Analysis of Total Phenols, Sugars, and Mineral Elements in Colored Tubers of Solanum tuberosum L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Studied Plant Material
2.2. Extraction and Dry-Weight
2.3. Determination of Total Phenols and Anthocyanins
2.4. Capillary Electrophoretic Analysis of Natural Sugars
2.5. Atomic Absobrance Analysis of Microelements
2.6. Statistical Analysis
3. Results and Discussion
3.1. Total Phenlos and Anthocyanins
3.2. Analysis of Sugars
3.3. Microelements in Tubers
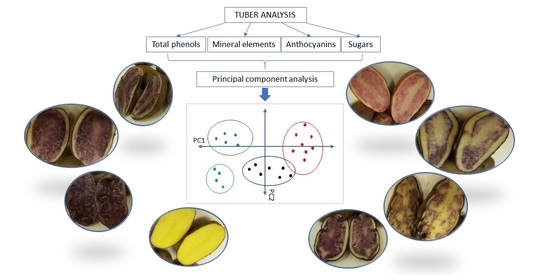
3.4. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Meulenaer, B.; Medeiros, R.; Mestdagh, F. Chapter 18 Acrylamide in Potato Products; Singh, J., Kaur, L.B.T., Second, E., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 527–562. ISBN 978-0-12-800002-1. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets-Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Schilling, G.; Eißner, H.; Schmidt, L.; Peiter, E. Yield formation of five crop species under water shortage and differential potassium supply. J. Plant Nutr. Soil Sci. 2016, 179, 234–243. [Google Scholar] [CrossRef]
- Usmani, A.; Mishra, A. The Globe’s Healthiest Food with Numerous Medicinal Properties—Solanum tuberosum. Res. Rev. A J. Pharmacol. 2016, 6, 1–10. [Google Scholar]
- Wierzbowska, J.; Rychcik, B.; Światły, A. The effect of different production systems on the content of micronutrients and trace elements in potato tubers. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 701–708. [Google Scholar] [CrossRef]
- Murniece, I.; Kruma, Z.; Skrabule, I.; Vaivode, A. Carotenoids and Phenols of Organically and Conventionally Cultivated Potato Varieties. Int. J. Chem. Eng. Appl. 2013, 4, 342–348. [Google Scholar] [CrossRef]
- Kita, A.; Bakowska-Barczak, A.; Hamouz, K.; Kułakowska, K.; Lisińska, G. The effect of frying on anthocyanin stability and antioxidant activity of crisps from red- and purple-fleshed potatoes (Solanum tuberosum L.). J. Food Compos. Anal. 2013, 32, 169–175. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S. Investigation of the anticoagulant and antithrombotic effects of chlorogenic acid. J. Biochem. Mol. Toxicol. 2017, 31, 1–6. [Google Scholar] [CrossRef]
- Strugała, P.; Dzydzan, O.; Brodyak, I.; Kucharska, A.Z.; Kuropka, P.; Liuta, M.; Kaleta-Kuratewicz, K.; Przewodowska, A.; Michałowska, D.; Gabrielska, J.; et al. Antidiabetic and antioxidative potential of the blue Congo variety of purple potato extract in streptozotocin-induced diabetic rats. Molecules 2019, 24, 3126. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.R.; Wrolstad, R.; Durst, R.; Yang, C.P.; Clevidence, B. Breeding studies in potatoes containing high concentrations of anthocyanins. Am. J. Potato Res. 2003, 80, 241–249. [Google Scholar] [CrossRef]
- Cummings, J.H.; Beatty, E.R.; Kingman, S.M.; Bingham, S.A.; Englyst, H.N. Digestion and physiological properties of resistant starch in the human large bowel. Br. J. Nutr. 1996, 75, 733–747. [Google Scholar] [CrossRef]
