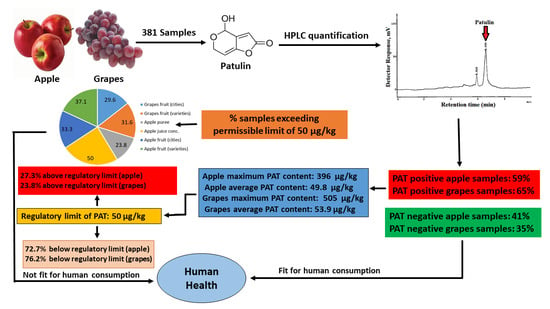
Surveillance of Patulin in Apple, Grapes, Juices and Value-Added Products for Sale in Pakistan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Chemicals and Reagents
2.3. Samples Preparation, Extraction and Cleanup
2.4. Apparatus and Conditions of HPLC for Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Method Validation
3.2. Occurrence of Patulin in Apple Fruits and Their Products
| Commodity | Country | Analytical Method | Incidence (%) | Concentration Range (µg/kg) | Reference |
|---|---|---|---|---|---|
| Apple juice for infants | Spain | MEKC 1 | 70 | LOD–29.6 | Murillo et al. [22] |
| Apple juice concentrate | Spain | HPLC-UV 2 | 42.4 | LOD–74.4 | Marín et al. [23] |
| Apple based food | Serbia | HPLC-UV | 43 | 3.2–30.2 | Torović et al. [24] |
| Apple juice | Sweden | HPLC-UV | 12.8 | LOD–50 | Thuvander et al. [25] |
| Apple juice | Italy | HPLC-UV | 37.5 | 5.8–56.4 | Ritieni et al. [26] |
| Apple puree | Italy | HPLC-UV | 50 | 15.9–16.7 | Ritieni et al. [26] |
| Apple juice | Portugal | HPLC-DAD 3 | 41 | 1.2–42 | Barreira et al. [27] |
| Apple juice | Belgium | HPLC-UV | 12 | 10.2–43.1 | Baert et al. [28] |
| Apple juice | Greece | HPLC-DAD | 100 | 0.9–36.8 | Moukas et al. [29] |
| Apple juice | Turkey | HPLC-UV | 60 | 19.1–732.8 | Yurdun et al. [30] |
| Apple juice | Tunisia | HPLC-UV | 64.3 | 4–122.4 | Zouaoui et al. [31] |
| Apple juice | Tunisia | HPLC-UV | 37 | 0–167 | Zaied et al. [32] |
| Apple juice | S. Africa | HPLC-UV | 23.5 | 5–45 | Leggot et al. [33] |
| Apple juice | S. Africa | HPLC-UV | 33.3 | 0–1650 | Shephard et al. [34] |
| Apple juice | USA | HPLC-DAD | 18.7 | LOD–467.4 | Harris et al. [35] |
| Apple juice | Brazil | HPLC-DAD | 3 | 3–7 | Iha et al. [36] |
| Apple fruit | Argentina | HPLC-DAD | 40.3 | LOD–19622 | Oteiza et al. [16] |
| Apple juice | Malaysia | HPLC-UV | 7.7 | LOD–26.9 | Lee et al. [37] |
| Apple juice | China | LC-MS 4 | 42.9 | LOD–1234.3 | Li et al. [38] |
| Apple juice | Japan | LC-MS | 19.7 | 1.4–45.6 | Ito et al. [39] |
| Apple juice | S. Korea | HPLC-DAD | 12.5 | LOD–8.9 | Cho et al. [40] |
| Apple juice | Iran | TLC 5 | 31 | 15–285.5 | Cheraghali et al. [41] |
| Apple fruit | Pakistan | HPLC-UV | 56.4 | LOD–396 | Present study |
| Apple juice | Pakistan | HPLC-UV | 62.9 | LOD–18 | Present study |
| Apple juice concentrate | Pakistan | HPLC-UV | 80 | LOD–328 | Present study |
| Apple puree | Pakistan | HPLC-UV | 71.4 | LOD–99 | Present study |
| Apple jam | Pakistan | HPLC-UV | 30 | LOD–6 | Present study |
3.3. Occurrence of Patulin in Grapes Fruits and Their Products
| Commodity | Country | Analytical Method | Incidence (%) | Conc. Range (µg/kg) | Reference |
|---|---|---|---|---|---|
| Grapes fruit | Argentina | HPLC-UV | 10 | 0–13,808 | Oteiza et al. [16] |
| Grapes juice | Austria | HPLC-UV | 45.3 | LOD–41 | SCOOP Task [55] |
| Grapes must | Austria | HPLC-UV | 52.4 | LOD–750 | SCOOP Task [55] |
| Grapes juice | Belgium | HPLC-UV | 20 | LOD–36 | SCOOP Task [55] |
| Grapes juice | Germany | HPLC-UV | 3.1 | LOD–31.5 | SCOOP Task [55] |
| Grapes must | Germany | HPLC-UV | 54 | 3.5–80 | Majerus at al. [56] |
| Grapes juice | Germany | GC-MS | 100 | 4.9–5.2 | Rychlik et al. [57] |
| Grapes fruit | Pakistan | HPLC-UV | 59.4 | LOD–505 | Present study |
| Grapes juice | Pakistan | HPLC-UV | 84.6 | LOD–39 | Present study |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Government of Pakistan. Fruit, Vegetables and Condiments Statistics of Pakistan 2017–2018; Government of Pakistan, Ministry of National Food Security & Research, Economic Wing: Islamabad, Pakistan, 2019.
