Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism
Abstract
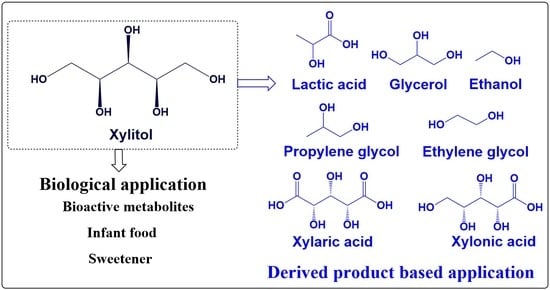
:1. Introduction
2. Xylitol Production
2.1. First Generation (Phyto-Extraction)
2.2. Second Generation (Catalytic Reduction)
2.3. Third Generation (Microbial Fermentation and Enzymatic Transformation)
2.4. Fourth Generation (Photo-Autotrophic Microbes)
3. Enhancement in Production
3.1. Strain Improvement
3.2. Alternate Substrates
4. Downstream Processing
4.1. Using Activated Charcoal and Ion Exchange Resins
4.2. Biphasic Extraction
4.3. Using Membrane Technology
5. Application of Xylitol
5.1. Food Industry
5.2. Pharmaceutical Industry
5.2.1. Oral Hygiene and Dental Caries
5.2.2. Respiratory Tract Infection
5.2.3. Acute Otitis Media
5.2.4. Hemolytic Anemia
5.2.5. Anti-Cancerous and Anti-Inflammatory Activity
5.2.6. Cardiovascular Diseases and Lipid Metabolism
5.2.7. Osteoporosis
5.3. Application in Other Industries
5.3.1. Personal Care
5.3.2. Biopolymer Synthesis and Tissue Regeneration
6. Side Effects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vishal, A.; Aashima, S.; Ranju Kumari, R.; Vaishali, S.; Nidhi, R.; Arvind Kumar, B. In-Vitro and In-Silico Characterization of Xylose Reductase from Emericella nidulans. Curr. Chem. Biol. 2019, 13, 159–170. [Google Scholar] [CrossRef]
- Ahuja, V.; Ranju, K.; Rathour, R.K.; Bhatia, B.K. Microbial Utilization of Municipal Solid Waste (Msw) For The Production Of Xylitol: A Highly Valuable Product. Life Sci. Int. Res. J. 2017, 4, 56–59. [Google Scholar]
- Barclay, A.; Sandall, P.; Shwide-Slavin, C.; Brand-Miller, J. The Ultimate Guide to Sugars and Sweeteners: Discover the Taste, Use, Nutrition, Science, and Lore of Everything from Agave Nectar to Xylitol; The Experiment: New York, NY, USA, 2014. [Google Scholar]
- Dasgupta, D.; Bandhu, S.; Adhikari, D.K.; Ghosh, D. Challenges and prospects of xylitol production with whole cell bio-catalysis: A review. Microbiol. Res. 2017, 197, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Salli, K.; Lehtinen, M.J.; Tiihonen, K.; Ouwehand, A.C. Xylitols Health Benefits beyond Dental Health: A Comprehensive Review. Nutrients 2019, 11, 1813. [Google Scholar] [CrossRef] [Green Version]
- Aliakbarian, B.; de Faveri, D.; Perego, P.; Converti, A. An Assessment on Xylitol Recovery Methods. In D-Xylitol: Fermentative Production, Application and Commercialization; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Grembecka, M. Sugar Alcohols. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 265–275. [Google Scholar] [CrossRef]
- Makinen, K.K.; Soderling, E. A Quantitative Study Of Mannitol, Sorbitol, Xylitol, And Xylose In Wild Berries And Commercial Fruits. J. Food Sci. 1980, 45, 367–371. [Google Scholar] [CrossRef]
- Meilany, D.; Kresnowati, M.T.; Setiadi, T.; Boopathy, R. Optimization of Xylose Recovery in Oil Palm Empty Fruit Bunches for Xylitol Production. Appl. Sci. 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- Albuquerque, T.L.; da Silva, I.J.; de Macedo, G.R.; Rocha, M.V.P. Biotechnological production of xylitol from lignocellulosic wastes: A review. Process Biochem. 2014, 49, 1779–1789. [Google Scholar] [CrossRef]
- Hernandez-Mejia, C.; Gnanakumar, E.S.; Olivos-Suarez, A.; Gascon, J.; Greer, H.F.; Zhou, W.; Rothenberg, G.; Raveendran Shiju, N. Ru/TiO2-catalysed hydrogenation of xylose: The role of the crystal structure of the support. Catal. Sci. Technol. 2016, 6, 577–582. [Google Scholar] [CrossRef] [Green Version]
- Morales, R.; Campos, C.H.; Fierro, J.L.G.; Fraga, M.A.; Pecchi, G. Stable reduced Ni catalysts for xylose hydrogenation in aqueous medium. Catal. Today 2018, 310, 59–67. [Google Scholar] [CrossRef]
- Audemar, M.; Ramdani, W.; Junhui, T.; Raluca Ifrim, A.; Ungureanu, A.; Jerome, F.; Royer, S.; de Oliveira Vigier, K. Selective Hydrogenation of Xylose to Xylitol over Co/SiO2 Catalysts. ChemCatChem 2020, 12, 1973–1978. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, S.K.; Seo, J.H. Recent Advances for Microbial Production of Xylitol. In Bioprocessing of Renewable Resources to Commodity Bioproducts; Bisaria, V.S., Kondo, A., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 497–518. [Google Scholar] [CrossRef]
- Belal, E. Optimization of xylitol production from rice straw by isolated strain of Aspergillus niger. In Proceedings of the 1st Mansoura International Food Congress (MIFC 2014), Mansoura, Egypt, 17–21 November 2014; pp. 36–54. [Google Scholar]
- Dorantes-Landa, D.N.; Cocotle-Ronzón, Y.; Morales-Cabrera, M.A.; Hernández-Martínez, E. Modeling of the xylitol production from sugarcane bagasse by immobilized cells. J. Chem. Technol. Biotechnol. 2020, 95, 1936–1945. [Google Scholar] [CrossRef]
- Rodrigues, R.C.L.B.; Lu, C.; Lin, B.; Jeffries, T.W. Fermentation Kinetics for Xylitol Production by a Pichia stipitisd-Xylulokinase Mutant Previously Grown in Spent Sulfite Liquor. Appl. Biochem. Biotechnol. 2008, 148, 199–209. [Google Scholar] [CrossRef]
