Antimicrobial Blue Light versus Pathogenic Bacteria: Mechanism, Application in the Food Industry, Hurdle Technologies and Potential Resistance
Abstract
:1. Introduction
2. Pathogenic Bacteria in Food
3. Antimicrobial Blue Light
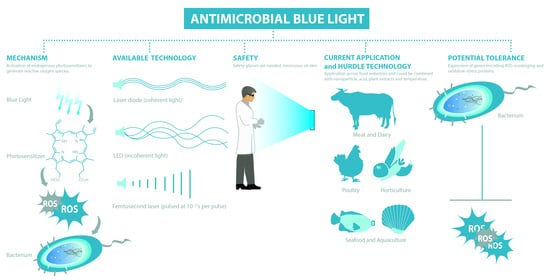
3.1. Mechanism
3.2. Available Technologies
3.3. Blue Light Regimes
3.4. Safety of Blue Light
4. Application of Antimicrobial Blue Light on Surfaces and in Food Matrixes
4.1. Inactivation of Bacteria on Food Packaging and Work Surfaces
4.2. Inactivation of Bacteria in Dairy and Liquid Foods: Milk, Cheese and Orange Juice
4.3. Inactivation of Bacteria in Horticultural Products
4.4. Inactivation of Bacteria in Meat Products and Seafood (Chicken, Beef and Fish)
5. Potential Application of Blue Light in Food Supply Chain
5.1. Food Processing and Farms: Airborne and Surface Inactivation
5.2. Aquaculture
5.3. Retail: Prolonging Shelf-Life
6. Hurdle Technology
6.1. Photosensitizers
6.2. Acidity and Temperature
6.3. Nanoparticle
6.4. Plant Extracts: Polyphenols and Essential Oils
7. Blue Light versus Antimicrobial Resistance and Consequences of Sub-Lethal Light Exposures
7.1. Resistant Bacteria: Improved Sensitivity to Antibiotics
7.2. Inactivation of Biofilms
7.3. Sub-Lethal Exposures Induce Cellular Processes Potentially Leading to Tolerance
8. Research Gap and Future Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO|WHO Estimates of the Global Burden of Foodborne Diseases. Available online: http://www.who.int/foodsafety/publications/foodborne_disease/fergreport/en/ (accessed on 10 October 2020).
- Dickson-Spillmann, M.; Siegrist, M.; Keller, C. Attitudes toward Chemicals Are Associated with Preference for Natural Food. Food Qual. Prefer. 2011, 22, 149–156. [Google Scholar] [CrossRef]
- Ha, T.M.; Shakur, S.; Pham Do, K.H. Consumer Concern about Food Safety in Hanoi, Vietnam. Food Control 2019, 98, 238–244. [Google Scholar] [CrossRef]
- Soni, A.; Oey, I.; Silcock, P.; Bremer, P. Bacillus Spores in the Food Industry: A Review on Resistance and Response to Novel Inactivation Technologies. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1139–1148. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.S.; Wang, L.H.; Bekhit, A.E.D.A.; Yang, J.; Hou, Z.P.; Wang, Y.Z.; Dai, Q.Z.; Zeng, X.A. A Review of Sublethal Effects of Pulsed Electric Field on Cells in Food Processing. J. Food Eng. 2018, 223, 32–41. [Google Scholar] [CrossRef]
- Huang, H.W.; Wu, S.J.; Lu, J.K.; Shyu, Y.T.; Wang, C.Y. Current Status and Future Trends of High-Pressure Processing in Food Industry. Food Control 2017, 72, 1–8. [Google Scholar] [CrossRef]
- Sampedro, F.; McAloon, A.; Yee, W.; Fan, X.; Geveke, D.J. Cost Analysis and Environmental Impact of Pulsed Electric Fields and High Pressure Processing in Comparison with Thermal Pasteurization. Food Bioprocess Technol. 2014, 7, 1928–1937. [Google Scholar] [CrossRef]
- D’Souza, C.; Yuk, H.G.; Khoo, G.H.; Zhou, W. Application of Light-Emitting Diodes in Food Production, Postharvest Preservation, and Microbiological Food Safety. Compr. Rev. Food Sci. Food Saf. 2015, 14, 719–740. [Google Scholar] [CrossRef]
- Bintsis, T.; Litopoulou-Tzanetaki, E.; Robinson, R.K. Existing and Potential Applications of Ultraviolet Light in the Food Industry—A Critical Review. J. Sci. Food Agric. 2000, 80, 637–645. [Google Scholar] [CrossRef]
- Koutchma, T. UV Light Microbial Inactivation in Foods. In Ultraviolet Light in Food Technology: Principles and Applications; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–344. [Google Scholar]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [Green Version]
- Kütting, B.; Drexler, H. UV-Induced Skin Cancer at Workplace and Evidence-Based Prevention. Int. Arch. Occup. Environ. Health 2010, 83, 843–854. [Google Scholar] [CrossRef]
- Zaffina, S.; Camisa, V.; Lembo, M.; Vinci, M.R.; Tucci, M.G.; Borra, M.; Napolitano, A.; Cannatã, V. Accidental Exposure to UV Radiation Produced by Germicidal Lamp: Case Report and Risk Assessment. Photochem. Photobiol. 2012, 88, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Dai, T.; Avci, P.; Jorge, A.E.S.; De Melo, W.C.M.A.; Vecchio, D.; Huang, Y.Y.; Gupta, A.; Hamblin, M.R. Light Based Anti-Infectives: Ultraviolet C Irradiation, Photodynamic Therapy, Blue Light, and Beyond. Curr. Opin. Pharmacol. 2013, 13, 731–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 17 December 2020).
- Møretrø, T.; Langsrud, S. Residential Bacteria on Surfaces in the Food Industry and Their Implications for Food Safety and Quality. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1022–1041. [Google Scholar] [CrossRef] [Green Version]
- Fox, E.M.; Jiang, Y.; Gobius, K.S. Key Pathogenic Bacteria Associated with Dairy Foods: On-Farm Ecology and Products Associated with Foodborne Pathogen Transmission. Int. Dairy J. 2018, 84, 28–35. [Google Scholar] [CrossRef]
- Cancino-Padilla, N.; Fellenberg, M.A.; Franco, W.; Ibáñez, R.A.; Vargas-Bello-Pérez, E. Foodborne Bacteria in Dairy Products: Detection by Molecular Techniques. Cien. Inv. Agr. 2017, 44, 215–229. [Google Scholar] [CrossRef] [Green Version]
- Castro, H.; Jaakkonen, A.; Hakkinen, M.; Korkeala, H.; Lindström, M. Occurrence, Persistence, and Contamination Routes of Listeria Monocytogenes Genotypes on Three Finnish Dairy Cattle Farms: A Longitudinal Study. Appl. Environ. Microbiol. 2018, 84, 2000–2017. [Google Scholar] [CrossRef] [Green Version]
- Haley, B.J.; Sonnier, J.; Schukken, Y.H.; Karns, J.S.; Van Kessel, J.A.S. Diversity of Listeria Monocytogenes Within a U.S. Dairy Herd, 2004–2010. Foodborne Pathog. Dis. 2015, 12, 844–850. [Google Scholar] [CrossRef]
- Browne, A.S.; Midwinter, A.C.; Withers, H.; Cookson, A.L.; Biggs, P.J.; Marshall, J.C.; Benschop, J.; Hathaway, S.; Haack, N.A.; Akhter, R.N.; et al. Molecular Epidemiology of Shiga Toxinproducing Escherichia Coli (STEC) on New Zealand Dairy Farms: Application of a Culture-Independent Assay and Whole-Genome Sequencing. Appl. Environ. Microbiol. 2018, 84, 481–499. [Google Scholar] [CrossRef] [Green Version]
- Venegas-Vargas, C.; Henderson, S.; Khare, A.; Mosci, R.E.; Lehnert, J.D.; Singh, P.; Ouellette, L.M.; Norby, B.; Funk, J.A.; Rust, S.; et al. Factors Associated with Shiga Toxin-Producing Escherichia Coli Shedding by Dairy and Beef Cattle. Appl. Environ. Microbiol. 2016, 82, 5049–5056. [Google Scholar] [CrossRef] [Green Version]
- Ross, C.M.; Rapp, D.; Cave, V.M.; Brightwell, G. Prevalence of Shiga Toxin-Producing Escherichia Coli in Pasture-Based Dairy Herds. Lett. Appl. Microbiol. 2019, 68, 112–119. [Google Scholar] [CrossRef]
- McAuley, C.M.; McMillan, K.E.; Moore, S.C.; Fegan, N.; Fox, E.M. Characterization of Escherichia Coli and Salmonella from Victoria, Australia, Dairy Farm Environments. J. Food Prot. 2017, 80, 2078–2082. [Google Scholar] [CrossRef]
- Rodriguez-Rivera, L.D.; Wright, E.M.; Siler, J.D.; Elton, M.; Cummings, K.J.; Warnick, L.D.; Wiedmann, M. Subtype Analysis of Salmonella Isolated from Subclinically Infected Dairy Cattle and Dairy Farm Environments Reveals the Presence of Both Human- and Bovine-Associated Subtypes. Vet. Microbiol. 2014, 170, 307–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuenberger, A.; Sartori, C.; Boss, R.; Resch, G.; Oechslin, F.; Steiner, A.; Moreillon, P.; Graber, H.U. Genotypes of Staphylococcus Aureus: On-Farm Epidemiology and the Consequences for Prevention of Intramammary Infections. J. Dairy Sci. 2019, 102, 3295–3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAuley, C.M.; McMillan, K.; Moore, S.C.; Fegan, N.; Fox, E.M. Prevalence and Characterization of Foodborne Pathogens from Australian Dairy Farm Environments. J. Dairy Sci. 2014, 97, 7402–7412. [Google Scholar] [CrossRef]
- Hunter, C.J.; Bean, J.F. Cronobacter: An Emerging Opportunistic Pathogen Associated with Neonatal Meningitis, Sepsis and Necrotizing Enterocolitis. J. Perinatol. 2013, 33, 581–585. [Google Scholar] [CrossRef] [Green Version]
- Norberg, S.; Stanton, C.; Ross, R.P.; Hill, C.; Fitzgerald, G.F.; Cotter, P.D. Cronobacter spp. in Powdered Infant Formula. J. Food Prot. 2012, 75, 607–620. [Google Scholar] [CrossRef]
- Vojkovska, H.; Karpiskova, R.; Orieskova, M.; Drahovska, H. Characterization of Cronobacter Spp. Isolated from Food of Plant Origin and Environmental Samples Collected from Farms and from Supermarkets in the Czech Republic. Int. J. Food Microbiol. 2016, 217, 130–136. [Google Scholar] [CrossRef]
- Jaradat, Z.W.; Ababneh, Q.O.; Saadoun, I.M.; Samara, N.A.; Rashdan, A.M. Isolation of Cronobacter spp. (Formerly Enterobacter Sakazakii) from Infant Food, Herbs and Environmental Samples and the Subsequent Identification and Confirmation of the Isolates Using Biochemical, Chromogenic Assays, PCR and 16S RRNA Sequencing. BMC Microbiol. 2009, 9, 225. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Iversen, C.; Gontia, I.; Stephan, R.; Hofmann, A.; Hartmann, A.; Jha, B.; Eberl, L.; Riedel, K.; Lehner, A. Evidence for a Plant-Associated Natural Habitat for Cronobacter spp. Res. Microbiol. 2009, 160, 608–614. [Google Scholar] [CrossRef] [Green Version]
- Molloy, C.; Cagney, C.; O’Brien, S.; Iversen, C.; Fanning, S.; Duffy, G. Surveillance and Characterisation by Pulsed-Field Gel Electrophoresis of Cronobacter Spp. in Farming and Domestic Environments, Food Production Animals and Retail Foods. Int. J. Food Microbiol. 2009, 136, 198–203. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The Complex Microbiota of Raw Milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef] [Green Version]
- Oliver, S.P.; Murinda, S.E. Milk and Raw Milk Consumption as a Vector for Human Disease. In Zoonotic Pathogens in the Food Chain; Krause, D., Hendrick, S., Eds.; CABI: Oxfordshire, UK, 2010; pp. 99–118. [Google Scholar]