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Ioi, J.D.; Zhou, T.; Tsao, R.F.; Marcone, M. Mitigation of Patulin in Fresh and Processed Foods and Beverages. Toxins 2017, 9, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Errampalli, D. Penicillium expansum (Blue Mold). In Postharvest Decay: Control Strategies; Chapter 6; Bautista-Baños, S., Ed.; Academic Press: London, UK, 2014; pp. 189–231. [Google Scholar]
- Barad, S.; Sionov, E.; Prusky, D. Role of patulin in post-harvest diseases. Fungal Biol. Rev. 2016, 30, 24–32. [Google Scholar] [CrossRef]
- Zdzieblo, A.P.; Reuter, W.M.; Shelton, C.T. Analysis of Patulin in Apple Juice by UHPLC with UV Detection. In Perkin Elmer Application Note—Liquid Chromatography; Perkin Elmer, Inc.: Waltham, MA, USA, 2015. [Google Scholar]
- Zhong, L.; Carere, J.; Lu, Z.; Lu, F.; Zhou, T. Patulin in Apples and Apple-Based Food Products: The Burdens and the Mitigation Strategies. Toxins 2018, 10, 475. [Google Scholar] [CrossRef] [Green Version]
- Snini, S.P.; Tannous, J.; Heuillard, P.; Bailly, S.; Lippi, Y.; Zehraoui, E.; Barreau, C.; Oswald, I.P.; Puel, O. Patulin is a cultivar-dependent aggressiveness factor favouring the colonization of apples by Penicillium expansum. Mol. Plant. Pathol. 2016, 17, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Shi, J.; Zhu, C. Fruit spoilage and ochratoxin a production by Aspergillus carbonarius in the berries of different grape cultivars. Food Control 2013, 30, 93–100. [Google Scholar] [CrossRef]
- Vidal, A.; Ouhibi, S.; Gali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. The mycotoxin patulin: An updated short review on occurrence, toxicity and analytical challenges. Food Chem. Toxicol. 2019, 129, 249–256. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission (CAC). Codex General Standard for Contaminants and Toxins in Food and Feed; Codex STAN 193-1995; CAC: Rome, Italy, 1995; p. 44. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 7 October 2020).