- Mukherji, R.; Joshi-Navare, K.; Prabhune, A. Crystalline Xylitol Production by a Novel Yeast, Pichia caribbica (HQ222812), and Its Application for Quorum Sensing Inhibition in Gram-Negative Marker Strain Chromobacterium violaceum CV026. Appl. Biochem. Biotechnol. 2013, 169, 1753–1763. [Google Scholar] [CrossRef]
- Silva, D.D.V.; Dussán, K.J.; Idarraga, A.; Grangeiro, L.; Silva, S.S.; Cardona, C.A.; Quintero, J.; Felipe, M.G.A. Production and purification of xylitol by Scheffersomyces amazonenses via sugarcane hemicellulosic hydrolysate. Biofuels Bioprod. Biorefining 2020, 14, 344–356. [Google Scholar] [CrossRef]
- de Albuquerque, T.L.; Gomes, S.D.L.; Marques, J.E., Jr.; Silva, I.J., Jr.; Rocha, M.V.P. Xylitol production from cashew apple bagasse by Kluyveromyces marxianus CCA510. Catal. Today 2015, 255, 33–40. [Google Scholar] [CrossRef]
- Yoshitake, J.; Ishizaki, H.; Shimamura, M.; Imai, T. Xylitol Production by an Enterobacter Species. Agric. Biol. Chem. 1973, 37, 2261–2267. [Google Scholar] [CrossRef]
- Rangaswamy, S.; Agblevor, F. Screening of facultative anaerobic bacteria utilizing D-xylose for xylitol production. Appl. Microbiol. Biotechnol. 2002, 60, 88–93. [Google Scholar] [CrossRef]
- Xiong, L.; Maki, M.; Guo, Z.; Mao, C.; Qin, W. Agave Biomass is Excellent for Production of Bioethanol and Xylitol Using Bacillus Strain 65S3 and Pseudomonas Strain CDS3. J. Biobased Mater. Bioenergy 2014, 8, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Wang, B.; Lv, J.; Jiang, M.; Lin, S.; Deng, Z. Xylitol production from xylose mother liquor: A novel strategy that combines the use of recombinant Bacillus subtilis and Candida maltosa. Microb. Cell Factories 2011, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Abd Rahman, N.H.; Jahim, J.; Abdul Munaim, M.S.; Rahman, R.A.; Fuzi, S.F.Z.; Illias, R. Immobilization of recombinant Escherichia coli on multi-walled carbon nanotubes for xylitol production. Enzym. Microb. Technol. 2020, 135, 109495. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jiang, Z.-H.; Chen, S.; Qin, W. Microbial and bioconversion production of D-xylitol and its detection and application. Int. J. Biol. Sci. 2010, 6, 834–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao Kang, T.; Mohammad, S.H.; Abd Murad, A.M.; Illias, R.; Jahim, J.M. Fermentative Production of Xylitol: A First Trial on Xylose Bifurcation. Indian J. Sci. Technol. 2016, 9, 1–5. [Google Scholar] [CrossRef]
- Dashtban, M.; Kepka, G.; Seiboth, B.; Qin, W. Xylitol Production by Genetically Engineered Trichoderma reesei Strains Using Barley Straw as Feedstock. Appl. Biochem. Biotechnol. 2013, 169, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Puri, A.K.; Wang, Z.; Singh, S.; Permaul, K. A unique xylose reductase from Thermomyces lanuginosus: Effect of lignocellulosic substrates and inhibitors and applicability in lignocellulosic bioconversion. Bioresour. Technol. 2019, 281, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Atzmuller, D.; Ullmann, N.; Zwirzitz, A. Identification of genes involved in xylose metabolism of Meyerozyma guilliermondii and their genetic engineering for increased xylitol production. Amb. Express 2020, 10, 78. [Google Scholar] [CrossRef]
- Sampaio, F.B.C.; Torre, P.; Passos, F.V.M.L.; Perego, P.; Passos, F.J.V.; Converti, A. Xylose Metabolism in Debaryomyces hansenii UFV-170. Effect of the Specific Oxygen Uptake Rate. Biotechnol. Prog. 2004, 20, 1641–1650. [Google Scholar] [CrossRef]
- Pappu, S.M.J.; Gummadi, S.N. Effect of cosubstrate on xylitol production by Debaryomyces nepalensis NCYC 3413: A cybernetic modelling approach. Process Biochem. 2018, 69, 12–21. [Google Scholar] [CrossRef]
- Mareczky, Z.; Fehér, A.; Fehér, C.; Barta, Z.; Réczey, K. Effects of pH and Aeration Conditions on Xylitol Production by Candida and Hansenula Yeasts. Period. Polytech. Chem. Eng. 2016, 60, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.-X.; Xie, C.-Y.; Xia, Z.-Y.; Wu, Y.-J.; Li, B.; Tang, Y.-Q. The effect of xylose reductase genes on xylitol production by industrial Saccharomyces cerevisiae in fermentation of glucose and xylose. Process Biochem. 2020, 95, 122–130. [Google Scholar] [CrossRef]
- Reshamwala, S.M.S.; Lali, A.M. Exploiting the NADPH pool for xylitol production using recombinant Saccharomyces cerevisiae. Biotechnol. Prog. 2020, 36, e2972. [Google Scholar] [CrossRef]
- da Silva, E.G.; Borges, A.S.; Maione, N.R.; Castiglioni, G.L.; Suarez, C.A.G.; Montano, I.D.C. Fermentation of hemicellulose liquor from Brewer’s spent grain using Scheffersomyces stipitis and Pachysolen tannophilus for production of 2G ethanol and xylitol. Biofuels Bioprod. Biorefining 2020, 14, 127–137. [Google Scholar] [CrossRef]