- Gaulin, C.; Ramsay, D.; Bekal, S. Widespread Listeriosis Outbreak Attributable to Pasteurized Cheese, Which Led to Extensive Cross-Contamination Affecting Cheese Retailers, Quebec, Canada, 2008. J. Food Prot. 2012, 75, 71–78. [Google Scholar] [CrossRef]
- Germinario, C.; Caprioli, A.; Giordano, M.; Chironna, M.; Gallone, M.S.; Tafuri, S.; Minelli, F.; Maugliani, A.; Michelacci, V.; Santangelo, L.; et al. Community-Wide Outbreak of Haemolytic Uraemic Syndrome Associated with Shiga Toxin 2-Producing Escherichia Coli O26: H11 in Southern Italy, Summer 2013. Eurosurveillance 2016, 21, 30343. [Google Scholar] [CrossRef] [Green Version]
- Oxaran, V.; Lee, S.H.I.; Chaul, L.T.; Corassin, C.H.; Barancelli, G.V.; Alves, V.F.; de Oliveira, C.A.F.; Gram, L.; De Martinis, E.C.P. Listeria Monocytogenes Incidence Changes and Diversity in Some Brazilian Dairy Industries and Retail Products. Food Microbiol. 2017, 68, 16–23. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shimamoto, T. Isolation and Molecular Characterization of Salmonella Enterica, Escherichia Coli O157: H7 and Shigella Spp. from Meat and Dairy Products in Egypt. Int. J. Food Microbiol. 2014, 168–169, 57–62. [Google Scholar] [CrossRef]
- Derra, F.A.; Karlsmose, S.; Monga, D.P.; Mache, A.; Svendsen, C.A.; Félix, B.; Granier, S.A.; Geyid, A.; Taye, G.; Hendriksen, R.S. Occurrence of Listeria Spp. in Retail Meat and Dairy Products in the Area of Addis Ababa, Ethiopia. Foodborne Pathog. Dis. 2013, 10, 577–579. [Google Scholar] [CrossRef] [Green Version]
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A Systematic Review of Bacterial Foodborne Outbreaks Related to Red Meat and Meat Products. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef] [Green Version]
- Tesson, V.; Federighi, M.; Cummins, E.; de Oliveira Mota, J.; Guillou, S.; Boué, G. A Systematic Review of Beef Meat Quantitative Microbial Risk Assessment Models. Int. J. Environ. Res. Public Health 2020, 17, 688. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Junior, C.A. Worldwide Epidemiology of Salmonella Serovars in Animal-Based Foods: A Meta-Analysis. Am. Soc. Microbiol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Mor-Mur, M.; Yuste, J. Emerging Bacterial Pathogens in Meat and Poultry: An Overview. Food Bioprocess Technol. 2010, 3, 24–35. [Google Scholar]
- Gieraltowski, L.; Higa, J.; Peralta, V.; Green, A.; Schwensohn, C.; Rosen, H.; Libby, T.; Kissler, B.; Marsden-Haug, N.; Booth, H.; et al. National Outbreak of Multidrug Resistant Salmonella Heidelberg Infections Linked to a Single Poultry Company. PLoS ONE 2016, 11, e0162369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grass, J.E.; Gould, L.H.; Mahon, B.E. Epidemiology of Foodborne Disease Outbreaks Caused by Clostridium Perfringens, United States, 1998–2010. Foodborne Pathog. Dis. 2013, 10, 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abid, M.; Wimalarathna, H.; Mills, J.; Saldana, L.; Pang, W.; Richardson, J.F.; Maiden, M.C.J.; McCarthy, N.D. Duck Liver–Associated Outbreak of Campylobacteriosis among Humans, United Kingdom, 2011. Emerg. Infect. Dis. 2013, 19, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.C.; Harding, O.; Fisher, L.; Cowden, J. A Continuous Common-Source Outbreak of Campylobacteriosis Associated with Changes to the Preparation of Chicken Liver Pâté. Epidemiol. Infect. 2009, 137, 383–388. [Google Scholar] [CrossRef] [Green Version]
- Chai, S.J.; Cole, D.; Nisler, A.; Mahon, B.E. Poultry: The Most Common Food in Outbreaks with Known Pathogens, United States, 1998–2012. Epidemiol. Infect. 2017, 145, 316–325. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention (CDC). Outbreak of Salmonella Heidelberg Infections Linked to a Single Poultry Producer—13 States, 2012-2013. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 553–556. [Google Scholar]
- Little, C.L.; Gormley, F.J.; Rawal, N.; Richardson, J.F. A Recipe for Disaster: Outbreaks of Campylobacteriosis Associated with Poultry Liver Pâté in England and Wales. Epidemiol. Infect. 2010, 138, 1691–1694. [Google Scholar] [CrossRef]
- Rouger, A.; Tresse, O.; Zagorec, M. Bacterial Contaminants of Poultry Meat: Sources, Species, and Dynamics. Microorganisms 2017, 5, 50. [Google Scholar] [CrossRef]
- Yang, B.; Qu, D.; Zhang, X.; Shen, J.; Cui, S.; Shi, Y.; Xi, M.; Sheng, M.; Zhi, S.; Meng, J. Prevalence and Characterization of Salmonella Serovars in Retail Meats of Marketplace in Shaanxi, China. Int. J. Food Microbiol. 2010, 141, 63–72. [Google Scholar] [CrossRef]
- Wu, S.; Huang, J.; Wu, Q.; Zhang, J.; Zhang, F.; Yang, X.; Wu, H.; Zeng, H.; Chen, M.; Ding, Y.; et al. Staphylococcus Aureusisolated from Retail Meat and Meat Products in China: Incidence, Antibiotic Resistance and Genetic Diversity. Front. Microbiol. 2018, 9, 2767. [Google Scholar] [CrossRef]
- Ali, N.H.; Farooqui, A.; Khan, A.; Khan, A.Y.; Kazmi, S.U. Microbial Contamination of Raw Meat and Its Environment in Retail Shops in Karachi, Pakistan. J. Infect. Dev. Ctries. 2010, 4, 382–388. [Google Scholar]
- Ge, B.; Mukherjee, S.; Hsu, C.H.; Davis, J.A.; Tran, T.T.T.; Yang, Q.; Abbott, J.W.; Ayers, S.L.; Young, S.R.; Crarey, E.T.; et al. MRSA and Multidrug-Resistant Staphylococcus Aureus in U.S. Retail Meats, 2010–2011. Food Microbiol. 2017, 62, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Ayers, T.; Mahon, B.E.; Swerdlow, D.L. Epidemiology of Seafood-Associated Infections in the United States. Clin. Microbiol. Rev. 2010, 23, 399–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoslavskij, A.; Terentjeva, M.; Eizenberga, I.; Valciņa, O.; Bartkevičs, V.; Bērziņš, A. Major Foodborne Pathogens in Fish and Fish Products: A Review. Ann. Microbiol. 2016, 66, 1–15. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, L.; Yang, Q.; Han, F.; Chen, S.; Pu, S.; Vance, A.; Ge, B. Prevalence and Antimicrobial Susceptibility of Major Foodborne Pathogens in Imported Seafood. J. Food Prot. 2011, 74, 1451–1461. [Google Scholar] [CrossRef]
- Dumen, E.; Ekici, G.; Ergin, S.; Bayrakal, G.M. Presence of Foodborne Pathogens in Seafood and Risk Ranking for Pathogens. Foodborne Pathog. Dis. 2020, 17, 541–546. [Google Scholar] [CrossRef]
- Novotny, L.; Dvorska, L.; Lorencova, A.; Beran, V.; Pavlik, I. Fish: A Potential Source of Bacterial Pathogens for Human Beings. Vet. Med. 2004, 49, 343–358. [Google Scholar] [CrossRef]
- Ali, A.; Parisi, A.; Conversano, M.C.; Iannacci, A.; D’Emilio, F.; Mercurio, V.; Normanno, G. Food-Borne Bacteria Associated with Seafoods: A Brief Review. J. Food Qual. Hazards Control. 2020, 7, 4–10. [Google Scholar] [CrossRef]
- Herwig, R. Pathogens Transmitted by Seafood. In Foodborne Disease Handbook: Volume IV: Seafood and Environmental Toxins; Hui, Y.H., Kitts, D., Stanfield, P.S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; p. 109. [Google Scholar]
- Jami, M.; Ghanbari, M.; Zunabovic, M.; Domig, K.J.; Kneifel, W. Listeria Monocytogenes in Aquatic Food Products—A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 798–813. [Google Scholar] [CrossRef]
- Jung, S.W. A Foodborne Outbreak of Gastroenteritis Caused by Vibrio Parahaemolyticus Associated with Cross-Contamination from Squid in Korea. Epidemiol. Health 2018, 40, e2018056. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, J.; Hong, S.; Lee, S.; Na, H.Y.; Jeong, Y.-I.; Choi, E.J.; Kim, J.; Kawk, H.S.; Cho, E. Cholera Outbreak Due to Raw Seafood Consumption in South Korea, 2016. Am. J. Trop. Med. Hyg. 2018, 99, 168–170. [Google Scholar] [CrossRef] [Green Version]
- Hassan, R.; Tecle, S.; Adcock, B.; Kellis, M.; Weiss, J.; Saupe, A.; Sorenson, A.; Klos, R.; Blankenship, J.; Blessington, T.; et al. Multistate Outbreak of Salmonella Paratyphi B Variant L(+) Tartrate(+) and Salmonella Weltevreden Infections Linked to Imported Frozen Raw Tuna: USA, March–July 2015. Epidemiol. Infect. 2018, 146, 1461–1467. [Google Scholar] [CrossRef] [Green Version]
- Heinitz, M.L.; Ruble, R.D.; Wagner, D.E.; Tatini, S.R. Incidence of Salmonella in Fish and Seafood. J. Food Prot. 2000, 63, 579–592. [Google Scholar] [CrossRef]
- Taylor, M.; Cheng, J.; Sharma, D.; Bitzikos, O.; Gustafson, R.; Fyfe, M.; Greve, R.; Murti, M.; Stone, J.; Honish, L.; et al. Outbreak of Vibrio Parahaemolyticus Associated with Consumption of Raw Oysters in Canada, 2015. Foodborne Pathog. Dis. 2018, 15, 554–559. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Factors Influencing the Microbial Safety of Fresh Produce: A Review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and Contamination Routes of Microbial Pathogens to Fresh Produce during Field Cultivation: A Review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Beuchat, L.R. Ecological Factors Influencing Survival and Growth of Human Pathogens on Raw Fruits and Vegetables. Microbes Infect. 2002, 4, 413–423. [Google Scholar] [CrossRef]
- Araújo, S.; Silva, I.A.T.; Tacão, M.; Patinha, C.; Alves, A.; Henriques, I. Characterization of Antibiotic Resistant and Pathogenic Escherichia Coli in Irrigation Water and Vegetables in Household Farms. Int. J. Food Microbiol. 2017, 257, 192–200. [Google Scholar] [CrossRef]
- Akinde, S.B.; Sunday, A.A.; Adeyemi, F.M.; Fakayode, I.B.; Oluwajide, O.O.; Adebunmi, A.A.; Oloke, J.K.; Adebooye, C.O. Microbes in Irrigation Water and Fresh Vegetables: Potential Pathogenic Bacteria Assessment and Implications for Food Safety. Appl. Biosaf. 2016, 21, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Steele, M.; Odumeru, J. Irrigation Water as Source of Foodborne Pathogens on Fruit and Vegetables. J. Food Prot. 2004, 67, 2839–2849. [Google Scholar] [CrossRef]