- European Commission (EC). European Union Commission Regulation No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- AOAC International. AOAC Official Method 2000.02 Patulin in clear and cloudy apple juices and apple puree. In Official Methods of Analysis of AOAC International, 18th ed.; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- European Commission (EC). European Union Commission Regulation No 401/2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 12–34. [Google Scholar]
- Iqbal, S.Z.; Malik, S.; Asi, M.R.; Selamat, J.; Malik, N. Natural occurrence of patulin in different fruits, juices and smoothies and evaluation of dietary intake in Punjab, Pakistan. Food Control 2018, 84, 370–374. [Google Scholar] [CrossRef]
- Oteiza, J.M.; Khaneghah, A.M.; Campagnollo, F.B.; Granato, D.; Mahmoudi, M.R.; Sant’Ana, A.S.; Gianuzzi, L. Influence of production on the presence of patulin and ochratoxin A in fruit juices and wines of Argentina. LWT Food Sci. Technol. 2017, 80, 200–207. [Google Scholar] [CrossRef]
- Vaclavikova, M.; Dzuman, Z.; Lacina, O.; Fenclova, M.; Veprikova, Z.; Zachariasova, M.; Hajslova, J. Monitoring survey of patulin in a variety of fruit-based products using a sensitive UHPLC–MS/MS analytical procedure. Food Control 2015, 47, 577–584. [Google Scholar] [CrossRef]
- Al-Hazmi, N.A. Determination of patulin and ochratoxin A using HPLC in apple juice samples in Saudi Arabia. Saudi J. Biol. Sci. 2010, 17, 353–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattar, A.; Riaz, A.; Ahmed, S.; Hassan, I. Efficacy of selected plant extracts for inhibition of Penicillium expansum growth on apple fruits. Pak. J. Phytopathol. 2014, 26, 63–66. [Google Scholar]
- Zhang, Z.; Li, M.; Wu, C.; Peng, B. Physical adsorption of patulin by Saccharomyces cerevisiae during fermentation. J. Food Sci. Technol. 2019, 56, 2326–2331. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Tariq, F.; Perveen, K.; Ayub, N.; Mansab, S.; Batool, S.Q.; Abbasi, M.; Iram, S.; Tabassum, N. Mycotoxins analysis in fresh and dry fruits from Pakistan—A review. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 2538–2545. [Google Scholar]
- Murillo, M.; González-Peñas, E.; Amezqueta, S. Comparison between capillary electrophoresis and high performance liquid chromatography for the study of the occurrence of patulin in apple juice intended for infants. Food Chem. Toxicol. 2010, 48, 2429–2434. [Google Scholar] [CrossRef]
- Marín, S.; Mateo, E.M.; Sanchis, V.; Valle-Algarra, F.M.; Ramos, A.J.; Jiménez, M. Patulin contamination in fruit derivatives, including baby food, from the Spanish market. Food Chem. 2011, 124, 563–568. [Google Scholar] [CrossRef]
- Torović, L.; Dimitrov, N.; Assunção, R.; Alvito, P. Risk assessment of patulin intake through apple-based food by infants and preschool children in Serbia. Food Addit. Contam. Part A 2017, 34, 2023–2032. [Google Scholar] [CrossRef]
- Thuvander, A.; Moller, T.; Enghardt, H.; Jansson, A.; Salomonsson, A.-C.; Olsen, M. Dietary intake of some important mycotoxins by the Swedish population. Food Addit. Contam. 2001, 18, 696–706. [Google Scholar] [CrossRef]
- Ritieni, A. Patulin in Italian commercial apple products. J. Agric. Food Chem. 2003, 1, 6086–6090. [Google Scholar] [CrossRef]
- Barreira, M.J.; Alvito, P.C.; Almeida, C.M.M. Occurrence of patulin in apple-based-foods in Portugal. Food Chem. 2010, 121, 653–658. [Google Scholar] [CrossRef]
