Simultaneous Quantitative Assessment of Ochratoxin A, Patulin, 5-Hydroxymethylfurfural, and Bisphenol A in Fruit Drinks Using HPLC with Diode Array-Fluorimetric Detection
Abstract
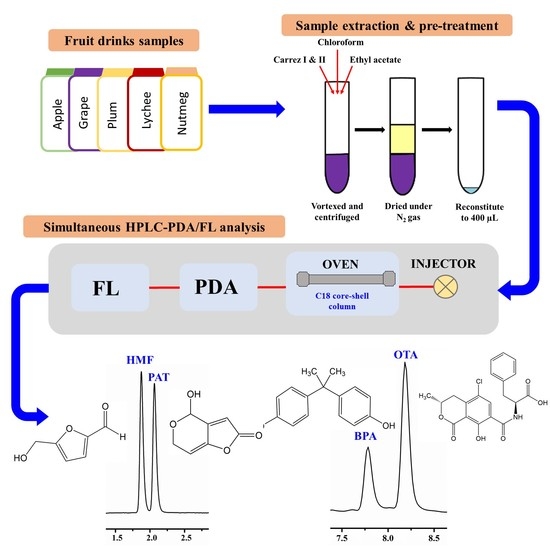
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fruit Beverage Samples
2.3. High-Performance Liquid Chromatographic System
2.4. Stock and Standard Solutions
2.5. Preparation of Fruit Drink Samples
2.6. Method Validation
2.7. Data Handling
3. Results and Discussion
3.1. HPLC-DAD/FL Method Development
3.2. Optimisation of Clean-Up and Preconcentration Strategies
3.3. Method Validation
3.3.1. Linearity
3.3.2. Limits of Detection and Quantitation
3.3.3. Precision and Accuracy Studies
3.4. Application to Fruit Beverages and Drinks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fruit and Vegetable Juices Global Market Trajectory & Analytics; Global Industry Analysts, Inc. Available online: https://www.researchandmarkets.com/reports/338669/fruit_and_vegetable_juices_global_market (accessed on 13 July 2020).
- National Institute of Environmental Health Sciences. Environmental Agents. Available online: https://www.niehs.nih.gov/health/topics/agents/index.cfm (accessed on 13 July 2020).
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarc’h, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609. [Google Scholar] [CrossRef] [PubMed]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [Green Version]
- Afsah-Hejri, L.; Jinap, S.; Hajeb, P.; Radu, S.; Shakibazadeh, S. A review on mycotoxins in food and feed: Malaysia case study. Compr. Rev. Food Sci. Food Saf. 2013, 12, 629–651. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. IARC Monograph on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1993; p. 489. [Google Scholar]
- EC (European Commission). Commission regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 324–365. [Google Scholar]
- Joint FAO/WHO Committee on Food Additives (JECFA). Evaluation of Certain Food Additives and Contaminants (Sixty-Eighth Report) of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Zouaoui, N.; Sbaii, N.; Bacha, H.; Abid-Essefi, S. Occurrence of patulin in various fruit juice marketed in Tunisia. Food Control 2015, 51, 356–360. [Google Scholar] [CrossRef]
- Marín, S.; Mateo, E.M.; Sanchis, V.; Valle-Algarra, F.M.; Ramos, A.J.; Jiménez, M. Patulin contamination in fruit derivatives, including baby food, from the Spanish market. Food Chem. 2011, 124, 563–568. [Google Scholar] [CrossRef]
- Oteiza, J.M.; Khaneghah, A.M.; Campagnollo, F.B.; Granato, D.; Mahmoudi, M.R.; Sant’Ana, A.S.; Gianuzzi, L. Influence of production on the presence of patulin and ochratoxin A in fruit juices and wines of Argentina. LWT 2017, 80, 200–207. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Malik, S.; Asi, M.R.; Selamat, J.; Malik, N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab, Pakistan. Food Control. 2018, 84, 370–374. [Google Scholar] [CrossRef]
- Sadok, I.; Stachniuk, A.; Staniszewska, M. Developments in the monitoring of patulin in fruits using liquid chromatography: An overview. Food Anal. Method. 2019, 12, 76–93. [Google Scholar] [CrossRef]