- Sanchez, S.; Bravo, V.; Moya, A.J.; Castro, E.; Camacho, F. Influence of temperature on the fermentation of d-xylose by Pachysolen tannophilus to produce ethanol and xylitol. Process Biochem. 2004, 39, 673–679. [Google Scholar] [CrossRef]
- Li, Z.; Qu, H.; Li, C.; Zhou, X. Direct and efficient xylitol production from xylan by Saccharomyces cerevisiae through transcriptional level and fermentation processing optimizations. Bioresour. Technol. 2013, 149, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, D.; Kurmi, A.K.; Adhikari, D.K.; Ghosh, D. Xylitol production from lignocellulosic pentosans using Kluyveromyces marxianus: Kinetic modelling of yeast growth and fermentation. Biofuels 2020, 11, 309–319. [Google Scholar] [CrossRef]
- Fan, E.S.; Lu, K.W.; Wen, R.C.; Shen, C.R. Photosynthetic Reduction of Xylose to Xylitol Using Cyanobacteria. Biotechnol. J. 2020, 15, 1900354. [Google Scholar] [CrossRef]
- Pourmir, A.; Noor-Mohammadi, S.; Johannes, T.W. Production of xylitol by recombinant microalgae. J. Biotechnol. 2013, 165, 178–183. [Google Scholar] [CrossRef]
- Carneiro, C.V.G.C.; Silva, F.V.C.; Almeida, J.R.M. Xylitol Production: Identification and Comparison of New Producing Yeasts. Microorganisms 2019, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijland, J.G.; Driessen, A.J.M. Engineering of Pentose Transport in Saccharomyces cerevisiae for Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 7. [Google Scholar] [CrossRef]
- Jang, S.H.; Kang, H.Y.; Kim, G.-J.; Seo, J.; Ryu, Y.W. Complete In vitro conversion of D-xylose to xylitol by coupling xylose reductase and formate dehydrogenase. J. Microbiol. Biotechnol. 2003, 13, 501–508. [Google Scholar]
- Marie, K.W.; Hussein Fadhil, K.; Khalida, A.S. Production of Xylitol from Agricultural Waste by Enzymatic Methods. Am. J. Agric. Biol. Sci. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- McEwen, J.T.; Machado, I.M.P.; Connor, M.R.; Atsumi, S. Engineering Synechococcus elongatus PCC 7942 for Continuous Growth under Diurnal Conditions. Appl. Environ. Microbiol. 2013, 79, 1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.-C.; Xiong, W.; Paddock, T.; Carrieri, D.; Chang, I.-F.; Chiu, H.-F.; Ungerer, J.; Hank Juo, S.-H.; Maness, P.-C.; Yu, J. Engineered xylose utilization enhances bio-products productivity in the cyanobacterium Synechocystis sp. PCC 6803. Metab. Eng. 2015, 30, 179–189. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Yu, X.; Li, T.; Xiong, X.; Chen, S. Induction of D-xylose uptake and expression of NAD(P)H-linked xylose reductase and NADP linked xylitol dehydrogenase in the oleaginous microalga Chlorella sorokiniana. Biotechnol. Biofuels 2014, 7, 125. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, M.; Inui, M.; Yukawa, H. Microorganisms for Xylitol Production: Focus on Strain Improvement. In D-Xylitol: Fermentative Production, Application and Commercialization; da Silva, S.S., Chandel, A.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Pal, S.; Choudhary, V.; Kumar, A.; Biswas, D.; Mondal, A.K.; Sahoo, D.K. Studies on xylitol production by metabolic pathway engineered Debaryomyces hansenii. Bioresour. Technol. 2013, 147, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, H.; Magocha, T.A.; An, Y.; Yun, J.; Yang, M.; Xue, Y.; Liang, S.; Sun, W.; Cao, Z. Improved xylitol production by expressing a novel D-arabitol dehydrogenase from isolated Gluconobacter sp. JX-05 and co-biotransformation of whole cells. Bioresour. Technol. 2017, 235, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-H.; Zhu, J.-F.; Yun, J.-H.; Lin, J.; Qi, Y.-L.; Guo, Q.; Xu, H. Enhanced xylitol production: Expression of xylitol dehydrogenase from Gluconobacter oxydans and mixed culture of resting cell. J. Biosci. Bioeng. 2016, 122, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, H.; Yun, J.; Yang, M.; Zhang, G.; Xue, Y.; Bai, X.; Qi, X.; Liu, Y.; Ran, L. Biosynthesis of Xylitol from Glucose: Microorganism, Key Enzymes and Genetically Engineered Strains. Am. J. Biosci. Bioeng. 2017, 5, 109–112. [Google Scholar] [CrossRef]
- Kogje, A.B.; Ghosalkar, A. Xylitol production by genetically modified industrial strain of Saccharomyces cerevisiae using glycerol as co-substrate. J. Ind. Microbiol. Biotechnol. 2017, 44, 961–971. [Google Scholar] [CrossRef]
- Guirimand, G.; Inokuma, K.; Bamba, T.; Matsuda, M.; Morita, K.; Sasaki, K.; Ogino, C.; Berrin, J.-G.; Hasunuma, T.; Kondo, A. Cell-surface display technology and metabolic engineering of Saccharomyces cerevisiae for enhancing xylitol production from woody biomass. Green Chem. 2019, 21, 1795–1808. [Google Scholar] [CrossRef]
- Hoshida, H.; Akada, R. High-Temperature Bioethanol Fermentation by Conventional and Nonconventional Yeasts. In Biotechnology of Yeasts and Filamentous Fungi; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Bhuvaneshwari, S.; Hettiarachchi, H.; Meegoda, J.N. Crop Residue Burning in India: Policy Challenges and Potential Solutions. Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef] [Green Version]