- Baert, K.; De Meulenaer, B.; Kamala, A.; Kasase, C.; Devlieghere, F. Occurrence of patulin in organic, conventional, and handcrafted apple juices marketed in Belgium. J. Food Prot. 2006, 69, 1371–1378. [Google Scholar] [CrossRef] [PubMed]
- Moukas, A.; Panagiotopoulou, V.; Markaki, P. Determination of patulin in fruit juices using HPLC-DAD and GC-MSD techniques. Food Chem. 2008, 109, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Yurdun, T.; Omurtag, G.Z.; Ersoy, O. Incidence of patulin in apple juices marketed in Turkey. J. Food Prot. 2001, 64, 1851–1853. [Google Scholar] [CrossRef] [PubMed]
- Zouaoui, N.; Sbaii, N.; Bacha, H.; Abid-Essefi, S. Occurrence of patulin in various fruit juice marketed in Tunisia. Food Control 2015, 51, 356–360. [Google Scholar] [CrossRef]
- Zaied, C.; Abid, S.; Hlel, W.; Bacha, H. Occurrence of patulin in apple-based-foods largely consumed in Tunisia. Food Control 2013, 31, 263–267. [Google Scholar] [CrossRef]
- Leggot, N.L.; Shephard, G.S. Patulin in South African commercial apple products. Food Control 2001, 2, 73–76. [Google Scholar] [CrossRef]
- Shephard, G.S.; van der Westhuizen, L.; Katerere, D.R.; Herbst, M.; Pineiro, M. Preliminary exposure assessment of deoxynivalenol and patulin in South Africa. Mycotoxin Res. 2010, 26, 181–185. [Google Scholar] [CrossRef]
- Harris, K.L.; Bobe, G.; Bourquin, L.D. Patulin surveillance in apple cider and juice marketed in Michigan. J. Food Prot. 2009, 72, 1255–1261. [Google Scholar] [CrossRef]
- Iha, M.H.; Sabino, M. Incidence of patulin in Brazilian apple-based drinks. Food Control 2008, 19, 417–422. [Google Scholar] [CrossRef]
- Lee, T.P.; Sakai, R.; Manaf, N.A.; Rodhi, A.M.; Saad, B. High performance liquid chromatography method for the determination of patulin and 5-hydroxymethylfurfural in fruit juices marketed in Malaysia. Food Control 2014, 38, 142–149. [Google Scholar] [CrossRef]
- Li, X.; Li, H.; Ma, W.; Guo, Z.; Li, X.; Li, X.; Zhang, Q. Determination of patulin in apple juice by single-drop liquid-liquid-liquid microextraction coupled with liquid chromatography-mass spectrometry. Food Chem. 2018, 257, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Yamazaki, H.; Inoue, K.; Yoshimura, Y.; Kawaguchi, M.; Nakazawa, H. Development of liquid chromatography–electrospray mass spectrometry for the determination of patulin in apple juice. Investigation of its contamination levels in Japan. J. Agric. Food Chem. 2004, 52, 7464–7468. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Kim, K.; Seo, E.; Kassim, N.; Mtenga, A.B.; Shim, W.B.; Lee, S.H.; Chung, D.H. Occurrence of patulin in various fruit juices from South Korea: An exposure assessment. Food Sci. Biotechnol. 2010, 19, 1–5. [Google Scholar] [CrossRef]
- Cheraghali, A.M.; Mohammadi, H.R.; Amirahmadi, M.; Yazdanpanah, H.; Abouhossain, G.; Zamaian, F. Incidence of patulin contamination in apple juice produced in Iran. Food Control 2005, 16, 165–167. [Google Scholar] [CrossRef]
- Salomão, B.C.; Aragao, G.M.; Churey, J.J.; Padilla-Zakour, O.I.; Worobo, R.W. Influence of storage temperature and apple variety on patulin production by Penicillium expansum. J. Food Prot. 2009, 72, 1030–1036. [Google Scholar] [CrossRef]
- Sattar, A.; Riaz, A.; Mehmood, N.; Altaf, R.; Afzal, A.; Jabeen, Z. Effect of incubation temperature on lesion diameter of Penicillium expansum on apple fruit varieties. Int. J. Biosci. 2018, 12, 1–5. [Google Scholar]
- Kumar, D.; Tannous, J.; Sionov, E.; Keller, N.; Prusky, D. Apple intrinsic factors modulating the global regulator, LaeA, the Patulin gene cluster and patulin accumulation during fruit colonization by Penicillium expansum. Front. Plant Sci. 2018, 9, 1094. [Google Scholar] [CrossRef] [Green Version]