- Okafo, C.N.; Umoh, V.J.; Galadima, M. Occurrence of Pathogens on Vegetables Harvested from Soils Irrigated with Contaminated Streams. Sci. Total Environ. 2003, 311, 49–56. [Google Scholar] [CrossRef]
- Hounmanou, Y.M.G.; Mdegela, R.H.; Dougnon, T.V.; Mhongole, O.J.; Mayila, E.S.; Malakalinga, J.; Makingi, G.; Dalsgaard, A. Toxigenic Vibrio Cholerae O1 in Vegetables and Fish Raised in Wastewater Irrigated Fields and Stabilization Ponds during a Non-Cholera Outbreak Period in Morogoro, Tanzania: An Environmental Health Study. BMC Res. Notes 2016, 9, 466. [Google Scholar] [CrossRef] [Green Version]
- Willis, C.; McLauchlin, J.; Aird, H.; Amar, C.; Barker, C.; Dallman, T.; Elviss, N.; Lai, S.; Sadler-Reeves, L. Occurrence of Listeria and Escherichia Coli in Frozen Fruit and Vegetables Collected from Retail and Catering Premises in England 2018–2019. Int. J. Food Microbiol. 2020, 334, 108849. [Google Scholar] [CrossRef]
- Sagoo, S.K.; Little, C.L.; Ward, L.; Gillespie, I.A.; Mitchell, R.T. Microbiological Study of Ready-to-Eat Salad Vegetables from Retail Establishments Uncovers a National Outbreak of Salmonellosis. J. Food Prot. 2003, 66, 403–409. [Google Scholar] [CrossRef]
- Ponniah, J.; Robin, T.; Paie, M.S.; Radu, S.; Ghazali, F.M.; Kqueen, C.Y.; Nishibuchi, M.; Nakaguchi, Y.; Malakar, P.K. Listeria Monocytogenes in Raw Salad Vegetables Sold at Retail Level in Malaysia. Food Control 2010, 21, 774–778. [Google Scholar] [CrossRef]
- Ajayeoba, T.A.; Atanda, O.O.; Obadina, A.O.; Bankole, M.O.; Adelowo, O.O. The Incidence and Distribution of Listeria Monocytogenes in Ready-to-Eat Vegetables in South-Western Nigeria. Food Sci. Nutr. 2016, 4, 59–66. [Google Scholar] [CrossRef]
- Gomez-Govea, M.; Solis, L.; Heredia, N.; García, S. Analysis of Microbial Contamination Levels of Fruits and Vegetables at Retail in Monterrey, Mexico. J. Food Agric. Environ. 2005, 10, 152–156. [Google Scholar]
- Denis, N.; Zhang, H.; Leroux, A.; Trudel, R.; Bietlot, H. Prevalence and Trends of Bacterial Contamination in Fresh Fruits and Vegetables Sold at Retail in Canada. Food Control 2016, 67, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.; Gooneratne, R.; Hussain, M.A. Detection of Listeria Species in Fresh Produce Samples from Different Retail Shops in Canterbury, New Zealand. Adv. Food Technol. Nutr. Sci.Open J. 2016, 2, 96–102. [Google Scholar] [CrossRef]
- Nunes Silva, B.; Cadavez, V.; Teixeira, J.A.; Gonzales-Barron, U. Meta-Analysis of the Incidence of Foodborne Pathogens in Vegetables and Fruits from Retail Establishments in Europe. Curr. Opin. Food Sci. 2017, 18, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Møretrø, T.; Hermansen, L.; Holck, A.L.; Sidhu, M.S.; Rudi, K.; Langsrud, S. Biofilm Formation and the Presence of the Intercellular Adhesion Locus Ica among Staphylococi from Food and Food Processing Environments. Appl. Environ. Microbiol. 2003, 69, 5648–5655. [Google Scholar] [PubMed] [Green Version]
- Fagerlund, A.; Langsrud, S.; Heir, E.; Mikkelsen, M.I.; Møretrø, T. Biofilm Matrix Composition Affects the Susceptibility of Food Associated Staphylococci to Cleaning and Disinfection Agents. Front. Microbiol. 2016, 7, 856. [Google Scholar] [PubMed] [Green Version]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide Intercellular Adhesin in Biofilm: Structural and Regulatory Aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [PubMed] [Green Version]
- Fratamico, P.M.; Annous, B.A.; Guenther, N.W. Biofilms in the Food and Beverage Industries; Woodhead Publishing Limited: Cambridge, UK, 2009. [Google Scholar]
- Furkanur, M.; Mizan, R.; Jahid, I.K.; Ha, S.-D. Microbial Biofilms in Seafood: A Food-Hygiene Challenge. Food Microbiol. 2015, 49, 41–55. [Google Scholar]
- Srey, S.; Jahid, I.K.; Ha, S.-D. Biofilm Formation in Food Industries: A Food Safety Concern. Food Control 2013, 31, 572–585. [Google Scholar]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the Food Industry: Health Aspects and Control Methods. Front. Microbiol. 2018, 9, 898. [Google Scholar]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar]
- Majed, R.; Faille, C.; Kallassy, M.; Gohar, M. Bacillus Cereus Biofilms-Same, Only Different. Front. Microbiol. 2016, 7, 1054. [Google Scholar]
- Liu, N.T.; Nou, X.; Lefcourt, A.M.; Shelton, D.R.; Lo, Y.M. Dual-Species Biofilm Formation by Escherichia Coli O157: H7 and Environmental Bacteria Isolated from Fresh-Cut Processing Facilities. Int. J. Food Microbiol. 2014, 171, 15–20. [Google Scholar]
- Alonso, A.N.; Perry, K.J.; Regeimbal, J.M.; Regan, P.M.; Higgins, D.E. Identification of Listeria Monocytogenes Determinants Required for Biofilm Formation. PLoS ONE 2014, 9, e113696. [Google Scholar]
- Zhang, T.; Bae, D.; Wang, C. Listeria Monocytogenes DNA Glycosylase AdlP Affects Flagellar Motility, Biofilm Formation, Virulence, and Stress Responses. Appl. Environ. Microbiol. 2016, 82, 5144–5152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, R.W.; Reeve, K.E.; Gunn, J.S. Flagellated but Not Hyperfimbriated Salmonella Enterica Serovar Typhimurium Attaches to and Forms Biofilms on Cholesterol-Coated Surfaces. J. Bacteriol. 2010, 192, 2981–2990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, K.; Tomenius, H.; Kader, A.; Normark, S.; Römling, U.; Belova, L.M.; Melefors, Ö. Roles of Curli, Cellulose and BapA in Salmonella Biofilm Morphology Studied by Atomic Force Microscopy. BMC Microbiol. 2007, 7, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, M.Q.; Louie, J.W.; Feng, D.; Zhong, W.; Brandl, M.T. Curli Fimbriae Are Conditionally Required in Escherichia Coli O157: H7 for Initial Attachment and Biofilm Formation. Food Microbiol. 2016, 57, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Giacomucci, S.; Cros, C.D.N.; Perron, X.; Mathieu-Denoncourt, A.; Duperthuy, M. Flagella-Dependent Inhibition of Biofilm Formation by Sub-Inhibitory Concentration of Polymyxin B in Vibrio Cholerae. PLoS ONE 2019, 14, e0221431. [Google Scholar] [CrossRef] [Green Version]
- Gwynne, P.J.; Gallagher, M.P. Light as a Broad-Spectrum Antimicrobial. Front. Microbiol. 2018, 9, 119. [Google Scholar] [CrossRef]
- Aurum, F.S.; Nguyen, L.T. Efficacy of Photoactivated Curcumin to Decontaminate Food Surfaces under Blue Light Emitting Diode. J. Food Process Eng. 2019, 42. [Google Scholar] [CrossRef]
- Josewin, S.W.; Kim, M.J.; Yuk, H.G. Inactivation of Listeria Monocytogenes and Salmonella spp. on Cantaloupe Rinds by Blue Light Emitting Diodes (LEDs). Food Microbiol. 2018, 76, 219–225. [Google Scholar] [CrossRef]
- Bumah, V.V.; Whelan, H.T.; Masson-Meyers, D.S.; Quirk, B.; Buchmann, E.; Enwemeka, C.S. The Bactericidal Effect of 470-Nm Light and Hyperbaric Oxygen on Methicillin-Resistant Staphylococcus Aureus (MRSA). Lasers Med. Sci. 2015, 30, 1153–1159. [Google Scholar] [CrossRef] [Green Version]
- De Lucca, A.J.; Carter-Wientjes, C.; Williams, K.A.; Bhatnagar, D. Blue Light (470 nm) Effectively Inhibits Bacterial and Fungal Growth. Lett. Appl. Microbiol. 2012, 55, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Dietel, W.; Pottier, R.; Pfister, W.; Schleier, P.; Zinner, K. 5-Aminolaevulinic Acid (ALA) Induced Formation of Different Fluorescent Porphyrins: A Study of the Biosynthesis of Porphyrins by Bacteria of the Human Digestive Tract. J. Photochem. Photobiol. B Biol. 2007, 86, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Choby, J.E.; Skaar, E.P. Heme Synthesis and Acquisition in Bacterial Pathogens. J. Mol. Biol. 2016, 428, 3408–3428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dailey, H.A.; Dailey, T.A.; Gerdes, S.; Jahn, D.; Jahn, M.; O’Brian, M.R.; Warren, M.J. Prokaryotic Heme Biosynthesis: Multiple Pathways to a Common Essential Product. Microbiol. Mol. Biol. Rev. 2017, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukšienè, Ž. New Approach to Inactivation of Harmful and Pathogenic Microorganisms by Photosensitization. Food Technol. Biotechnol. 2005, 43, 411–418. [Google Scholar]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Mikš-Krajnik, M.; Kumar, A.; Yuk, H.G. Inactivation by 405 ± 5 nm Light Emitting Diode on Escherichia Coli O157: H7, Salmonella Typhimurium, and Shigella Sonnei under Refrigerated Condition Might Be Due to the Loss of Membrane Integrity. Food Control 2016, 59, 99–107. [Google Scholar] [CrossRef]
- Wu, J.; Chu, Z.; Ruan, Z.; Wang, X.; Dai, T.; Hu, X. Changes of Intracellular Porphyrin, Reactive Oxygen Species, and Fatty Acids Profiles During Inactivation of Methicillin-Resistant Staphylococcus Aureus by Antimicrobial Blue Light. Front. Physiol. 2018, 9, 1658. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Umesh Hebbar, H. Efficacy of Blue LED in Microbial Inactivation: Effect of Photosensitization and Process Parameters. Int. J. Food Microbiol. 2019, 290, 296–304. [Google Scholar] [CrossRef]
- Chu, Z.; Hu, X.; Wang, X.; Wu, J.; Dai, T.; Wang, X. Inactivation of Cronobacter Sakazakii by Blue Light Illumination and the Resulting Oxidative Damage to Fatty Acids. Can. J. Microbiol. 2019, 65, 922–929. [Google Scholar] [CrossRef]