- Huda, A.S.N.; Mekhilef, S.; Ahsan, A. Biomass energy in Bangladesh: Current status and prospects. Renew Sustain. Energy Rev. 2014, 30, 504–517. [Google Scholar] [CrossRef]
- Ji, L.-Q. An assessment of agricultural residue resources for liquid biofuel production in China. Renew. Sustain. Energy Rev. 2015, 44, 561–575. [Google Scholar] [CrossRef]
- Kashif, M.; Awan, M.B.; Nawaz, S.; Amjad, M.; Talib, B.; Farooq, M.; Nizami, A.S.; Rehan, M. Untapped renewable energy potential of crop residues in Pakistan: Challenges and future directions. J. Environ. Manag. 2020, 256, 109924. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Leitao, V.; Gottschalk, L.M.F.; Ferrara, M.A.; Nepomuceno, A.L.; Molinari, H.B.C.; Bon, E.P.S. Biomass Residues in Brazil: Availability and Potential Uses. Waste Biomass Valorization 2010, 1, 65–76. [Google Scholar] [CrossRef]
- Neh, A. Agricultural Waste Management System [AWMS] in Malaysia. Open Access J. Waste Manag. Xenobiotics 2020, 3. [Google Scholar] [CrossRef]
- Japhet, J.A.; Luka, B.S.; Maren, I.B.; Datau, S.G. The potential of wood and agricultural waste for pellet fuel development in nigeria—A technical review. Int. J. Eng. Appl. Sci. Technol. 2020, 4, 598–607. [Google Scholar]
- Ahuja, V.; Bhatt, A.K. Trichoderma viride (MTCC 800): A potential candidate for agri-horti waste utilization by solid state fermentation. Int. J. Environ. Sci. Technol. 2018, 15, 2679–2684. [Google Scholar] [CrossRef]
- Joshi, R.; Ahmed, S. Status and challenges of municipal solid waste management in India: A review. Cogent Environ. Sci. 2016, 2. [Google Scholar] [CrossRef]
- Marriott, P.E.; Gamez, L.D.; McQueen-Mason, S.J. Unlocking the potential of lignocellulosic biomass through plant science. N. Phytol. 2016, 209, 1366–1381. [Google Scholar] [CrossRef] [Green Version]
- Putro, J.N.; Soetaredjo, F.E.; Lin, S.-Y.; Ju, Y.-H.; Ismadji, S. Pretreatment and conversion of lignocellulose biomass into valuable chemicals. Rsc. Adv. 2016, 6, 46834–46852. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.D.; de Vries, H.; Luque, R. Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production. Rsc. Adv. 2014, 4, 48109–48127. [Google Scholar] [CrossRef]
- Dell’Omo, P.P.; Spena, V.A. Mechanical pretreatment of lignocellulosic biomass to improve biogas production: Comparison of results for giant reed and wheat straw. Energy 2020, 203, 117798. [Google Scholar] [CrossRef]
- Betiku, E.; Adetunji, O.A.; Ojumu, T.V.; Solomon, B.O. A comparative study of the hydrolysis of gamma irradiated lignocelluloses. Braz. J. Chem. Eng. 2009, 26, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Guo, L.; Wang, L.; Zhan, W.; Zhou, H. Irradiation pretreatment facilitates the achievement of high total sugars concentration from lignocellulose biomass. Bioresour. Technol. 2017, 232, 270–277. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Rabemanolontsoa, H.; Saka, S. Various pretreatments of lignocellulosics. Bioresour. Technol. 2016, 199, 83–91. [Google Scholar] [CrossRef]
- Bichot, A.; Lerosty, M.; Radoiu, M.; Mchin, V.; Bernet, N.; Delgenes, J.-P.; Garcia-Bernet, D. Decoupling thermal and non-thermal effects of the microwaves for lignocellulosic biomass pretreatment. Energy Convers. Manag. 2020, 203, 112220. [Google Scholar] [CrossRef]
- Camargo, D.; Sydney, E.B.; Leonel, L.V.; Pintro, T.C.; Sene, L. Dilute acid hydrolysis of sweet sorghum bagasse and fermentability of the hemicellulosic hydrolysate. Braz. J. Chem. Eng. 2019, 36, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Badiei, M.; Asim, N.; Jahim, J.M.; Sopian, K. Comparison of Chemical Pretreatment Methods for Cellulosic Biomass. Apcbee Procedia 2014, 9, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Balan, V.; Bals, B.; Chundawat, S.P.S.; Marshall, D.; Dale, B.E. Lignocellulosic Biomass Pretreatment Using AFEX. In Biofuels: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2009. [Google Scholar]
- Sharma, S.; Nandal, P.; Arora, A. Ethanol Production from NaOH Pretreated Rice Straw: A Cost Effective Option to Manage Rice Crop Residue. Waste Biomass Valorization 2019, 10, 3427–3434. [Google Scholar] [CrossRef]
- Arora, A.; Nandal, P.; Singh, J.; Verma, M.L. Nanobiotechnological advancements in lignocellulosic biomass pretreatment. Mater. Sci. Energy Technol. 2020, 3, 308–318. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.U.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. Amb Express 2017, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Sankaran, R.; Parra Cruz, R.A.; Pakalapati, H.; Show, P.L.; Ling, T.C.; Chen, W.-H.; Tao, Y. Recent advances in the pretreatment of microalgal and lignocellulosic biomass: A comprehensive review. Bioresour. Technol. 2020, 298, 122476. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Enzyme Immobilization on Chitin and Chitosan-Based Supports for Biotechnological Applications. In Sustainable Agriculture Reviews 35: Chitin and Chitosan: History, Fundamentals and Innovations; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ. Chem. Lett. 2020, 18, 315–323. [Google Scholar] [CrossRef]