- Vidal, A.; Ouhibi, S.; Ghali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Committee on Food Additives (JECFA). Evaluations of Certain Food Additives and Contaminants; World Health Organization: Geneva, Switzerland, 1995; Series 859. [Google Scholar]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-Hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef]
- Czerwonka, M.; Opiłka, J.; Tokarz, A. Evaluation of 5-hydroxymethylfurfural content in non-alcoholic drinks. Eur. Food Res. Technol. 2018, 244, 11–18. [Google Scholar] [CrossRef]
- Gaspar, E.M.; Lucena, A.F. Improved HPLC methodology for food control–furfurals and patulin as markers of quality. Food Chem. 2009, 114, 1576–1582. [Google Scholar] [CrossRef]
- EC (European Commission). Commission regulation (EC) no 1881/2006. Directive 2001/110/EC. Off. J. Eur. Union 2001, 47–52. [Google Scholar]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, nonylphenols, benzophenones, and benzotriazoles in soils, groundwater, surface water, sediments, and food: A review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baluka, S.A.; Rumbeiha, W.K. Bisphenol A and food safety: Lessons from developed to developing countries. Food Chem. Toxicol. 2016, 92, 58–63. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the risks to public health related to the presence of bisphenol A in foodstuffs: EFSA Panel on Food Contact Materials, Enzymes, Flavorings and Processing Aids. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- Zhang, Z.; Alomirah, H.; Cho, H.-S.; Li, Y.-F.; Liao, C.; Minh, T.B.; Mohd, M.A.; Nakata, H.; Ren, N.; Kannan, K. Urinary bisphenol A concentrations and their implications for human exposure in several Asian countries. Environ. Sci. Technol. 2011, 45, 7044–7050. [Google Scholar] [CrossRef]
- Karami-Osboo, R.; Miri, R.; Javidnia, K.; Kobarfard, F.; AliAbadi, M.H.S.; Maham, M. A validated dispersive liquid-liquid microextraction method for extraction of ochratoxin A from raisin samples. J. Food Sci. Tech. 2015, 52, 2440–2445. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.P.; Sakai, R.; Manaf, N.A.; Rodhi, A.M.; Saad, B. High performance liquid chromatography method for the determination of patulin and 5-hydroxymethylfurfural in fruit juices marketed in Malaysia. Food Control. 2014, 38, 142–149. [Google Scholar] [CrossRef]
- Mignot, M.; Tchapla, A.; Mercier, O.; Couvrat, N.; Tisse, S.; Cardinael, P.; Peulon-Agasse, V. High-density octadecyl chemically bonded core–shell silica phases for HPLC: Comparison of microwave-assisted and classical synthetic routes, structural characterization and chromatographic evaluation. Chromatographia 2014, 77, 1577–1588. [Google Scholar] [CrossRef]
- Sowa, I.; Zielińska, S.; Sawicki, J.; Bogucka-Kocka, A.; Staniak, M.; Bartusiak-Szcześniak, E.; Podolska-Fajks, M.; Kocjan, R.; Wójciak-Kosior, M. Systematic evaluation of chromatographic parameters for isoquinoline alkaloids on XB-C18 core-shell column using different mobile phase compositions. J. Anal. Methods. Chem. 2018, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sungur, Ş.; Köroğlu, M.; Özkan, A. Determinatıon of bisphenol a migrating from canned food and beverages in markets. Food Chem. 2014, 142, 87–91. [Google Scholar] [CrossRef]