- Heinmaa, L.; Põldma, P.; Mirmajlessi, S.M.; Kaldmäe, H.; Vangdal, E.; Kidmose, U.; Bertelsen, M.; Lo Scalzo, R.; Fibiani, M.; Moor, U. Occurrence of mycotoxin patulin and polyphenol profile of Nordic apple juices in relation to apple cultivation system and pre-processing storage temperature. Agric. Food Sci. 2019, 28, 126–136. [Google Scholar] [CrossRef]
- Ahmadi-Afzadi, M.; Orsel, M.; Pelletier, S.; Bruneau, M.; Proux-Wéra, E.; Nybom, H.; Renou, J.P. Genome-wide expression analysis suggests a role for jasmonates in the resistance to blue mold in apple. Plant Growth Regul. 2018, 85, 375–387. [Google Scholar] [CrossRef]
- Olsen, M.; Lindqvist, R.; Bakeeva, A.; Su-lin, L.L.; Sulyok, M. Distribution of mycotoxins produced by Penicillium spp. inoculated in apple jam and crème fraiche during chilled storage. Int. J. Food Microbiol. 2019, 292, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Asi, M.R.; Iqbal, M.; Khalid, N.; Wajih-ul-Hassan, S.; Ariño, A. Patulin Mycotoxin in Mango and Orange Fruits, Juices, Pulps, and Jams Marketed in Pakistan. Toxins 2020, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Sanzani, S.M.; Miazzi, M.M.; Di Rienzo, V.; Fanelli, V.; Gambacorta, G.; Taurino, M.R.; Montemurro, C. A rapid assay to detect toxigenic Penicillium spp. contamination in wine and musts. Toxins 2016, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- Ostry, V.; Malir, F.; Cumova, M.; Kyrova, V.; Toman, J.; Grosse, Y.; Pospichalova, M.; Ruprich, J. Investigation of patulin and citrinin in grape must and wine from grapes naturally contaminated by strains of Penicillium expansum. Food Chem. Toxicol. 2018, 118, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Chunmei, J.; Junling, S.; Qi’an, H.; Yanlin, L. Occurrence of toxin-producing fungi in intact and rotten table and wine grapes and related influencing factors. Food Control 2013, 31, 5–13. [Google Scholar] [CrossRef]
- Battilani, P.; Logrieco, A.; Giorni, P.; Cozzi, G.; Bertuzzi, T.; Pietri, A. Ochratoxin A production by Aspergillus carbonarius on some grape varieties grown in Italy. J. Sci. Food Agric. 2004, 84, 1736–1740. [Google Scholar] [CrossRef]
- El-Samawaty, A.E.R.M.; Moslem, M.A.; Yassin, M.A.; Sayed, S.R.; El-Shikh, M.S. Control of grape blue molding Penicillia by Allium sativum. J. Pure Appl. Microbiol. 2013, 7, 1047–1053. [Google Scholar]
- Welke, J.E. Fungal and mycotoxin problems in grape juice and wine industries. Curr. Opin. Food Sci. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- SCOOP Task 3.2.8. Assessment of Dietary Intake of Patulin by the Population of EU Member States; Majerus, P., Kapp, K., Eds.; Reports on Tasks for Scientific Cooperation, Task 3.2.8; SCOOP Report: Brussels, Belgium, 2002. [Google Scholar]
- Majerus, P.; Hain, J.; Kölb, C. Patulin in grape must and new, still fermenting wine (Federweißer). Mycotoxin Res. 2008, 24, 135–139. [Google Scholar] [CrossRef]
- Rychlik, M. Quantification of the mycotoxin patulin in foods. Ernährung/Nutrition 2005, 29, 9–15. [Google Scholar]
| Sample Type | Variety/Location | n Total (Positive) | Incidence % | Mean ± SD | Max. | n (%) > 50 µg/kg |
|---|---|---|---|---|---|---|
| Apple fruit | Amri | 9 (5) | 55.6 | 66.9 ± 94.7 | 221 | 3 (33.3) |
| Apple fruit | Kashmiri | 11 (7) | 63.6 | 100.6 ± 111.3 | 264 | 5 (45.5) |
| Apple fruit | Kala Kulu | 15 (4) | 26.7 | 32.3 ± 82.2 | 308 | 3 (20) |