- Cavka, A.; Jonsson, L.J. Detoxification of lignocellulosic hydrolysates using sodium borohydride. Bioresour. Technol. 2013, 136, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Martinez, E.A.; Canettieri, E.V.; Bispo, J.A.C.; Giulietti, M.; de Almeida e Silva, J.B.; Converti, A. Strategies for Xylitol Purification and Crystallization: A Review. Sep. Sci. Technol. 2015, 50, 2087–2098. [Google Scholar] [CrossRef] [Green Version]
- Mun, L.W.; Rafiqul, I.S.M.; Sakinah, A.M.M.; Zularisam, A.W. Purification of bioxylitol by liquid–liquid extraction from enzymatic reaction mixture. Sep. Sci. Technol. 2016, 51, 2369–2377. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.F.D.; Suzuki, E.Y.; Ferreira, A.S.; Oliveira, M.G.; Silva, S.l.S.D.; Raposo, N.D.R.B. In vitro inhibition of adhesion of Escherichia coli strains by Xylitol. Braz. Arch. Biol. Technol. 2011, 54, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Faneer, K.A.; Rohani, R.; Mohammad, A.W.; Ba-Abbad, M.M. Evaluation of the operating parameters for the separation of xylitol from a mixed sugar solution by using a polyethersulfone nanofiltration membrane. Korean J. Chem. Eng. 2017, 34, 2944–2957. [Google Scholar] [CrossRef]
- Gede Wenten, R.D.M.T.A.P.K.I. Membrane-Based Downstream Processing of Microbial Xylitol Production. Int. J. Technol. 2017, 8, 291–319. [Google Scholar] [CrossRef]
- Mussatto, S.I. Application of Xylitol in Food Formulations and Benefits for Health. In D-Xylitol: Fermentative Production, Application and Commercialization; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Sadowska-Rociek, A.; Cieslik, E.; Sieja, K. Mitigation role of erythritol and xylitol in the formation of 3-monochloropropane-1,2-diol and its esters in glycerol and shortbread model systems. Eur. Food Res. Technol. 2015, 243, 2055–2063. [Google Scholar] [CrossRef] [Green Version]
- Ur-Rehman, S.; Mushtaq, Z.; Zahoor, T.; Jamil, A.; Murtaza, M.A. Xylitol: A Review on Bioproduction, Application, Health Benefits, and Related Safety Issues. Crit. Rev. Food Sci. Nutr. 2015, 55, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, N.A.; Madar, A. Artificial sweeteners versus natural sweeteners. Bull. Transilv. Univ. Brasov Ser. V Econ. Sci. 2014, 7, 59–64. [Google Scholar]
- Ruiz-Ojeda, F.J.; Plaza-Diaz, J.; Saez-Lara, M.J.; Gil, A. Effects of Sweeteners on the Gut Microbiota: A Review of Experimental Studies and Clinical Trials. Adv. Nutr. 2019, 10, S31–S48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touster, O. The Uronic Acid Pathway and its Defect in Essential Pentosuria; Springer: Berlin/Heidelberg, Germany, 1969; pp. 79–96. [Google Scholar]
- Lang, K. Xylit, Stoffwechsel und klinische Verwendung. Klin. Wochenschr. 1971, 49, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Makinen, K.K. Long-term tolerance of healthy human subjects to high amounts of xylitol and fructose: General and biochemical findings. Int. Z Vitam. Ernahr. Beih. 1976, 15, 92–104. [Google Scholar]
- Ylikahri, R.H.; Leino, T. Metabolic interactions of xylitol and ethanol in healthy males. Metabolism 1979, 28, 25–29. [Google Scholar] [CrossRef]
- Islam, M.S.; Indrajit, M. Effects of Xylitol on Blood Glucose, Glucose Tolerance, Serum Insulin and Lipid Profile in a Type 2 Diabetes Model of Rats. Ann. Nutr. Metab. 2012, 61, 57–64. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Islam, M.S. Effects of xylitol on carbohydrate digesting enzymes activity, intestinal glucose absorption and muscle glucose uptake: A multi-mode study. Food Funct. 2015, 6, 955–962. [Google Scholar] [CrossRef]
- Wolnerhanssen, B.K.; Meyer-Gerspach, A.C.; Beglinger, C.; Islam, M.S. Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1986–1998. [Google Scholar] [CrossRef]
- Gargouri, W.; Zmantar, T.; Kammoun, R.; Kechaou, N.; Ghoul-Mazgar, S. Coupling xylitol with remineralizing agents improves tooth protection against demineralization but reduces antibiofilm effect. Microb. Pathog. 2018, 123, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Goli, J.K.; Panda, S.H.; Linga, V.R. Molecular Mechanism of d-Xylitol Production in Yeasts: Focus on Molecular Transportation, Catabolic Sensing and Stress Response. In D-Xylitol: Fermentative Production, Application and Commercialization; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Janakiram, C.; Deepan Kumar, C.; Joseph, J. Xylitol in preventing dental caries: A systematic review and meta-analyses. J. Nat. Sci. Biol. Med. 2017, 8, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marttinen, A.M.; Ruas-Madiedo, P.; Hidalgo-Cantabrana, C.; Saari, M.A.; Ihalin, R.A.; Soderling, E.M. Effects of Xylitol on Xylitol-Sensitive Versus Xylitol-Resistant Streptococcus mutans Strains in a Three-Species in Vitro Biofilm. Curr. Microbiol. 2012, 65, 237–243. [Google Scholar] [CrossRef]