- Kim, M.J.; Bang, W.S.; Yuk, H.G. 405 ± 5 nm Light Emitting Diode Illumination Causes Photodynamic Inactivation of Salmonella Spp. on Fresh-Cut Papaya without Deterioration. Food Microbiol. 2017, 62, 124–132. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial Blue Light Inactivation of Pathogenic Microbes: State of the Art. Drug Resist. Updat. 2017, 33–35, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dosselli, R.; Millioni, R.; Puricelli, L.; Tessari, P.; Arrigoni, G.; Franchin, C.; Segalla, A.; Teardo, E.; Reddi, E. Molecular Targets of Antimicrobial Photodynamic Therapy Identified by a Proteomic Approach. J. Proteomics 2012, 77, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Almeida, A.; Cunha, A. Photodynamic Inactivation of Bacterial and Yeast Biofilms with a Cationic Porphyrin. Photochem. Photobiol. 2014, 90, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Gupta, A.; Huang, Y.Y.; Sherwood, M.E.; Murray, C.K.; Vrahas, M.S.; Kielian, T.; Hamblin, M.R. Blue Light Eliminates Community-Acquired Methicillin-Resistant Staphylococcus Aureus in Infected Mouse Skin Abrasions. Photomed. Laser Surg. 2013, 31, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhu, Y.; Gupta, A.; Huang, Y.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Baer, D.G.; Hamblin, M.R.; Dai, T. Antimicrobial Blue Light Therapy for Multidrug-Resistant Acinetobacter Baumannii Infection in a Mouse Burn Model: Implications for Prophylaxis and Treatment of Combat-Related Wound Infections. J. Infect. Dis. 2014, 209, 1963–1971. [Google Scholar] [CrossRef] [Green Version]
- Giannelli, M.; Landini, G.; Materassi, F.; Chellini, F.; Antonelli, A.; Tani, A.; Nosi, D.; Zecchi-Orlandini, S.; Rossolini, G.M.; Bani, D. Effects of Photodynamic Laser and Violet-Blue Led Irradiation on Staphylococcus Aureus Biofilm and Escherichia Coli Lipopolysaccharide Attached to Moderately Rough Titanium Surface: In Vitro Study. Lasers Med. Sci. 2017, 32, 857–864. [Google Scholar] [CrossRef]
- Tschowri, N.; Lindenberg, S.; Hengge, R. Molecular Function and Potential Evolution of the Biofilm-Modulating Blue Light-Signalling Pathway of Escherichia Coli. Mol. Microbiol. 2012, 85, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Tschowri, N.; Busse, S.; Hengge, R. The BLUF-EAL Protein YcgF Acts as a Direct Anti-Repressor in a Blue-Light Response of Escherichia Coli. Genes Dev. 2009, 23, 522–534. [Google Scholar] [CrossRef] [Green Version]
- Mussi, M.A.; Gaddy, J.A.; Cabruja, M.; Arivett, B.A.; Viale, A.M.; Rasia, R.; Actis, L.A. The Opportunistic Human Pathogen Acinetobacter Baumannii Senses and Responds to Light. J. Bacteriol. 2010, 192, 6336–6345. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Wang, N.; Wang, C.; Yao, Y.; Fu, X.; Yu, W.; Cai, R.; Yao, M. 460 Nm Visible Light Irradiation Eradicates MRSA via Inducing Prophage Activation. J. Photochem. Photobiol. B Biol. 2017, 166, 311–322. [Google Scholar] [CrossRef]
- Jayaraman, P.; Devarajan, K.; Chua, T.K.; Zhang, H.; Gunawan, E.; Poh, C.L. Blue Light-Mediated Transcriptional Activation and Repression of Gene Expression in Bacteria. Nucleic Acids Res. 2016, 44, 6994–7005. [Google Scholar] [CrossRef] [PubMed]
- Braatsch, S.; Klug, G. Blue Light Perception in Bacteria. Photosynth. Res. 2004, 79, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Gomelsky, M.; Hoff, W.D. Light Helps Bacteria Make Important Lifestyle Decisions. Trends Microbiol. 2011, 19, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Yeh, N.; Chung, J.P. High-Brightness LEDs-Energy Efficient Lighting Sources and Their Potential in Indoor Plant Cultivation. Renew. Sustain. Energy Rev. 2009, 13, 2175–2180. [Google Scholar] [CrossRef]
- Prasad, A.; Du, L.; Zubair, M.; Subedi, S.; Ullah, A.; Roopesh, M.S. Applications of Light-Emitting Diodes (LEDs) in Food Processing and Water Treatment. Food Eng. Rev. 2020, 12, 268–289. [Google Scholar] [CrossRef]
- Feezell, D.; Nakamura, S. Invention, Development, and Status of the Blue Light-Emitting Diode, the Enabler of Solid-State Lighting. Comptes Rendus Phys. 2018, 19, 113–133. [Google Scholar] [CrossRef]
- Dall Agnol, M.A.; Nicolau, R.A.; De Lima, C.J.; Munin, E. Comparative Analysis of Coherent Light Action (Laser) versus Non-Coherent Light (Light-Emitting Diode) for Tissue Repair in Diabetic Rats. Lasers Med. Sci. 2009, 24, 909–916. [Google Scholar] [CrossRef]
- Corazza, A.V.; Jorge, J.; Kurachi, C.; Bagnato, V.S. Photobiomodulation on the Angiogenesis of Skin Wounds in Rats Using Different Light Sources. Photomed. Laser Surg. 2007, 25, 102–106. [Google Scholar] [CrossRef]
- de Abreu Chaves, M.E.; Piancastelli, A.C.C.; de Araújo, A.R.; Pinotti, M. Effects of Low-Power Light Therapy on Wound Healing: LASER x LED. An. Bras. Dermatol. 2014, 89, 616–623. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Bumah, V.V.; Biener, G.; Raicu, V.; Enwemeka, C.S. The Relative Antimicrobial Effect of Blue 405 Nm LED and Blue 405 Nm Laser on Methicillin-Resistant Staphylococcus Aureus in Vitro. Lasers Med. Sci. 2015, 30, 2265–2271. [Google Scholar] [CrossRef]
- Feltin, E.; Castiglia, A.; Cosendey, G.; Sulmoni, L.; Carlin, J.F.; Grandjean, N.; Rossetti, M.; Dorsaz, J.; Laino, V.; Duelk, M.; et al. Broadband Blue Superluminescent Light-Emitting Diodes Based on GaN. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
- Rossetti, M.; Napierala, J.; Matuschek, N.; Achatz, U.; Duelk, M.; Vélez, C.; Castiglia, A.; Grandjean, N.; Dorsaz, J.; Feltin, E. Superluminescent Light Emitting Diodes: The Best out of Two Worlds. MOEMS Miniaturized Syst. XI 2012, 8252, 825208. [Google Scholar]
- Zhang, Z.; Hogg, R.; Lv, X.; Wang, Z. Self-Assembled Quantum-Dot Superluminescent Light-Emitting Diodes. Adv. Opt. Photonics 2010, 2, 201–228. [Google Scholar] [CrossRef]
- Enwemeka, C.S.; Williams, D.; Hollosi, S.; Yens, D.; Enwemeka, S.K. Visible 405 Nm SLD Light Photo-Destroys Methicillin-Resistant Staphylococcus Aureus (MRSA) in Vitro. Lasers Surg. Med. 2008, 40, 734–737. [Google Scholar] [CrossRef]
- Guffey, J.S.; Wilborn, J. Effects of Combined 405-Nm and 880-Nm Light on Staphylococcus Aureus and Pseudomonas Aeruginosa in Vitro. Photomed. Laser Surg. 2006, 24, 680–683. [Google Scholar]
- Guffey, J.S.; Wilborn, J. In Vitro Bactericidal Effects of 405-Nm and 470-Nm Blue Light. Photomed. Laser Surg. 2006, 24, 684–688. [Google Scholar] [CrossRef]
- Ahmed, E.; El-Gendy, A.O.; Moniem Radi, N.A.; Mohamed, T. The Bactericidal Efficacy of Femtosecond Laser-Based Therapy on the Most Common Infectious Bacterial Pathogens in Chronic Wounds: An in Vitro Study. Lasers Med. Sci. 2020. [Google Scholar] [CrossRef]
- Tsen, K.T.; Tsen, S.-W.D.; Fu, Q.; Lindsay, S.M.; Li, Z.; Cope, S.; Vaiana, S.; Kiang, J.G. Studies of Inactivation of Encephalomyocarditis Virus, M13 Bacteriophage, and Salmonella Typhimurium by Using a Visible Femtosecond Laser: Insight into the Possible Inactivation Mechanisms. J. Biomed. Opt. 2011, 16, 078003. [Google Scholar]
- Barneck, M.D.; Rhodes, N.L.R.; de la Presa, M.; Allen, J.P.; Poursaid, A.E.; Nourian, M.M.; Firpo, M.A.; Langell, J.T. Violet 405-Nm Light: A Novel Therapeutic Agent against Common Pathogenic Bacteria. J. Surg. Res. 2016, 206, 316–324. [Google Scholar]
- Murdoch, L.E.; MacLean, M.; Endarko, E.; MacGregor, S.J.; Anderson, J.G. Bactericidal Effects of 405nm Light Exposure Demonstrated by Inactivation of Escherichia, Salmonella, Shigella, Listeria, and Mycobacterium Species in Liquid Suspensions and on Exposed Surfaces. Sci. World J. 2012, 2012, 137805. [Google Scholar]
- Bumah, V.V.; Masson-Meyers, D.S.; Enwemeka, C.S. Pulsed 450 nm Blue Light Suppresses MRSA and Propionibacterium Acnes in Planktonic Cultures and Bacterial Biofilms. J. Photochem. Photobiol. B Biol. 2020, 202, 111702. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Bumah, V.V.; Castel, C.; Castel, D.; Enwemeka, C.S. Pulsed 450 Nm Blue Light Significantly Inactivates Propionibacterium Acnes More than Continuous Wave Blue Light. J. Photochem. Photobiol. B Biol. 2020, 202, 111719. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.B.; Maclean, M.; Given, M.J.; Wilson, M.P.; Judd, M.D.; Timoshkin, I.V.; Macgregor, S.J. Efficacy of Pulsed 405-Nm Light-Emitting Diodes for Antimicrobial Photodynamic Inactivation: Effects of Intensity, Frequency, and Duty Cycle. Photomed. Laser Surg. 2017, 35, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinpenning, M.M.; Smits, T.; Frunt, M.H.A.; van Erp, P.E.J.; van de Kerkhof, P.C.M.; Gerritsen, R.M.J.P. Clinical and Histological Effects of Blue Light on Normal Skin. Photodermatol. Photoimmunol. Photomed. 2010, 26, 16–21. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Huang, Y.Y.; Yin, R.; Murray, C.K.; Vrahas, M.S.; Sherwood, M.E.; Tegos, G.P.; Hamblin, M.R. Blue Light Rescues Mice from Potentially Fatal Pseudomonas Aeruginosa Burn Infection: Efficacy, Safety, and Mechanism of Action. Antimicrob. Agents Chemother. 2013, 57, 1238–1245. [Google Scholar] [CrossRef] [Green Version]
- Liebmann, J.; Born, M.; Kolb-Bachofen, V. Blue-Light Irradiation Regulates Proliferation and Differentiation in Human Skin Cells. J. Investig. Dermatol. 2010, 130, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Marek, V.; Mélik-Parsadaniantz, S.; Villette, T.; Montoya, F.; Baudouin, C.; Brignole-Baudouin, F.; Denoyer, A. Blue Light Phototoxicity toward Human Corneal and Conjunctival Epithelial Cells in Basal and Hyperosmolar Conditions. Free Radic. Biol. Med. 2018, 126, 27–40. [Google Scholar] [CrossRef]
- Zhao, Z.C.; Zhou, Y.; Tan, G.; Li, J. Research Progress about the Effect and Prevention of Blue Light on Eyes. Int. J. Ophthalmol. 2018, 11, 1999–2003. [Google Scholar]
- West, K.E.; Jablonski, M.R.; Warfield, B.; Cecil, K.S.; James, M.; Ayers, M.A.; Maida, J.; Bowen, C.; Sliney, D.H.; Rollag, M.D.; et al. Blue Light from Light-Emitting Diodes Elicits a Dose-Dependent Suppression of Melatonin in Humans. J. Appl. Physiol. 2011, 110, 619–626. [Google Scholar] [CrossRef] [Green Version]
- American Conference of Governmental Industrial Hygienists. ACGIH—Threshold Limit Values (TLVs) and Biological Exposure Indices (BEIs). Available online: https://www.nsc.org/Portals/0/Documents/facultyportal/Documents/fih-6e-appendix-b.pdf (accessed on 21 October 2020).