- Lee, T.P.; Saad, B.; Salleh, B.; Mat, I. Micro-solid phase extraction of ochratoxin A, and its determination in urine using capillary electrophoresis. Microchim. Acta 2013, 180, 1149–1156. [Google Scholar] [CrossRef]
- Gallart-Ayala, H.; Núñez, O.; Moyano, E.; Galceran, M.T. Field-amplified sample injection-micellar electrokinetic capillary chromatography for the analysis of bisphenol A, bisphenol F, and their diglycidyl ethers and derivatives in canned soft drinks. Electrophoresis 2010, 31, 1550–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bationo, R.; Jordana, F.; Boileau, M.-J.; Colat-Parros, J. Release of monomers from orthodontic adhesives. Am. J. Orthod. Dentofac. 2016, 150, 491–498. [Google Scholar] [CrossRef]
- Al-Taher, F.; Banaszewski, K.; Jackson, L.; Zweigenbaum, J.; Ryu, D.; Cappozzo, J. Rapid method for the determination of multiple mycotoxins in wines and beers by LC-MS/MS using a stable isotope dilution assay. J. Agric. Food Chem. 2013, 61, 2378–2384. [Google Scholar] [CrossRef]
- Gallo, P.; Di Marco Pisciottano, I.; Fattore, M.; Rimoli, M.G.; Seccia, S.; Albrizio, S. A method to determine BPA, BPB, and BPF levels in fruit juices by liquid chromatography coupled to tandem mass spectrometry. Food Addit. Contam. Part A 2019, 36, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Terra, M.F.; Prado, G.; Pereira, G.E.; Ematné, H.J.; Batista, L.R. Detection of ochratoxin A in tropical wine and grape juice from Brazil. J. Sci. Food Agric. 2013, 93, 890–894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Shan, T.; Yuan, Y.; Zhang, Z.; Guo, C.; Yue, T. Evaluation of Penicillium expansum for growth, patulin accumulation, nonvolatile compounds and volatile profile in kiwi juices of different cultivars. Food Chem. 2017, 228, 211–218. [Google Scholar] [CrossRef]
- Derouich, M.; Meziani, R.; Bourkhis, B.; Filali-Zegzouti, Y.; Chakib, A. Nutritional, mineral and organic acid composition of syrups produced from six Moroccan date fruit (Phoenix dactylifera L.) varieties. J. Food Compos. Anal. 2020, 93, 103591. [Google Scholar]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, S.H. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35–52. [Google Scholar] [CrossRef]
- Geens, T.; Apelbaum, T.Z.; Goeyens, L.; Neels, H.; Covaci, A. Intake of bisphenol A from canned beverages and foods on the Belgian market. Food Addit. Contam. 2010, 27, 1627–1637. [Google Scholar] [CrossRef]
- Noonan, G.O.; Ackerman, L.K.; Begley, T.H. Concentration of bisphenol A in highly consumed canned foods on the US market. J. Agric. Food Chem. 2011, 59, 7178–7185. [Google Scholar] [CrossRef]
- Lorber, M.; Schecter, A.; Paepke, O.; Shropshire, W.; Christensen, K.; Birnbaum, L. Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ. Int. 2015, 77, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Adopted HPLC Conditions | ||
|---|---|---|
| Variable | Optimum Value | |
| Flow rate | 1.2 mL min−1 | |
| Temperature | 35 °C | |
| Initial mobile phase composition (%) | MeOH: Acidified water (18:82) | |