| Apple fruit | Golden delicious | 14 (9) | 64.3 | 68.1 ± 74.1 | 189 | 6 (42.9) |
| Apple fruit | Red delicious | 13 (6) | 46.2 | 67.6 ± 103.4 | 276 | 5 (38.5) |
| Apple fruit | Gaja | 8 (8) | 100.0 | 107.3 ± 106.1 | 299 | 4 (50) |
| Apple fruit | Rawalpindi City | 10 (7) | 70.0 | 55.3 ± 68.4 | 182 | 4 (40) |
| Apple fruit | Faisalabad City | 15 (10) | 66.7 | 52.2 ± 75.7 | 283 | 6 (40) |
| Apple fruit | Lahore City | 8 (4) | 50.0 | 57.9 ± 102.3 | 277 | 2 (25) |
| Apple fruit | Gilgit City | 7 (3) | 42.9 | 16.9 ± 35.6 | 97 | 1 (14.3) |
| Apple fruit | Swat City | 8 (2) | 25.0 | 9.4 ± 24 | 68 | 1 (12.5) |
| Apple fruit | Muzafarabad City | 9 (4) | 44.4 | 59.2 ± 110.7 | 311 | 2 (22.2) |
| Apple fruit | Murree City | 6 (6) | 100.0 | 183.8 ± 144.2 | 396 | 5 (83.3) |
| Apple juice | General stores | 35 (22) | 62.9 | 5.6 ± 5.1 | 18 | 0 (0) |
| Apple juice concentrate | Supermarket | 10 (8) | 80.0 | 107.5 ± 115.5 | 328 | 5 (50) |
| Apple puree | City market | 21 (15) | 71.4 | 29.4 ± 32.3 | 99 | 5 (23.8) |
| Apple jam | General stores | 10 (3) | 30.0 | 1.7 ± 2.7 | 6 | 0 (0) |
| Total | --- | 209 (123) | 58.9 | 49.8 ± 83.6 | 396 | 57 (27.3) |
| Sample Type | Variety/Location | n Total (Positive) | Incidence % | Mean ± SD | Max. | n (%) > 50 µg/kg |
|---|---|---|---|---|---|---|
| Grapes fruit | White Thompson 1 | 6 (3) | 50 | 33.8 ± 50.3 | 119 | 2 (33.3) |
| Grapes fruit | King Ruby 1 | 9 (6) | 66.7 | 52.7 ± 60.6 | 152 | 4 (44.4) |
| Grapes fruit | Narc Black 1 | 12 (7) | 58.3 | 60.4 ± 93.2 | 274 | 4 (33.3) |
| Grapes fruit | Perlette 1 | 8 (5) | 62.5 | 53.4 ± 102.3 | 298 | 2 (25) |
| Grapes fruit | Sundar Khani | 14 (8) | 57.1 | 90.9 ± 140.9 | 490 | 6 (42.9) |
| Grapes fruit | Vitro Black | 10 (6) | 60 | 63.6 ± 112.9 | 356 | 3 (30) |
| Grapes fruit | Cardinal 1 | 9 (5) | 55.6 | 62.9 ± 114.2 | 310 | 2(22.2) |
| Grapes fruit | Flame 1 | 11 (6) | 54.5 | 40.5 ± 68.8 | 183 | 2 (18.2) |
| Grapes fruit | Faisalabad City | 16 (10) | 62.5 | 99.4 ± 169.2 | 505 | 5 (31.3) |
| Grapes fruit | Lahore City | 10 (5) | 50 | 57.7 ± 107.1 | 306 | 2 (20) |
| Grapes fruit | Rawalpindi City | 10 (7) | 70 | 101.1 ± 139.7 | 416 | 4 (40) |
| Grapes fruit | Attock City | 7 (4) | 57.1 | 22.5 ± 38.7 | 107 | 1 (14.3) |
| Grapes fruit | Dunyapur City | 5 (3) | 60 | 55 ± 89.2 | 210 | 2 (40) |
| Grapes fruit | Chakwal City | 6 (4) | 66.7 | 44.6 ± 70.7 | 180 | 2 (33.3) |
| Grapes juice | Supermarket | 39 (33) | 84.6 | 16.3 ± 11.9 | 39 | 0 |
| Total | --- | 172 (112) | 65.1 | 53.9 ± 98.5 | 505 | 41 (23.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Asi, M.R.; Iqbal, M.; Akhtar, M.; Imran, M.; Ariño, A. Surveillance of Patulin in Apple, Grapes, Juices and Value-Added Products for Sale in Pakistan. Foods 2020, 9, 1744. https://doi.org/10.3390/foods9121744
Hussain S, Asi MR, Iqbal M, Akhtar M, Imran M, Ariño A. Surveillance of Patulin in Apple, Grapes, Juices and Value-Added Products for Sale in Pakistan. Foods. 2020; 9(12):1744. https://doi.org/10.3390/foods9121744
Chicago/Turabian StyleHussain, Shabbir, Muhammad Rafique Asi, Mazhar Iqbal, Muhammad Akhtar, Muhammad Imran, and Agustín Ariño. 2020. "Surveillance of Patulin in Apple, Grapes, Juices and Value-Added Products for Sale in Pakistan" Foods 9, no. 12: 1744. https://doi.org/10.3390/foods9121744
APA StyleHussain, S., Asi, M. R., Iqbal, M., Akhtar, M., Imran, M., & Ariño, A. (2020). Surveillance of Patulin in Apple, Grapes, Juices and Value-Added Products for Sale in Pakistan. Foods, 9(12), 1744. https://doi.org/10.3390/foods9121744