- Leccese, F.; Vandelanotte, V.; Salvadori, G.; Rocca, M. Blue Light Hazard and Risk Group Classification of 8 W LED Tubes, Replacing Fluorescent Tubes, through Optical Radiation Measurements. Sustainability 2015, 7, 13454–13468. [Google Scholar] [CrossRef] [Green Version]
- Soares, C.J.; de Paula Rodrigues, M.; Vilela, A.B.F.; Rizo, E.R.C.; Ferreira, L.B.; Giannini, M.; Price, R.B. Evaluation of Eye Protection Filters Used with Broad-Spectrum and Conventional LED Curing Lights. Braz. Dent. J. 2017, 28, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimberly, B.; James, R.P. Amber Lenses to Block Blue Light and Improve Sleep: A Randomized Trial. Chronobiol. Int. 2009, 26, 1602–1612. [Google Scholar] [CrossRef] [PubMed]
- Esaki, Y.; Kitajima, T.; Ito, Y.; Koike, S.; Nakao, Y.; Tsuchiya, A.; Hirose, M.; Iwata, N. Wearing Blue Light-Blocking Glasses in the Evening Advances Circadian Rhythms in the Patients with Delayed Sleep Phase Disorder: An Open-Label Trial. Chronobiol. Int. 2016, 33, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.; Gunther, N.W.; Sheen, S. Inactivation of Salmonella Spp., Pathogenic Escherichia Coli, Staphylococcus Spp., or Listeria Monocytogenes in Chicken Purge or Skin Using a 405-nm LED Array. Food Microbiol. 2017, 64, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Gunther, N.W.; Phillips, J.G.; Sommers, C. The Effects of 405-Nm Visible Light on the Survival of Campylobacter on Chicken Skin and Stainless Steel. Foodborne Pathog. Dis. 2016, 13, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, M.J.; Bang, W.S.; Yuk, H.G. Anti-Biofilm Effect of 405-Nm LEDs against Listeria Monocytogenes in Simulated Ready-to-Eat Fresh Salmon Storage Conditions. Food Control 2018, 84, 513–521. [Google Scholar] [CrossRef]
- McKenzie, K.; Maclean, M.; Timoshkin, I.V.; Endarko, E.; Macgregor, S.J.; Anderson, J.G. Photoinactivation of Bacteria Attached to Glass and Acrylic Surfaces by 405 Nm Light: Potential Application for Biofilm Decontamination. Photochem. Photobiol. 2013, 89, 927–935. [Google Scholar] [CrossRef] [Green Version]
- Hyun, J.E.; Lee, S.Y. Antibacterial Effect and Mechanisms of Action of 460–470 nm Light-Emitting Diode against Listeria Monocytogenes and Pseudomonas Fluorescens on the Surface of Packaged Sliced Cheese. Food Microbiol. 2020, 86, 103314. [Google Scholar] [CrossRef]
- Luksiene, Z.; Buchovec, I.; Paskeviciute, E. Inactivation of Food Pathogen Bacillus Cereus by Photosensitization in Vitro and on the Surface of Packaging Material. J. Appl. Microbiol. 2009, 107, 2037–2046. [Google Scholar] [CrossRef]
- Luksiene, Z.; Paskeviciute, E. Reprint of: Novel Approach to Decontaminate Food-Packaging from Pathogens in Non-Thermal and Not Chemical Way: Chlorophyllin-Based Photosensitization Q. J. Food Eng. 2012, 110, 317–323. [Google Scholar] [CrossRef]
- Buchovec, I.; Paskeviciute, E.; Luksiene, Z. Photosensitization-Based Inactivation of Food Pathogen Listeria Monocytogenes in Vitro and on the Surface of Packaging Material. J. Photochem. Photobiol. B Biol. 2010, 99, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Haughton, P.N.; Grau, E.G.; Lyng, J.; Cronin, D.; Fanning, S.; Whyte, P. Susceptibility of Campylobacter to High Intensity near Ultraviolet/Visible 395 ± 5 nm Light and Its Effectiveness for the Decontamination of Raw Chicken and Contact Surfaces. Int. J. Food Microbiol. 2012, 159, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.M.; Sauer, A.; Moraru, C.I. Inactivation of Escherichia Coli in Milk and Concentrated Milk Using Pulsed-Light Treatment. J. Dairy Sci. 2012, 95, 5597–5603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- dos Anjos, C.; Sellera, F.P.; de Freitas, L.M.; Gargano, R.G.; Telles, E.O.; Freitas, R.O.; Baptista, M.S.; Ribeiro, M.S.; Lincopan, N.; Pogliani, F.C.; et al. Inactivation of Milk-Borne Pathogens by Blue Light Exposure. J. Dairy Sci. 2020, 103, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, L.E.; MacLean, M.; MacGregor, S.J.; Anderson, J.G. Inactivation of Campylobacter Jejuni by Exposure to High-Intensity 405-Nm Visible Light. Foodborne Pathog. Dis. 2010, 7, 1211–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endarko, E.; Maclean, M.; Timoshkin, I.V.; MacGregor, S.J.; Anderson, J.G. High-Intensity 405 Nm Light Inactivation of Listeria Monocytogenes. Photochem. Photobiol. 2012, 88, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Srimagal, A.; Ramesh, T.; Sahu, J.K. Effect of Light Emitting Diode Treatment on Inactivation of Escherichia Coli in Milk. LWT-Food Sci. Technol. 2016, 71, 378–385. [Google Scholar] [CrossRef]
- Ghate, V.; Kumar, A.; Zhou, W.; Yuk, H.G. Irradiance and Temperature Influence the Bactericidal Effect of 460-Nanometer Light-Emitting Diodes on Salmonella in Orange Juice. J. Food Prot. 2016, 79, 553–560. [Google Scholar] [CrossRef]
- Ricciardi, E.F.; Pedros-Garrido, S.; Papoutsis, K.; Lyng, J.G.; Conte, A.; Del Nobile, M.A. Novel Technologies for Preserving Ricotta Cheese: Effects of Ultraviolet and near-Ultraviolet–Visible Light. Foods 2020, 9, 580. [Google Scholar] [CrossRef]
- Glueck, M.; Schamberger, B.; Eckl, P.; Plaetzer, K. New Horizons in Microbiological Food Safety: Photodynamic Decontamination Based on a Curcumin Derivative. Photochem. Photobiol. Sci. 2017, 16, 1784–1791. [Google Scholar] [CrossRef] [Green Version]
- Tortik, N.; Spaeth, A.; Plaetzer, K. Photodynamic Decontamination of Foodstuff from Staphylococcus Aureus Based on Novel Formulations of Curcumin. Photochem. Photobiol. Sci. 2014, 13, 1402–1409. [Google Scholar] [CrossRef]
- Winter, S.; Tortik, N.; Kubin, A.; Krammer, B.; Plaetzer, K. Back to the Roots: Photodynamic Inactivation of Bacteria Based on Water-Soluble Curcumin Bound to Polyvinylpyrrolidone as a Photosensitizer. Photochem. Photobiol. Sci. 2013, 12, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Buchovec, I.; Lukseviciute, V.; Marsalka, A.; Reklaitis, I.; Luksiene, Z. Effective Photosensitization-Based Inactivation of Gram (−) Food Pathogens and Molds Using the Chlorophyllin-Chitosan Complex: Towards Photoactive Edible Coatings to Preserve Strawberries. Photochem. Photobiol. Sci. 2016, 15, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Paskeviciute, E.; Zudyte, B.; Luksiene, Z. Towards Better Microbial Safety of Fresh Produce: Chlorophyllin-Based Photosensitization for Microbial Control of Foodborne Pathogens on Cherry Tomatoes. J. Photochem. Photobiol. B Biol. 2018, 182, 130–136. [Google Scholar] [CrossRef] [PubMed]
- MinJeong, K.; CheeHwa, T.; WooSuk, B.; HyunGyun, Y. Antibacterial Effect of 405±5 Nm Light Emitting Diode Illumination against Escherichia Coli O157:H7, Listeria Monocytogenes, and Salmonella on the Surface of Fresh-Cut Mango and Its Influence on Fruit Quality. Int. J. Food Microbiol. 2017, 244, 82–89. [Google Scholar]
- Tao, R.; Zhang, F.; Tang, Q.-J.; Xu, C.-S.; Ni, Z.J.; Meng, X.-H. Effects of Curcumin-Based Photodynamic Treatment on the Storage Quality of Fresh-Cut Apples. Food Chem. 2019, 274, 415–421. [Google Scholar] [CrossRef]
- Ghate, V.; Kumar, A.; Kim, M.J.; Bang, W.S.; Zhou, W.; Yuk, H.G. Effect of 460 Nm Light Emitting Diode Illumination on Survival of Salmonella Spp. on Fresh-Cut Pineapples at Different Irradiances and Temperatures. J. Food Eng. 2017, 196, 130–138. [Google Scholar] [CrossRef]
- Lacombe, A.; Niemira, B.A.; Sites, J.; Boyd, G.; Gurtler, J.B.; Tyrell, B.; Fleck, M. Reduction of Bacterial Pathogens and Potential Surrogates on the Surface of Almonds Using High-Intensity 405-Nanometer Light. J. Food Prot. 2016, 79, 1840–1845. [Google Scholar] [CrossRef]
- Paskeviciute, E.; Zudyte, B.; Luksiene, Z. Innovative Nonthermal Technologies: Chlorophyllin and Visible Light Significantly Reduce Microbial Load on Basil. Food Technol. Biotechnol. 2019, 57, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Guffey, J.S.; Payne, W.C.; Motts, S.D.; Towery, P.; Hobson, T.; Harrell, G.; Meurer, L.; Lancaster, K. Inactivation of Salmonella on Tainted Foods: Using Blue Light to Disinfect Cucumbers and Processed Meat Products. Food Sci. Nutr. 2016, 4, 878–887. [Google Scholar] [CrossRef]
- Luksiene, Z.; Paskeviciute, E. Novel Approach to the Microbial Decontamination of Strawberries: Chlorophyllin-Based Photosensitization. J. Appl. Microbiol. 2011, 110, 1274–1283. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Adeline Ng, B.X.; Zwe, Y.H.; Yuk, H.G. Photodynamic Inactivation of Salmonella Enterica Enteritidis by 405 ± 5-Nm Light-Emitting Diode and Its Application to Control Salmonellosis on Cooked Chicken. Food Control 2017, 82, 305–315. [Google Scholar] [CrossRef]
- Li, X.; Kim, M.J.; Yuk, H.G. Influence of 405 nm Light-Emitting Diode Illumination on the Inactivation of Listeria Monocytogenes and Salmonella Spp. on Ready-to-Eat Fresh Salmon Surface at Chilling Storage for 8 h and Their Susceptibility to Simulated Gastric Fluid. Food Control 2018, 88, 61–68. [Google Scholar] [CrossRef]
