 1.18 | 1.37 × 109 ± 0.65 |
| S. thermophilus L. bulgaricus subsp. bulgaricus | 4.37 × 106 ± 0.95 | 1.19 × 109 ± 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Hong, E.; Oh, S.; Kim, E. Non-Viable Lactobacillus johnsonii JNU3402 Protects against Diet-Induced Obesity. Foods 2020, 9, 1494. https://doi.org/10.3390/foods9101494
Yang G, Hong E, Oh S, Kim E. Non-Viable Lactobacillus johnsonii JNU3402 Protects against Diet-Induced Obesity. Foods. 2020; 9(10):1494. https://doi.org/10.3390/foods9101494
Chicago/Turabian StyleYang, Garam, Eunjeong Hong, Sejong Oh, and Eungseok Kim. 2020. "Non-Viable Lactobacillus johnsonii JNU3402 Protects against Diet-Induced Obesity" Foods 9, no. 10: 1494. https://doi.org/10.3390/foods9101494
APA StyleYang, G., Hong, E., Oh, S., & Kim, E. (2020). Non-Viable Lactobacillus johnsonii JNU3402 Protects against Diet-Induced Obesity. Foods, 9(10), 1494. https://doi.org/10.3390/foods9101494