| Gradient programming (Methanol composition, %) | 0–2.0 min | (18%) |
| 2.0–8.0 min | (95%) | |
| 8.0–10 min | (95%) | |
| 10–10.5 min | (18%) | |
| 10.5–12.5 min | (18%) | |
| Injection volume | 20 µL | |
| PDA configuration | HMF 1 | 284 nm |
| PAT 2 | 276 nm | |
| FL configuration | BPA 3 | 275 nm (excitation), 300 nm (emission) |
| OTA 4 | 333 nm (excitation), 443 nm (emission) | |
| Analytes | Linear Range (ng mL−1) | Regression Equation | Linearity, r2 | LOD (ng mL−1) | LOQ (ng mL−1) |
|---|---|---|---|---|---|
| OTA 1 | 0.5–50 | y = 4629.4x + 5522.6 | 0.9984 | 0.5 | 1.7 |
| PAT 2 | 10–200 | y = 77.499x − 57.109 | 0.9998 | 1.1 | 3.4 |
| HMF 3 | 10–1000 | y = 302.62x − 2243.6 | 0.9993 | 7.9 | 24.0 |
| BPA 4 | 1–100 | y = 8820.4x + 115511 | 0.9988 | 1.0 | 3.2 |
| Types of Fruit Drinks | Average Concentration ± SD 1 | |||
|---|---|---|---|---|
| OTA 2 (ng mL−1) | PAT 3 (ng mL−1) | HMF 4 (µg mL−1) | BPA 5 (ng mL−1) | |
| Ambarella | 1.55 ± 0.08 | <LOD 6 | 18.59 ± 0.54 | 1.18 ± 0.07 |
| Apple | <LOD | 23.80 ± 0.82 | 6.80 ± 0.03 | <LOD |
| Dates | 0.98 ± 0.11 | <LOD | 27.73 ± 0.64 | 1.02 ± 0.01 |
| Grapes | 0.92 ± 0.06 | 13.06 ± 0.50 | <LOD | <LOD |
| Guava | <LOD | 14.53 ± 1.19 | 12.31 ± 0.06 | <LOD |
| Kiwi | <LOD | 93.28 ± 0.12 | 2.80 ± 0.01 | <LOD |
| Lychee | <LOD | <LOD | 15.94 ± 0.67 | 1.54 ± 0.06 |
| Mixed fruit | <LOD | 12.08 ± 0.94 | <LOD | 1.16 ± 0.19 |
| Nutmeg | <LOD | 7.93 ± 0.83 | 25.72 ± 2.60 | 1.25 ± 0.07 |
| Pineapple | <LOD | 47.78 ± 1.79 | 17.04 ± 0.49 | <LOD |
| Plum 1 | 1.47 ± 0.04 | <LOD | 11.81 ± 0.66 | <LOD |
| Plum 2 | <LOD | <LOD | <LOD | 8.59 ± 0.45 |
| Roselle | <LOD | 36.72 ± 0.46 | <LOD | <LOD |
| Soursop | <LOD | <LOD | 0.13 ± 0.01 | <LOD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, N.H.; Othman, H.I.A.A.; Abdul Malek, N.R.; Zulkurnain, M.; Saad, B.; Wong, Y.F. Simultaneous Quantitative Assessment of Ochratoxin A, Patulin, 5-Hydroxymethylfurfural, and Bisphenol A in Fruit Drinks Using HPLC with Diode Array-Fluorimetric Detection. Foods 2020, 9, 1633. https://doi.org/10.3390/foods9111633
Hassan NH, Othman HIAA, Abdul Malek NR, Zulkurnain M, Saad B, Wong YF. Simultaneous Quantitative Assessment of Ochratoxin A, Patulin, 5-Hydroxymethylfurfural, and Bisphenol A in Fruit Drinks Using HPLC with Diode Array-Fluorimetric Detection. Foods. 2020; 9(11):1633. https://doi.org/10.3390/foods9111633
Chicago/Turabian StyleHassan, Norfarizah Hanim, Haneen Ibrahim Ahmad Al Othman, Nur Rabiatutadawiah Abdul Malek, Musfirah Zulkurnain, Bahruddin Saad, and Yong Foo Wong. 2020. "Simultaneous Quantitative Assessment of Ochratoxin A, Patulin, 5-Hydroxymethylfurfural, and Bisphenol A in Fruit Drinks Using HPLC with Diode Array-Fluorimetric Detection" Foods 9, no. 11: 1633. https://doi.org/10.3390/foods9111633
APA StyleHassan, N. H., Othman, H. I. A. A., Abdul Malek, N. R., Zulkurnain, M., Saad, B., & Wong, Y. F. (2020). Simultaneous Quantitative Assessment of Ochratoxin A, Patulin, 5-Hydroxymethylfurfural, and Bisphenol A in Fruit Drinks Using HPLC with Diode Array-Fluorimetric Detection. Foods, 9(11), 1633. https://doi.org/10.3390/foods9111633