- Josewin, S.W.; Ghate, V.; Kim, M.J.; Yuk, H.G. Antibacterial Effect of 460 Nm Light-Emitting Diode in Combination with Riboflavin against Listeria Monocytogenes on Smoked Salmon. Food Control 2018, 84, 354–361. [Google Scholar] [CrossRef]
- Stephen Guffey, J.; Stephen, J.; Professor, A. The Use of 405nm and 464nm Blue Light to Inhibit Listeria Monocytogenes in Ready-to-Eat (RTE) Meat. Eur. J. Acad. Essays ISSN 2016, 3, 76–80. [Google Scholar]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G.A.; Coia, J.E.; Hamilton, K.; Taggart, I.; Watson, S.B.; Thakker, B.; Gettinby, G. Environmental Decontamination of a Hospital Isolation Room Using High-Intensity Narrow-Spectrum Light. J. Hosp. Infect. 2010, 76, 247–251. [Google Scholar] [CrossRef]
- Bache, S.E.; MacLean, M.; MacGregor, S.J.; Anderson, J.G.; Gettinby, G.; Coia, J.E.; Taggart, I. Clinical Studies of the High-Intensity Narrow-Spectrum Light Environmental Decontamination System (HINS-Light EDS), for Continuous Disinfection in the Burn Unit Inpatient and Outpatient Settings. Burns 2012, 38, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Maclean, M.; Booth, M.; Anderson, J.; MacGregor, S.; Woolsey, G.; Coia, J.; Hamilton, K.; Gettinby, G. Continuous Decontamination of an Intensive Care Isolation Room during Patient Occupancy Using 405 Nm Light Technology. J. Infect. Prev. 2013, 14, 176–181. [Google Scholar] [CrossRef]
- MacLean, M.; Murdoch, L.E.; MacGregor, S.J.; Anderson, J.G. Sporicidal Effects of High-Intensity 405 nm Visible Light on Endospore-Forming Bacteria. Photochem. Photobiol. 2013, 89, 120–126. [Google Scholar] [CrossRef]
- Dougall, L.; Anderson, J.G.; Timoshkin, I.V.; MacGregor, S.J.; Maclean, M. Efficacy of Antimicrobial 405 nm Blue-Light for Inactivation of Airborne Bacteria; Dai, T., Ed.; International Society for Optics and Photonics: San Fransisco, CA, USA, 2018; Volume 10479, p. 10479G. [Google Scholar]
- Moreira, L.H.; de Souza, J.C.P.; de Lima, C.J.; Salgado, M.A.C.; Fernandes, A.B.; Andreani, D.I.K.; Villaverde, A.B.; Zângaro, R.A. Use of Photodynamic Therapy in the Treatment of Bovine Subclinical Mastitis. Photodiagn. Photodyn. Ther. 2018, 21, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Sellera, F.P.; Sabino, C.P.; Ribeiro, M.S.; Gargano, R.G.; Benites, N.R.; Melville, P.A.; Pogliani, F.C. In Vitro Photoinactivation of Bovine Mastitis Related Pathogens. Photodiagn. Photodyn. Ther. 2016, 13, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, P.S.; Al-Ghabshi, A.; Al-Mazrooei, N.; Al-Habsi, S. Comparative Pathogenomics of Bacteria Causing Infectious Diseases in Fish. Int. J. Evol. Biol. 2012, 2012, 16. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.J.; Kim, A.; Kang, G.S.; Kim, D.H. Photoinactivation of Major Bacterial Pathogens in Aquaculture. Fish. Aquat. Sci. 2016, 19, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Alves, E.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Photodynamic Antimicrobial Chemotherapy in Aquaculture: Photoinactivation Studies of Vibrio Fischeri. PLoS ONE 2011, 6, e20970. [Google Scholar] [CrossRef] [Green Version]
- Arrojado, C.; Pereira, C.; Tomé, J.P.C.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Calado, R.; Gomes, N.C.M.; et al. Applicability of Photodynamic Antimicrobial Chemotherapy as an Alternative to Inactivate Fish Pathogenic Bacteria in Aquaculture Systems. Photochem. Photobiol. Sci. 2011, 10, 1691–1700. [Google Scholar] [CrossRef]
- Roh, H.J.; Kang, G.S.; Kim, A.; Kim, N.E.; Nguyen, T.L.; Kim, D.H. Blue Light-Emitting Diode Photoinactivation Inhibits Edwardsiellosis in Fancy Carp (Cyprinus Carpio). Aquaculture 2018, 483, 1–7. [Google Scholar] [CrossRef]
- Villamizar, N.; Blanco-Vives, B.; Migaud, H.; Davie, A.; Carboni, S.; Sánchez-Vázquez, F.J. Effects of Light during Early Larval Development of Some Aquacultured Teleosts: A Review. Aquaculture 2011, 315, 86–94. [Google Scholar] [CrossRef]
- Machado, S.G.; Baglinière, F.; Marchand, S.; Van Coillie, E.; Vanetti, M.C.D.; De Block, J.; Heyndrickx, M. The Biodiversity of the Microbiota Producing Heat-Resistant Enzymes Responsible for Spoilage in Processed Bovine Milk and Dairy Products. Front. Microbiol. 2017, 8, 302. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Sadiq, F.A.; Burmølle, M.; Wang, N.; He, G. Insights into Psychrotrophic Bacteria in Raw Milk: A Review. J. Food Prot. 2019, 82, 1148–1159. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Avery, S.V.; Singleton, I. Microbes Associated with Fresh Produce: Sources, Types and Methods to Reduce Spoilage and Contamination. Adv. Appl. Microbiol. 2019, 107, 29–82. [Google Scholar]
- Pellissery, A.J.; Vinayamohan, P.G.; Amalaradjou, M.A.R.; Venkitanarayanan, K. Spoilage Bacteria and Meat Quality. In Meat Quality Analysis: Advanced Evaluation Methods, Techniques, and Technologies; Biswas, A.K., Mandal, P.K., Eds.; Academic Press: London, UK, 2019; pp. 307–334. [Google Scholar]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood Spoilage Microbiota and Associated Volatile Organic Compounds at Different Storage Temperatures and Packaging Conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hu, J.; Li, S.; Hamzah, S.S.; Jiang, H.; Zhou, A.; Zeng, S.; Lin, S. Curcumin-Based Photodynamic Sterilization for Preservation of Fresh-Cut Hami Melon. Molecules 2019, 24, 2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Li, Z.; Cao, B.; Wu, J.; Wang, Y.; Xue, Y.; Xu, J.; Xue, C.; Tang, Q.J. The Effect of a Novel Photodynamic Activation Method Mediated by Curcumin on Oyster Shelf Life and Quality. Food Res. Int. 2016, 87, 204–210. [Google Scholar] [CrossRef]
- Gong, C.; Li, Y.; Gao, R.; Xiao, F.; Zhou, X.; Wang, H.; Xu, H.; Wang, R.; Huang, P.; Zhao, Y. Inactivation of Specific Spoilage Organism (Pseudomonas) of Sturgeon by Curcumin-Mediated Photodynamic Inactivation. Photodiagn. Photodyn. Ther. 2020, 31, 101827. [Google Scholar] [CrossRef] [PubMed]
- Chui, C.; Aoki, A.; Takeuchi, Y.; Sasaki, Y.; Hiratsuka, K.; Abiko, Y.; Izumi, Y. Antimicrobial Effect of Photodynamic Therapy Using High-Power Blue Light-Emitting Diode and Red-Dye Agent on Porphyromonas Gingivalis. J. Periodontal Res. 2013, 48, 696–705. [Google Scholar] [PubMed]
- Hamblin, M.R.; Abrahamse, H. Inorganic Salts and Antimicrobial Photodynamic Therapy: Mechanistic Conundrums? Molecules 2018, 23, 3190. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Szewczyk, G.; Sarna, T.; Hamblin, M.R. Potassium Iodide Potentiates Broad-Spectrum Antimicrobial Photodynamic Inactivation Using Photofrin. ACS Infect. Dis. 2017, 3, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Fotinos, N.; Convert, M.; Piffaretti, J.C.; Gurny, R.; Lange, N. Effects on Gram-Negative and Gram-Positive Bacteria Mediated by 5-Aminolevulinic Acid and 5-Aminolevulinic Acid Derivatives. Antimicrob. Agents Chemother. 2008, 52, 1366–1373. [Google Scholar] [CrossRef] [Green Version]
- Nitzan, Y.; Salmon-Divon, M.; Shporen, E.; Malik, Z. ALA Induced Photodynamic Effects on Gram Positive and Negative Bacteria. Photochem. Photobiol. Sci. 2004, 3, 430–435. [Google Scholar] [CrossRef]
- McKenzie, K.; Maclean, M.; Timoshkin, I.V.; MacGregor, S.J.; Anderson, J.G. Enhanced Inactivation of Escherichia Coli and Listeria Monocytogenes by Exposure to 405nm Light under Sub-Lethal Temperature, Salt and Acid Stress Conditions. Int. J. Food Microbiol. 2014, 170, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Ghate, V.; Kumar, A.; Zhou, W.; Yuk, H.G. Effect of Organic Acids on the Photodynamic Inactivation of Selected Foodborne Pathogens Using 461 nm LEDs. Food Control 2015, 57, 333–340. [Google Scholar] [CrossRef]
- Kumar, A.; Ghate, V.; Kim, M.J.; Zhou, W.; Khoo, G.H.; Yuk, H.G. Antibacterial Efficacy of 405, 460 and 520 Nm Light Emitting Diodes on Lactobacillus Plantarum, Staphylococcus Aureus and Vibrio Parahaemolyticus. J. Appl. Microbiol. 2016, 120, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver Nanoparticles in Polymeric Matrices for Fresh Food Packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Akram, F.E.; El-Tayeb, T.; Abou-Aisha, K.; El-Azizi, M. A Combination of Silver Nanoparticles and Visible Blue Light Enhances the Antibacterial Efficacy of Ineffective Antibiotics against Methicillin-Resistant Staphylococcus Aureus (MRSA). Ann. Clin. Microbiol. Antimicrob. 2016, 15, 48. [Google Scholar] [CrossRef] [Green Version]
- El Din, S.N.; El-Tayeb, T.A.; Abou-Aisha, K.; El-Azizi, M. In Vitro and in Vivo Antimicrobial Activity of Combined Therapy of Silver Nanoparticles and Visible Blue Light against Pseudomonas Aeruginosa. Int. J. Nanomed. 2016, 11, 1749–1758. [Google Scholar]
- Yang, M.Y.; Chang, K.C.; Chen, L.Y.; Wang, P.C.; Chou, C.C.; Wu, Z.-B.; Hu, A. Blue Light Irradiation Triggers the Antimicrobial Potential of ZnO Nanoparticles on Drug-Resistant Acinetobacter Baumannii. J. Photochem. Photobiol. B Biol. 2018, 180, 235–242. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Yamada, Y.; Ikai, H.; Kanno, T.; Sasaki, K.; Niwano, Y. Bactericidal Action of Photoirradiated Gallic Acid via Reactive Oxygen Species Formation. J. Agric. Food Chem. 2012, 60, 10048–10054. [Google Scholar] [CrossRef]