- Zyzak, D.V.; Sanders, R.A.; Stojanovic, M.; Tallmadge, D.H.; Eberhart, B.L.; Ewald, D.K.; Gruber, D.C.; Morsch, T.R.; Strothers, M.A.; Rizzi, G.P.; et al. Acrylamide formation mechanism in heated foods. J. Agric. Food Chem. 2003, 51, 4782–4787. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singh, B.P.; Kumar, P. An overview of the factors affecting sugar content of potatoes. Ann. Appl. Biol. 2004, 145, 247–256. [Google Scholar] [CrossRef]
- Clements, R.S.; Darnell, B. Myo-inositol content of common foods: Development of a high-myo-inositol diet. Am. J. Clin. Nutr. 1955, 33, 1954–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO Standard no. 10390:2005. Soil Quality—Determination of pH; International Organization for Standardization: Geneva, Switzerland, 2005. [Google Scholar]
- Jankauskas, B.; Jankauskiene, G.; Slepetiene, A.; Fullen, M.A.; Booth, C.A. International comparison of analytical methods of determining the soil organic matter content of Lithuanian Eutric Albeluvisols. Commun. Soil Sci. Plant Anal. 2006, 37, 707–720. [Google Scholar] [CrossRef]
- AgroEcoLab Mehlich 3 Extraction Protocol. Available online: http://www.agroecologylab.com/uploads/2/7/2/8/27281831/mehlich3_extraction.pdf (accessed on 9 September 2020).
- Kotkas, K.; Rosenberg, V. The Methods for Potato Virus Eradication and Creation of Meristem Clones with Improved Traits, Virus-Free Potato Meristem Plants and Virus-Free Potato. International Patent WO 2009/143856 A1, 3 December 2009. [Google Scholar]
- Waterhouse, A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar] [CrossRef]
- Vaher, M.; Borissova, M.; Seiman, A.; Aid, T.; Kolde, H.; Kazarjan, J.; Kaljurand, M. Automatic spot preparation and image processing of paper microzone-based assays for analysis of bioactive compounds in plant extracts. Food Chem. 2014, 143, 465–471. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- Vaher, M.; Helmja, K.; Käsper, A.; Kurašin, M.; Väljamäe, P.; Kudrjašova, M.; Koel, M.; Kaljurand, M. Capillary electrophoretic monitoring of hydrothermal pre-treatment and enzymatic hydrolysis of willow: Comparison with HPLC and NMR. Catal. Today 2012, 196, 34–41. [Google Scholar] [CrossRef]
- Ru, W.; Pang, Y.; Gan, Y.; Liu, Q.; Bao, J. Phenolic compounds and antioxidant activities of potato cultivars with white, yellow, red and purple flesh. Antioxidants 2019, 8, 419. [Google Scholar] [CrossRef] [Green Version]
- Jansen, G.; Flamme, W. Coloured potatoes (Solanum tuberosum L.)-Anthocyanin content and tuber quality. Genet. Resour. Crop Evol. 2006, 53, 1321–1331. [Google Scholar] [CrossRef]
- Wang, S.; Nie, S.; Zhu, F. Chemical constituents and health effects of sweet potato. Food Res. Int. 2016, 89, 90–116. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Delgado, D.; Ñústez-López, C.E.; Narváez-Cuenca, C.E.; Restrepo-Sánchez, L.P.; Melo, S.E.; Sarmiento, F.; Kushalappa, A.C.; Mosquera-Vásquez, T. Natural variation of sucrose, glucose and fructose contents in Colombian genotypes of Solanum tuberosum Group Phureja at harvest. J. Sci. Food Agric. 2016, 96, 4288–4294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piikki, K.; Vorne, V.; Ojanperä, K.; Pleijel, H. Potato tuber sugars, starch and organic acids in relation to ozone exposure. Potato Res. 2003, 46, 67–79. [Google Scholar] [CrossRef]