- Cocco, F.; Cagetti, M.G.; Majdub, O.; Campus, G. Concentration in Saliva and Antibacterial Effect of Xylitol Chewing Gum: In Vivo and In Vitro Study. Appl. Sci. 2020, 10. [Google Scholar] [CrossRef]
- Cagetti, M.G.; Cocco, F.; Carta, G.; Maspero, C.; Campus, G. Long-term efficacy of Magnolia Bark Extract and Xylitol administered through chewing gums on caries in adults: A 2-year randomized controlled intervention trial. J. Funct. Foods 2020, 68, 103891. [Google Scholar] [CrossRef]
- Ganter, J.; Hellwig, E.; Doerken, S.; Al-Ahmad, A. In vitro evaluation of the cariogenic potential of rebaudioside A compared to sucrose and xylitol. Clin. Oral Investig. 2020, 24, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Loimaranta, V.; Mazurel, D.; Deng, D.; Soderling, E. Xylitol and erythritol inhibit real-time biofilm formation of Streptococcus mutans. BMC Microbiol. 2020, 20, 184. [Google Scholar] [CrossRef]
- Zabner, J.; Seiler, M.P.; Launspach, J.L.; Karp, P.H.; Kearney, W.R.; Look, D.C.; Smith, J.J.; Welsh, M.J. The osmolyte xylitol reduces the salt concentration of airway surface liquid and may enhance bacterial killing. Proc. Natl. Acad. Sci. 2000, 97, 11614. [Google Scholar] [CrossRef] [Green Version]
- Sajjan, U.; Moreira, J.; Liu, M.; Humar, A.; Chaparro, C.; Forstner, J.; Keshavjee, S. A novel model to study bacterial adherence to the transplanted airway: Inhibition of Burkholderia cepacia adherence to human airway by dextran and xylitol. J. Heart Lung Transplant. 2004, 23, 1382–1391. [Google Scholar] [CrossRef]
- Yin, S.Y.; Kim, H.J.; Kim, H.-J. Protective Effect of Dietary Xylitol on Influenza A Virus Infection. PLOS ONE 2014, 9, e84633. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Lee, T.; Hardcastle, T.; Biswas, K.; Radcliff, F.; Douglas, R. The in vitro effect of xylitol on chronic rhinosinusitis biofilms. Rhinology 2016, 54, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.L.; Wi, G.R.; Kim, H.J.; Kim, H.-J. Ameliorating Effect of Dietary Xylitol on Human Respiratory Syncytial Virus (hRSV) Infection. Biol. Pharm. Bull. 2016, 39, 540–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azarpazhooh, A.; Lawrence, H.P.; Shah, P.S. Xylitol for preventing acute otitis media in children up to 12 years of age. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kontiokari, T.; Uhari, M.; Koskela, M. Antiadhesive effects of xylitol on otopathogenic bacteria. J Antimicrob. Chemother 1998, 41, 563–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkinen, K.K. Biochemical Principles of the Use of Xylitol in Medicine and Nutrition with Special Consideration of Dental Aspects; Birkhäuser: Basel, Switzerland, 1978; Volume 30. [Google Scholar]
- Akram, M.; Ali Shah, S.M.; Munir, N.; Daniyal, M.; Tahir, I.M.; Mahmood, Z.; Irshad, M.; Akhlaq, M.; Sultana, S.; Zainab, R. Hexose monophosphate shunt, the role of its metabolites and associated disorders: A review. J. Cell. Physiol. 2019, 234, 14473–14482. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.F.; Jofre, F.M.; de Souza Queiroz, S.; Vaz de Arruda, P.; Chandel, A.K.; das Graças de Almeida Felipea, M. Biotechnological production of sweeteners. Biotechnol. Prod. Bioactive Compd. 2020, 261–292. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [Green Version]
- Park, E.; Park, M.H.; Na, H.S.; Chung, J. Xylitol induces cell death in lung cancer A549 cells by autophagy. Biotechnol. Lett. 2015, 37, 983–990. [Google Scholar] [CrossRef]
- Szel, E.; Polyanka, H.; Szabo, K.; Hartmann, P.; Degovics, D.; Balazs, B.; Nemeth, I.B.; Korponyai, C.; Csa¡nyi, E.; Kaszaki, J.; et al. Anti-irritant and anti-inflammatory effects of glycerol and xylitol in sodium lauryl sulphate-induced acute irritation. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2333–2341. [Google Scholar] [CrossRef] [Green Version]
- Szél, E.; Danis, J.; Sőrés, E.; Tóth, D.; Korponyai, C.; Degovics, D.; Prorok, J.; Acsai, K.; Dikstein, S.; Kemény, L.; et al. Protective effects of glycerol and xylitol in keratinocytes exposed to hyperosmotic stress. Clin. Cosmet. Investig. Dermatol. 2019, 12, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Kikuko, A.; Arai, H.; Takashi, U.; Fukaya, M.; Koganei, M.; Sasaki, H.; Yamamoto, H.; Taketani, Y.; Takeda, E. Effects of xylitol on metabolic parameters and visceral fat accumulation. J. Clin. Biochem. Nutr. 2011, 49, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Ide, Y.; Nasu, M.; Numabe, Y. The effects of oral xylitol administration on bone density in rat femur. Odontology 2011, 99, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Delgado Arcaño, Y.; Valmaña García, O.D.; Mandelli, D.; Carvalho, W.A.; Magalhães Pontes, L.A. Xylitol: A review on the progress and challenges of its production by chemical route. Catal. Today 2020, 344, 2–14. [Google Scholar] [CrossRef]