- Nakamura, K.; Ishiyama, K.; Sheng, H.; Ikai, H.; Kanno, T.; Niwano, Y. Bactericidal Activity and Mechanism of Photoirradiated Polyphenols against Gram-Positive and -Negative Bacteria. J. Agric. Food Chem. 2015, 63, 7707–7713. [Google Scholar] [CrossRef]
- Nakamura, K.; Shirato, M.; Kanno, T.; Lingström, P.; Örtengren, U.; Niwano, Y. Photo-Irradiated Caffeic Acid Exhibits Antimicrobial Activity against Streptococcus Mutans Biofilms via Hydroxyl Radical Formation. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukada, M.; Sheng, H.; Tada, M.; Mokudai, T.; Oizumi, S.; Kamachi, T.; Niwano, Y. Bactericidal Action of Photo-Irradiated Aqueous Extracts from the Residue of Crushed Grapes from Winemaking. Biocontrol Sci. 2016, 21, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukada, M.; Sheng, H.; Kamachi, T.; Niwano, Y. Microbicidal Action of Photoirradiated Aqueous Extracts from Wine Lees. J. Food Sci. Technol. 2016, 53, 3020–3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra-Rosas, M.I.; Morales-Castro, J.; Cubero-Márquez, M.A.; Salvia-Trujillo, L.; Martín-Belloso, O. Antimicrobial Activity of Nanoemulsions Containing Essential Oils and High Methoxyl Pectin during Long-Term Storage. Food Control 2017, 77, 131–138. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, K.P.; Zhang, X.; Pan, D.D.; Sun, Y.Y.; Cao, J.X. Antibacterial Activity and Mechanism of Action of Black Pepper Essential Oil on Meat-Borne Escherichia Coli. Front. Microbiol. 2017, 7, 2094. [Google Scholar] [CrossRef] [Green Version]
- Marqués-Calvo, M.S.; Codony, F.; Agustí, G.; Lahera, C. Visible Light Enhances the Antimicrobial Effect of Some Essential Oils. Photodiagn. Photodyn. Ther. 2017, 17, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; De Schaetzen, M.-A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial Resistance in the Food Chain: A Review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef] [Green Version]
- Zekar, F.M.; Granier, S.A.; Marault, M.; Yaici, L.; Gassilloud, B.; Manceau, C.; Touati, A.; Millemann, Y. From Farms to Markets: Gram-Negative Bacteria Resistant to Third-Generation Cephalosporins in Fruits and Vegetables in a Region of North Africa. Front. Microbiol. 2017, 8, 1569. [Google Scholar] [CrossRef] [PubMed]
- Boss, R.; Overesch, G.; Baumgartner, A. Antimicrobial Resistance of Escherichia Coli, Enterococci, Pseudomonas Aeruginosa, and Staphylococcus Aureus from Raw Fish and Seafood Imported into Switzerland. J. Food Prot. 2016, 79, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, R.; Stijntjes, M.; Rodríguez-Baño, J.; Tacconelli, E.; Babu Rajendran, N.; Voss, A. Review of Antimicrobial Resistance Surveillance Programmes in Livestock and Meat in EU with Focus on Humans. Clin. Microbiol. Infect. 2018, 24, 577–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reygaert, W. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Rapacka-Zdonczyk, A.; Wozniak, A.; Pieranski, M.; Woziwodzka, A.; Bielawski, K.P.; Grinholc, M. Development of Staphylococcus Aureus Tolerance to Antimicrobial Photodynamic Inactivation and Antimicrobial Blue Light upon Sub-Lethal Treatment. Sci. Rep. 2019, 9, 9423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fila, G.; Kawiak, A.; Grinholc, M.S. Blue Light Treatment of Pseudomonas Aeruginosa: Strong Bactericidal Activity, Synergism with Antibiotics and Inactivation of Virulence Factors. Virulence 2017, 8, 938–958. [Google Scholar] [CrossRef] [Green Version]
- Tomb, R.M.; Maclean, M.; Coia, J.E.; MacGregor, S.J.; Anderson, J.G. Assessment of the Potential for Resistance to Antimicrobial Violet-Blue Light in Staphylococcus Aureus. Antimicrob. Resist. Infect. Control 2017, 6, 100. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Huang, Y.Y.; Xi, L.; Gelfand, J.A.; Hamblin, M.R. Tetracyclines Function as Dual-Action Light-Activated Antibiotics. PLoS ONE 2018, 13, e0196485. [Google Scholar] [CrossRef] [Green Version]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The Interconnection between Biofilm Formation and Horizontal Gene Transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Espada, R.; Liu, X.; Goh, X.S.; Dai, T. Antimicrobial Blue Light Inactivation of Polymicrobial Biofilms. Front. Microbiol. 2019, 10, 721. [Google Scholar] [CrossRef]
- Ferrer-Espada, R.; Wang, Y.; Goh, X.S.; Dai, T. Antimicrobial Blue Light Inactivation of Microbial Isolates in Biofilms. Lasers Surg. Med. 2020, 52, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Leanse, L.G.; Harrington, O.D.; Fang, Y.; Ahmed, I.; Goh, X.S.; Dai, T. Evaluating the Potential for Resistance Development to Antimicrobial Blue Light (at 405 nm) in Gram-Negative Bacteria: In Vitro and in Vivo Studies. Front. Microbiol. 2018, 9, 2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adair, T.L.; Drum, B.E. RNA-Seq Reveals Changes in the Staphylococcus Aureus Transcriptome Following Blue Light Illumination. Genom. Data 2016, 9, 4–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardu, M.; Bulut, S.; Kavakli, I.H. MerR and ChrR Mediate Blue Light Induced Photo-Oxidative Stress Response at the Transcriptional Level in Vibrio Cholerae. Sci. Rep. 2017, 7, 40817. [Google Scholar] [CrossRef]
- Leanse, L.; Goh, X.; Cheng, J.; Hooper, D.; Dai, T. Dual-Wavelength Photo-Killing of Methicillin-Resistant Staphylococcus Aureus. JCl Insight 2020, 5, e134343. [Google Scholar] [CrossRef]
- Cieplik, F.; Späth, A.; Leibl, C.; Gollmer, A.; Regensburger, J.; Tabenski, L.; Hiller, K.A.; Maisch, T.; Schmalz, G. Blue Light Kills Aggregatibacter Actinomycetemcomitans Due to Its Endogenous Photosensitizers. Clin. Oral Investig. 2014, 18, 1763–1769. [Google Scholar] [CrossRef]
- Battisti, A.; Morici, P.; Ghetti, F.; Sgarbossa, A. Spectroscopic Characterization and Fluorescence Imaging of Helicobacter Pylori Endogenous Porphyrins. Biophys. Chem. 2017, 229, 19–24. [Google Scholar] [CrossRef]
- Amin, R.M.; Bhayana, B.; Hamblin, M.R.; Dai, T. Antimicrobial Blue Light Inactivation of Pseudomonas Aeruginosa by Photo-Excitation of Endogenous Porphyrins: In Vitro and in Vivo Studies. Lasers Surg. Med. 2016, 48, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, X.; Chen, J.; Amin, R.; Lu, M.; Bhayana, B.; Zhao, J.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; et al. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and in Vivo Studies. J. Infect. Dis. 2016, 213, 1380–1387. [Google Scholar] [CrossRef] [Green Version]
- Hope, C.K.; Strother, M.; Creber, H.K.; Higham, S.M. Lethal Photosensitisation of Prevotellaceae under Anaerobic Conditions by Their Endogenous Porphyrins. Photodiagn. Photodyn. Ther. 2016, 13, 344–346. [Google Scholar] [CrossRef]
- Hope, C.K.; Hindley, J.A.; Khan, Z.; de Josselin de Jong, E.; Higham, S.M. Lethal Photosensitization of Porphyromonas Gingivalis by Their Endogenous Porphyrins under Anaerobic Conditions: An in Vitro Study. Photodiagn. Photodyn. Ther. 2013, 10, 677–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bumah, V.V.; Aboualizadeh, E.; Masson-Meyers, D.S.; Eells, J.T.; Enwemeka, C.S.; Hirschmugl, C.J. Spectrally Resolved Infrared Microscopy and Chemometric Tools to Reveal the Interaction between Blue Light (470 Nm) and Methicillin-Resistant Staphylococcus Aureus. J. Photochem. Photobiol. B Biol. 2017, 167, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Kaidzu, S.; Sugihara, K.; Sasaki, M.; Nishiaki, A.; Igarashi, T.; Tanito, M. Evaluation of Acute Corneal Damage Induced by 222-nm and 254-nm Ultraviolet Light in Sprague–Dawley Rats. Free Radic. Res. 2019, 53, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Asano, K.; Morimoto, Y.; Igarashi, T.; Nakane, A. Chronic Irradiation with 222-nm UVC Light Induces Neither DNA Damage nor Epidermal Lesions in Mouse Skin, Even at High Doses. PLoS ONE 2018, 13, e0201259. [Google Scholar] [CrossRef]
- Buonanno, M.; Ponnaiya, B.; Welch, D.; Stanislauskas, M.; Randers-Pehrson, G.; Smilenov, L.; Lowy, F.D.; Owens, D.M.; Brenner, D.J. Germicidal Efficacy and Mammalian Skin Safety of 222-nm UV Light. Radiat. Res. 2017, 187, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Yamano, N.; Kunisada, M.; Kaidzu, S.; Sugihara, K.; Nishiaki-Sawada, A.; Ohashi, H.; Yoshioka, A.; Igarashi, T.; Ohira, A.; Tanito, M.; et al. Long-term Effects of 222-nm Ultraviolet Radiation C Sterilizing Lamps on Mice Susceptible to Ultraviolet Radiation. Photochem. Photobiol. 2020, 96, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Buonanno, M.; Randers-Pehrson, G.; Bigelow, A.W.; Trivedi, S.; Lowy, F.D.; Spotnitz, H.M.; Hammer, S.M.; Brenner, D.J. 207-nm UV Light—A Promising Tool for Safe Low-Cost Reduction of Surgical Site Infections. I: In Vitro Studies. PLoS ONE 2013, 8, e76968. [Google Scholar] [CrossRef] [Green Version]