- Andre, C.M.; Ghislain, M.; Bertin, P.; Oufir, M.; Herrera, M.D.R.; Hoffmann, L.; Hausman, J.F.; Larondelle, Y.; Evers, D. Andean potato cultivars (Solarium tuberosum L.) as a source of antioxidant and mineral micronutrients. J. Agric. Food Chem. 2007, 55, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Navarre, D.A.; Goyer, A.; Shakya, R. Chapter 14-Nutritional Value of Potatoes: Vitamin, Phytonutrient, and Mineral Content. In Advances in Potato Chemistry and Technology; Singh, J., Kaur, L.B.T.-A., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 395–424. ISBN 978-0-12-374349-7. [Google Scholar]
- Wszelaki, A.L.; Delwiche, J.F.; Walker, S.D.; Liggett, R.E.; Scheerens, J.C.; Kleinhenz, M.D. Sensory quality and mineral and glycoalkaloid concentrations in organically and conventionally grown redskin potatoes (Solanum tuberosum). J. Sci. Food Agric. 2005, 85, 720–726. [Google Scholar] [CrossRef]
- Brown, C.R. Breeding for phytonutrient enhancement of potato. Am. J. Potato Res. 2008, 85, 298–307. [Google Scholar] [CrossRef]
- Gąsiorowska, B.; Płaza, A.; Rzążewska, E.; Cybulska, A.; Górski, R. The potato tuber content of microelements as affected by organic fertilisation and production system. Environ. Monit. Assess. 2018, 190, 522. [Google Scholar] [CrossRef]
- Olsen, N.L.; Hiller, L.K.; Mikitzel, L.J. The dependence of internal brown spot development upon calcium fertility in potato tubers. Potato Res. 1996, 39, 165–178. [Google Scholar] [CrossRef]
- Pedreschi, F.; Mariotti, S.; Granby, K.; Risum, J. Acrylamide reduction in potato chips by using commercial asparaginase in combination with conventional blanching. LWT Food Sci. Technol. 2011, 44, 1473–1476. [Google Scholar] [CrossRef]




| Sample No | Material Number | Descriptors of Tubers | ||
|---|---|---|---|---|
| Skin Color | Flesh Color | Tuber’s Shape | ||
| Botanical Seeds | ||||
| 1 | 25 | Violet | Oak leaf form with white border around | Ovate |
| 2 | 34 | Violet | Violet with white border | Elongate, big |
| 3 | 51 | Dark violet | Dark violet marble | Ovate |
| 4 | 53 | Dark violet | Dark violet marble | Elongate |
| 5 | 76 | Pale violet | Pale reddish violet, white border | Ovate |
| 6 | 89 | Pale violet | White border, beautiful violet oak form | Round |
| 7 | 116 | Reddish violet | Pale reddish violet with white border | Ovate |
| Cross-Breeding Blue Congo and Desiree | ||||
| 8 | 41 | Dark violet | White border, violet vary-colored oak leaf form | Ovate |
| 9 | 47 | Dark pink | Pale pink border white in middle | Ovate |
| Cross-Breeding Blue Congo and Granola | ||||
| 10 | 9 | Dark violet | Dark violet pale border | Elongate big |
| 11 | 16 | Dark violet | Strong white border violet vary-color | Elongate big |
| 12 | 28 | Dark violet | Vary-color violet narrow border | Elongate-ovate |
| 13 | 30 | Dark pink | Netting pink violet | Uniform ovate |