- Paula, V.A.C.; Modesto, A.; Santos, K.R.N.; Gleiser, R. Antimicrobial effects of the combination of chlorhexidine and xylitol. Br. Dent. J. 2010, 209, E19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anglenius, H.; Tiihonen, K. Evaluation of xylitol as an agent that controls the growth of skin microbes: Staphylococcus aureus, Staphylococcus epidermidis, and Cutibacterium acnes. Korean J. Microbiol. 2020, 56, 54–58. [Google Scholar] [CrossRef]
- Piątek-Hnat, M.; Bomba, K. The influence of of cross-linking process on the physicochemical properties of new copolyesters containing xylitol. Mater. Today Commun. 2020, 22, 100734. [Google Scholar] [CrossRef]
- Firoozi, N.; Kang, Y. Immobilization of FGF on Poly (xylitol dodecanedioic Acid) Polymer for Tissue Regeneration. Sci. Rep. 2020, 10, 10419. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.D.; Hovda, L.R. Acute Hepatic Failure in a Dog after Xylitol Ingestion. J. Med Toxicol. 2016, 12, 201–205. [Google Scholar] [CrossRef]
- FAO. Evaluation of certain food additives and contaminants. In Twenty-Seventh Report of the Joint FAO/WHO Expert Committee on Food Additives, Geneva, Switzerland, 11–20 April 1983; World Health Organization: Geneva, Switzerland, 1983. [Google Scholar]
- Ylikahri, R. Metabolic and Nutritional Aspects of Xylitol. In Advances in Food Research; Chichester, C.O., Ed.; Academic Press: Cambridge, MA, USA, 1979; Volume 25, pp. 159–180. [Google Scholar]





| Manufacturer | Country | Substrate | Metric Tons/Year |
|---|---|---|---|
| Futaste pharmaceutical Co., Ltd. | China | Corn cobs | 31,000 |
| Shandong Lujian Biological Technology Co., Ltd. | 16,000 | ||
| Anhui elite industrial Co., Ltd. | 5400 | ||
| Hefei reachever import and export limited company | 5400 | ||
| Hunan JK international trade corporation | 12,000 | ||
| Shanghai just import and export Co., Ltd. | 55,000 | ||
| 5000 | |||
| Hangzhou Shouxing Biotechnology Co., Ltd. | 4000 | ||
| Shandong Biobridge Technology Co., Ltd. | 6000 | ||
| Tangyin Hung Industrial Co., Ltd. | 2500 | ||
| Thomson Biotech (Xiamen) Pte., Ltd. | 10,000 | ||
| Yucheng Lujian Biological Technology Co., Ltd. | 6000 | ||
| Zhejiang Huakang Enterprise Co., Ltd. | 20,000 | ||
| Shijiazhuang Acid Chemical Co., Ltd | 10,000 | ||
| Shengquan Healtang Biotech Co., Ltd. | 8000 | ||
| Xylitol USA, Inc | USA | Birch trees | - |
| DuPont (Danisco) USA | Waste side stream of a pulp and paper plant to extract xylose | 2000 | |
| Superior Supplement Manufacturing | - | - | |
| Avansecure | India | Corn husks, sugar cane bagasse and birch | - |
| Salvavidas | - | ||
| Herboveda | - | ||
| Geno Chem, Ltd | - | ||
| Leisha Pharma Solutions Pvt., Ltd. | - |
| Product | Xylitol Content (mg/100 g Dry Weight) |
|---|---|
| Carrot juice | 12 |
| Chestnut | 14 |
| Banana | 21 |
| Carrot | 86.5 |
| Onion | 89 |
| Lettuce | 96.5 |
| Pumpkin | 96.5 |
| Spinach | 107 |
| White mushroom | 128 |
| Eggplant | 180 |
| Raspberry | 268 |
| Cauliflower | 300 |
| Strawberry | 362 |
| Yellow plum | 935 |
| Lingon berry | 64 |
| Cran berry | 37 |
| Bilberrya | 38 |
| Sea buckthorn | 91 |
| Rowan berry | 160 |
| Apple | 128 |
| Organisms | Substrate | Yield (YP/S) and Productivity | References |
|---|---|---|---|
| Bacteria | |||
| Bacillus subtilis | Xylose | 0.85 g/g xylose (213 g/L) | [24] |
| Escherichia coli | Xylose | 0.612 g/g xylose (6.325 g/L) | [25] |
| Cellulomonas cellulans NRRL B-4567 | Xylose | 1.76 g/L xylitol, 1.67 g/L ethanol | [22] |
| Mycobacterium smegmatis | D-xylulose, D-mannitol | 0.7 g/g xylulose | [26] |
| Fungi | |||
| Aspergillus niger | D-glucose and D-xylose | 0.211 g/g biomass (1.139 g/L) | [27] |
| Trichoderma reesei | Barely straw | 0.122g/g biomass (6.1 g/L) and 26.44 g/g biomass (13.22 g/L) | [28] |
| Thermomyces lanuginosus SSBP | Xylose from sugarcane bagasse | 0.22 g/g xylose (4.4 ± 0.13 g/L) | [29] |
| Yeast | |||
| Meyerozyma guilliermondii | Xylose | 0.27 g/g xylose (4.28 ± 1.30 g/L) | [30] |
| Debaromyces hansenii UFV-170 | Xylose | 0.73 g/g (76.6 g/L) | [31] |
| Debaryomyces nepalensis NCYC 3413 | Xylose + Glucose | 0.54 g/g (48.6 g/L) | [32] |
| Hansunela anomala NCAIM Y.01499 | Xylose | 0.174 g/g xylose (8.7 g/L) | [33] |
| Saccharomyces cerevisiae | Pretreated corn stover | 0.99 g/g-consumed xylose (45.41g/L xylitol) and 50.19g/L ethanol | [34] |
| Saccharomyces cerevisiae | Wheat stalk | 3.47 g/L | [35] |
| Pachysolen tannophilus | Brewer’s spent grain | 0.47 ± 0.06 g xylitol/g xylose and 0.09 ± 0.002 g ethanol/g xylose | [36] |
| Scheffersomyces amazonensis UFMG-HMD-26.3 | sugarcane bagasse and straw hemicellulose hydrolysate | 0.5 g/g xylose (28.56 g/L) | [19] |
| Kluyveromyces marxianus CCA510 | Cashew apple bagasse | 0.50 g/g (6.01 g/L) | [20] |
| Pachysolan tannephilus ATTC 32691 | Xylose | 0.14 g xylitol/g and 0.39 g ethanol/g | [37] |
| Saccharomyces cerevisiae | Xylan | 0.71 g/g xylan (1.94 g/L) | [38] |
| Kluyveromyces marxianus IIPE453t | Sugarcane bagasse | 0.42 g/g biomass (25.6 g/L) | [39] |
| Cyanobacteria and Algae (Photoautotrophs) | |||
| Synechococcus elongatus PCC794 | Xylose | 0.85 g/g (33 g/L) | [40] |
| Chlamydomonas reinhardtii (expressing XR from Neurospora crassa) | Xylose | 0.05 g/g xylose (0.38 g/L) | [41] |
| Country | Major Crops-Residues Fraction | Agricultural Waste Generated (≈Million Tons/Year) | References |
|---|---|---|---|
| India | Rice, wheat, sugarcane, maize | 500 | [57] |
| Bangladesh | Maize, rice | 72 | [58] |
| Indonesia | Rice, maize | 55 | [57] |
| Myanmar | Rice | 19 | [57] |
| China | Rice, sugarcane, maize, soybean | 930.8 | [59] |
| Pakistan | Wheat, sugarcane, rice | 40 | [60] |
| Brazil | Sugarcane, maize | 597 | [61] |
| Malaysia | Rice | 1.2 | [62] |
| Nigeria | Barley, maize | 145.62 | [63] |
| Pre-Treatment Strategies | Operating Conditions | Mechanism | References |
|---|---|---|---|
| Physical | |||
| Milling and grinding | Drying, milling to fine or coarse powder | More surface area, improve flow properties, increase the bulk density and porosity | [68,69] |
| Irradiation | γ-radiation and electron beam | Scission of glycosidic bonds in polysaccharides and destruction of the cell wall | [70,71] |
| Physico-chemical treatment | |||
| Autohydrolysis and steam explosion | 160–260 °C and 5–50 atm pressure 1% acid may be added | The complex structure of LCB is disrupted due to the expansion of steam | [72,73] |
| Microwave radiation (MWR) | MWR/water, MWR/alkali, MWR/acid, MWR/ionic liquid, MWR/salt | Accelerates cellulose dissolution in ionic liquids, removes hemicellulose and lignin | [74] |
| Chemical treatment | |||
| Acid | CH3COOH, HCl and H2SO4 (Dilute or concentrated acid) | Disruption of the hydrogen bonds and covalent bonds, solubilization of hemicellulose and reduction of cellulose complexity | [75] |
| Alkali | KOH, NaOH, Ca(OH)2, Ammonia (ammonia fiber expansion) | Destruction of lignin, reduction of the degree of polymerization of hemicellulose, lower crystallinity of cellulose | [76,77,78] |
| Ionic liquids | 1-butyl-3-methyl-imidazolium acetate, cholinium ionic liquid, etc. | Attachment of hydrogen bonds to dissociate the lignocellulose complex | [79] |
| Biological treatment | |||
| Microbiological treatment | Yeast, fungi, micro-algae, bacteria | Enzymes break respective bonds and depolymerize/solubilize polymers | [79,80,81] |
| Enzymatic hydrolysis | Xylanases and cellulases | ||
| Nanotechnology in biomass pretreatment | |||
| Nanoparticles of metal/biopolymers | Acid/base/enzymes/microbes | Nanoparticles improve the delivery of agents and enhance the activity | [79,82,83] |
| Genre | Brand Name | Trademark | Concentration (%) |
|---|---|---|---|
| Chewing gum | Trident | Trident, USA | 1 |
| Epic Dental | Epic, USA | 1 | |
| Xylitol Sugar Free Chewing Gum | Lotte, Thailand | - | |
| Xylitol Chewing gum | Hager Werken, Germany | - | |
| Candies and drops | Xylipop | Hager Werken, Germany | - |
| Xylitol drops | Hager Werken, Germany | 94 | |
| Xylitol candy | Ice Chips candy, USA | ||
| Xylitol | Epic, USA | 1 | |
| Xyla | Xylitol, USA | 0.4 | |
| Snowflakes | Snowflakes, USA | 2 | |
| Mouthwashes and toothpastes | Spry mouth wash | Xlear, USA | - |
| Act Braces Care | Chattem, USA | - | |
| TheraMints | 3M, USA | 1 | |
| Xyli White | New Food Solutions, USA | 25 | |
| Bioxtra | Hetero Healthcare Ltd., India | - | |
| Bioxtra-T | Hetero Healthcare Ltd., India | - | |
| Beverages | Lime refresher | Naturally sweet, Australia | 6.6 |
| Citron tea | Yesan-nongsan Co., Ltd., Korea | - | |
| Honey | Health Garden, USA | 10 | |
| Xylitol Real birds nest | Scotch Real, Thailand | 10 | |
| Sweetener | Xylitol alternative | Suganon, South Africa | - |
| Xylitol plus | Now, USA | 1.7 | |
| So Sweet xylitol | Ankur drugs and pharma Ltd., India | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahuja, V.; Macho, M.; Ewe, D.; Singh, M.; Saha, S.; Saurav, K. Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism. Foods 2020, 9, 1592. https://doi.org/10.3390/foods9111592
Ahuja V, Macho M, Ewe D, Singh M, Saha S, Saurav K. Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism. Foods. 2020; 9(11):1592. https://doi.org/10.3390/foods9111592
Chicago/Turabian StyleAhuja, Vishal, Markéta Macho, Daniela Ewe, Manoj Singh, Subhasish Saha, and Kumar Saurav. 2020. "Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism" Foods 9, no. 11: 1592. https://doi.org/10.3390/foods9111592
APA StyleAhuja, V., Macho, M., Ewe, D., Singh, M., Saha, S., & Saurav, K. (2020). Biological and Pharmacological Potential of Xylitol: A Molecular Insight of Unique Metabolism. Foods, 9(11), 1592. https://doi.org/10.3390/foods9111592