| 14 | 36 | Dark pink | Netting blanched yellow middle | Ovate |
| 15 | 41 | Dark violet | Violet netting | Uniform ovate |
| Meristem Clones of Blue Congo | ||||
| 16 | 40 | Dark violet netting | Pale violet middle with pale border | Round |
| 17 | 195 | Dark violet netting | Dark violet with white border | Ovate |
| 18 | 197 | Dark violet netting | Pale violet with wider white border | Round |
| Commercial Varieties | ||||
| 19 | Teele | Yellow netting | Yellow | Round-ovate |
| 20 | Laura | Smooth dark red | Dark yellow | Round-ovate |
| 21 | Sweet Potato | Purple | Purple | Elongate |
| Sample No | Cu, mg/kg | Zn, mg/kg | Mn, mg/kg | Fe, mg/kg | Mg, mg/kg | Ca, mg/kg | K, g/kg |
|---|---|---|---|---|---|---|---|
| 1 | 2.477 | 11.02 | 8.68 | 77.2 | 973.6 | 349.2 | 19.49 |
| 2 | 3.212 | 17.02 | 7.21 | 80.3 | 880.8 | 437.5 | 17.05 |
| 3 | 4.000 | 14.11 | 8.02 | 57.0 | 1253.6 | 554.1 | 22.83 |
| 4 | 2.146 | 12.14 | 7.18 | 61.2 | 921.4 | 583.0 | 17.36 |
| 5 | 3.016 | 17.31 | 8.63 | 109.9 | 1264.3 | 508.8 | 21.54 |
| 6 | 2.793 | 13.51 | 7.16 | 74.3 | 926.6 | 466.7 | 17.19 |
| 7 | 4.244 | 12.64 | 8.73 | 70.6 | 976.1 | 375.6 | 14.27 |
| 8 | 2.952 | 10.91 | 8.56 | 85.6 | 1082.8 | 623.9 | 17.78 |
| 9 | 4.116 | 26.01 | 9.25 | 86.1 | 865.3 | 331.8 | 11.81 |
| 10 | 4.335 | 15.69 | 12.04 | 85.0 | 1004.8 | 668.9 | 16.53 |
| 11 | 5.390 | 14.53 | 7.30 | 115.1 | 1138.4 | 591.8 | 18.86 |
| 12 | 1.994 | 14.64 | 9.89 | 133.0 | 1240.2 | 316.8 | 18.84 |
| 13 | 3.708 | 14.74 | 9.82 | 82.5 | 1087.7 | 377.8 | 14.19 |
| 14 | 5.937 | 18.35 | 9.37 | 120.3 | 1225.3 | 729.1 | 18.67 |
| 15 | 4.689 | 14.95 | 6.75 | 54.4 | 1029.6 | 392.2 | 13.71 |
| 16 | 3.943 | 9.81 | 10.29 | 56.2 | 692.4 | 319.4 | 10.35 |
| 17 | 4.688 | 14.20 | 6.74 | 58.9 | 921.0 | 501.3 | 14.47 |
| 18 | 3.153 | 13.32 | 7.86 | 77.7 | 1045.0 | 517.5 | 15.11 |
| 19 | 3.353 | 12.41 | 5.26 | 48.3 | 867.7 | 489.2 | 13.06 |
| 20 | 4.385 | 14.62 | 10.60 | 53.4 | 922.2 | 270.5 | 15.81 |
| 21 | 9.630 | 8.17 | 6.79 | 45.8 | 988.8 | 679.1 | 8.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saar-Reismaa, P.; Kotkas, K.; Rosenberg, V.; Kulp, M.; Kuhtinskaja, M.; Vaher, M. Analysis of Total Phenols, Sugars, and Mineral Elements in Colored Tubers of Solanum tuberosum L. Foods 2020, 9, 1862. https://doi.org/10.3390/foods9121862
Saar-Reismaa P, Kotkas K, Rosenberg V, Kulp M, Kuhtinskaja M, Vaher M. Analysis of Total Phenols, Sugars, and Mineral Elements in Colored Tubers of Solanum tuberosum L. Foods. 2020; 9(12):1862. https://doi.org/10.3390/foods9121862
Chicago/Turabian StyleSaar-Reismaa, Piret, Katrin Kotkas, Viive Rosenberg, Maria Kulp, Maria Kuhtinskaja, and Merike Vaher. 2020. "Analysis of Total Phenols, Sugars, and Mineral Elements in Colored Tubers of Solanum tuberosum L." Foods 9, no. 12: 1862. https://doi.org/10.3390/foods9121862
APA StyleSaar-Reismaa, P., Kotkas, K., Rosenberg, V., Kulp, M., Kuhtinskaja, M., & Vaher, M. (2020). Analysis of Total Phenols, Sugars, and Mineral Elements in Colored Tubers of Solanum tuberosum L. Foods, 9(12), 1862. https://doi.org/10.3390/foods9121862