- Buonanno, M.; Stanislauskas, M.; Ponnaiya, B.; Bigelow, A.W.; Randers-Pehrson, G.; Xu, Y.; Shuryak, I.; Smilenov, L.; Owens, D.M.; Brenner, D.J. 207-Nm UV Light—A Promising Tool for Safe Low-Cost Reduction of Surgical Site Infections. II: In-Vivo Safety Studies. PLoS ONE 2016, 11, e0138418. [Google Scholar] [CrossRef] [Green Version]


| Bacteria | Surface | Reduction (CFU, CFU/mL or CFU/g) | Light Dosage | Light Source; Temperature; Distance; Photosensitizer β | Reference |
|---|---|---|---|---|---|
| B. cereus (vegetative or spores) | Food packaging (yellow trays; LINPAC) | 4 log (vegetative); 2.7 log (spores) | 18 J/cm2 | Blue LED (400 nm; 20 mW/cm2); ALA 1 (3 mmol/L) | [166] |
| B. cereus (vegetative or spores) L. monocytogenes (planktonic or biofilms) | Polyolefin food packaging (yellow trays; LINPAC) | 4–4.5 log (vegetative); 2–5 log (spores) 4.2 log | 3600–10,800 J (vegetative); 3600 J (spores) 3600 J | Blue LED (405 nm; 12 mW/cm2); CHL 2 (7.5 × 10−7–1.5 × 10−6 M for vegetative cells or 7.5 × 10−6–7.5 × 10−5 M for spores) Blue LED (405 nm; 12 mW/cm2); CHL 2 (7.5 × 10−7 M for planktonic cells or 1.5 × 10−4 M for biofilms) | [167] |
| L. monocytogenes (planktonic or biofilms) | Polyolefin food packaging (yellow trays; LINPAC) | 2.3–3.7 log (planktonic); 1.7–3 log (biofilm) | 18 J/cm2 | Blue LED (400 nm; 20 mW/cm2); ALA 1 (7.5 or 10 mM) | [168] |
| Uropathogenic E. coli; E. coli O157:H7; Salmonella spp.; L. monocytogenes; S. aureus | STC 2 contaminated with bacteria-laden chicken purge | 0.23–1.01 log | 180 J/cm2 | Blue LED (405 nm; 150 or 300 mW/cm2); 10 °C; 23 cm | [161] |
| Campylobacter jejuni Campylobacter coli | STC 3 contaminated with bacteria-laden chicken exudate | 1.1 or 4.9 log 3.1 or 5.1 log | 91.7 or 183.4 J/cm2 89.2 or 185.8 J/cm2 | Blue LED (405 nm; 151, 226 or 306 mW/cm2); 10 °C; 20.3 cm | [162] |
| E. coli L. monocytogenes S. aureus P. aeruginosa | Glass or acrylic surfaces | 7–8 log (glass); 5 log (acrylic) α 2.48 (glass) 2.75 (glass) 3.72 (glass) | 504 J/cm2 (E. coli) or 168 J/cm2 (other bacterial species) | Blue LED (405 nm; 141.48 mW/cm2); RT 4; 5 cm | [164] |
| C. jejuni | Stainless steel Cutting board (polyvinylchloride) | 2 log 4 log | 1.20–2.10 J/cm2 | NUV–vis 5 LED (395 nm); RT 4; 3, 12 or 23 cm | [169] |
| Salmonella enterica subsp. enterica serovar Enteritidis L. monocytogenes | Polyvinylchloride (PVC) or acrylic surfaces | 1.90–2.19 log (PVC); 1.18–1.63 log (acrylic) 0.68–0.90 log (PVC); 0.21–0.42 log (acrylic) | 15–45 J/cm2 (PVC); 15–60 J/cm2 (acrylic) | Blue LED (405 nm; 110 mW/cm2) | [146] |
| L. monocytogenes (planktonic or biofilm) | STC 3 or AC 6 contaminated with bacteria-laden salmon exudate | Planktonic: 1.9–2.4 log (STC 3); 2.4–2.8 log (AC 6) Biofilm: 0.7–1.6 log | 748.8 J/cm2 | Blue LED (405 nm; 26 mW/cm2); 4, 15, 25 °C; 4.5 cm | [163] |
| Bacteria | Food Matrix | Reduction (CFU/mL or CFU/g) | Light Dosage | Light Source; Temperature; Distance β | Reference |
|---|---|---|---|---|---|
| E. coli | UHT skim milk |
4.69–5.27 log (405 nm); 4.11–5.04 log (433 nm); 3.41–4.64 log (460 nm) |
Approx. 250 J/cm2 (405 nm); 313 J/cm2 (433 nm); 376 J/cm2 (460 nm) α | Blue LED (405, 433 or 460 nm; 10 W); 5–15 °C; 30 mm | [174] |
| S. aureus; E. coli; P. aeruginosa; S. Typhimurium; M. fortuitum | Whole milk | 5 log | 228.84–583.5 J/cm2 | Blue LED (413 nm; 100 mW/cm2); 1 mm | [171] |
| P. fluorescens (spoilage bacteria) | Ricotta cheese | 3–5 log | 6.36 J/cm2 | Near UV–vis LED (395 nm; 16 mW/cm2); 6 cm | [176] |
| L. monocytogenes
P. fluorescens (spoilage bacteria) | Sliced cheese (packaged) |
5.14 log (4 °C); 1.95 log (25 °C) 3.60 log (4 °C); 1.85 log (25 °C) |
604.8 J/cm2 (4 °C); 172.8 J/cm2 (25 °C) |
Blue LED (460–470 nm; 1 mW/cm2); 4 or 25 °C; 10 mm | [165] |
| S. enterica (Gaminara, Montevideo, Newport, Typhimurium and Saintpaul) | Orange juice | 2–5 log | 4500 J/cm2 |
Blue LED (460 nm; 92, 147.7 or 254.7 mW/cm2); 4, 12 or 20 °C | [175] |
| Bacteria | Food Matrix | Reduction (CFU, CFU/mL or CFU/g) | Light Dosage | Light Source; Temperature; Distance; Photosensitizer β | Reference |
|---|---|---|---|---|---|
| L. monocytogenes | Basil | 1.6 log | 9 J/cm2 | Blue LED (405 nm; 10 mW/cm2); RT 1; 6 cm; chlorophyllin (1.5 × 10−4 M) | [186] |
| E. coli | Grape | 2.4 log | 36.3 J/cm2 | Blue LED (465–470 nm; 4.5–30.2 mW/cm2); RT 1; 19 cm; curcumin (1.6 × 10−3 M) | [103] |
| L. monocytogenes
Salmonella spp. | Cantaloupe rinds |
At 405 nm: 2.4–2.9 log (no CHL); 2.8–3 log (CHL) At 460 nm: 2.7 log (no CHL); 2.2–2.3 log (CHL) At 405 nm: 2.3 (no CHL); 2.9 (CHL) At 460 nm: 1.1 log | 1210 J/cm2 (405 nm); 5360 J (460 nm) | Blue LED (405 or 460 nm; 7 or 31 mW/cm2); 4 or 20 °C; CHL2 (100 µM) | [104] |
| Salmonella spp. | Fresh-cut papaya | 1–1.2 log (4 °C); 0.3–1.3 log (10 °C); 0.8–1.6 log (20 °C) | 900–1700 J/cm2 | Blue LED (405 nm); 4, 10 or 20 °C; 2.3 or 4.5 cm | [116] |
|
Mesophilic bacteria B. cereus L. monocytogenes | Cherry tomatoes |
2.4 log 1.5 log 1.6 log | 3–9 J/cm2 | Blue LED (405 nm; 10 mW/cm2); RT 1; 6 cm NCCHL 3 (1.5 × 10−4 M) | [181] |
| S. Typhimurium | Strawberries | 2.2. log | 38 J/cm2 | Blue LED (405 nm; 10–11 mW/cm2); 37 °C; 3.5 or 6 cm; CHL-CHN 4 | [180] |
| S. Typhimurium | Cucumber peels | Approx. 3.9 log | 18 J/cm2 | Supra-luminous diode (SLD; 464 nm; 16.6 mW/cm2) | [187] |
| E. coli
O157:H7 E. coli K-12 S. Enteritidis non-pathogenic S. Typhimurium | Almond kernel |
1.43–2.44 log 1.64–1.84 log 0.55–0.70 log 0.64–0.96 log | 2000 J § | Blue LED (405 nm; 3.4 W); RT 1; 7 cm | [185] |
| S. aureus | Cucumber Pepper (green, red or yellow) |
2.6 log 2.5 log | 33.8 J/cm2 | Blue LED (435 nm; 9.4 mW/cm2); RT 1; PVP-C 5 (50 or 100 µM) | [178] |
| E. coli | Cucumber Tomatoes Lettuce |
3 log (10 µM); 4 log (50 µM); 4.5 log (100 µM) Approx. 3 log (10 µM); 6 log (50 µM); 3 log (100 µM) Approx. 3 log (10 µM); 7 log (50 µM); 6 log (100 µM) | 33.8 J/cm2 | Blue LED (435 nm; 9.4 mW/cm2);15 cm; cationic curcumin derivative (10, 50 or 100 µM) | [177] |
| E. coli |
Fenugreek seeds
Mung beans Mung bean germling |
Approx. 3 log (10 µM); 5 log (50 µM); 4.5 log (100 µM) Approx. 2.5 log (10 µM); 2 log (50 µM); 3.5 log (100 µM) Approx. 0.5 log (10 µM); 1 log (50 µM); 0.5 log (100 µM) | 33.8 J/cm2 | Blue LED (435 nm; 9.4 mW/cm2);15 cm; cationic curcumin derivative (10, 50 or 100 µM) | [177] |
| Salmonella spp. | Fresh-cut pineapple | 0.61–1.72 log | Approx. 8000 J/cm2 | Blue LED (460 nm; 92–257 mW/cm2); 7, 16 or 25 °C; 2.5–4.5 cm | [184] |
| E. coli O157:H7, Salmonella spp. or L. monocytogenes | Fresh-cut mangoes | 1–1.6 log | 1700–3500 J/cm2 | Blue LED (405 nm; 20 mW/cm2); 4, 10 or 20 °C; 4.5 cm | [182] |
| L. monocytogenes
Mesophilic bacteria Yeasts and microfungi | Strawberries |
1.8 log 1.7 log 0.87 log | 14.4 J/cm2 | Blue LED (400 nm; 12 mW/cm2); NCCHL 3 (1 mM) | [188] |
| E. coli | Fresh-cut Fuji apple | 0.95 log | 152 J/cm2 | Blue LED (420 nm; 298 mW/cm2); 4 cm; curcumin (2 µM) | [183] |
| Bacteria | Food Matrix | Reduction (CFU, CFU/mL or CFU/g) | Light Dosage | Light Source; Temperature; Distance; Photosensitizer β | Reference |
|---|---|---|---|---|---|
| Uropathogenic E. coli; E. coli O157:H7; Salmonella spp.; L. monocytogenes; S. aureus | Chicken skin | 0.19–0.40 log | 180 J/cm2 | Blue LED (405 nm; 150 or 300 mW/cm2); 10 °C; 23 cm | [161] |
| C. jejuni
C. coli | Chicken skin |
1.7 log 2.1 log |
184 J/cm2 185.8 J/cm2 | Blue LED (405 nm; 151, 226 or 306 mW/cm2); 10 °C; 20.3 cm | [162] |
| L. monocytogenes | Hot dog | <1 log | 120 J/cm2 | SLD (405 or 464 nm); 3–5 mm | [192] |
| E. coli | Hot dog | 2.43 log | 100 J/cm2 | SLD (405 nm; 83.3 mW/cm2); 3–5 mm | [187] |
| L. monocytogenes
Salmonella spp. | Fresh salmon |
0.4 log (4 °C); 0.3 log (12 °C) 0.5 log (4 °C); 0.4 log (12 °C) | 460.8 J/cm2 | Blue LED (405 nm; 16 mW/cm2); 4 or 12 °C; 7.9 cm | [190] |
| C. jejuni |
Skinless chicken fillet Chicken skin |
1.43–2.62 log Approx. 6.7 log (3 cm); 1 log (12 cm); 0.7 log (23 cm) |
1.20–2.10 J/cm2 9 J/cm2 (3 cm); 4.23 J/cm2 (12 cm); 1.20 J/cm2 (23 cm) | NUV–vis 1 LED (395 nm); RT 2; 3, 12 or 23 cm | [169] |
| L. monocytogenes | Smoked salmon fillets | 0.7–1.2 log | 2400 J/cm2 | Blue LED (460 nm; 15, 31 or 58 mW/cm2); 4 or 12 °C; 5.4–9 cm; riboflavin (25, 50 or 100 µM) | [191] |
| S. aureus | Chicken meat (with skin) | 1.7 log | 33.8 J/cm2 | Blue LED (435 nm; 9.4 mW/cm2); RT 2; curcumin (50 or 100 µM) | [178] |
| S. Enteritidis | Cooked chicken | 0.8–0.9 log | 1.58–3.80 J/cm2 | Blue LED (405 nm; 22 mW/cm2); 4 °C; 4 cm | [189] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadi, J.; Wu, S.; Brightwell, G. Antimicrobial Blue Light versus Pathogenic Bacteria: Mechanism, Application in the Food Industry, Hurdle Technologies and Potential Resistance. Foods 2020, 9, 1895. https://doi.org/10.3390/foods9121895
Hadi J, Wu S, Brightwell G. Antimicrobial Blue Light versus Pathogenic Bacteria: Mechanism, Application in the Food Industry, Hurdle Technologies and Potential Resistance. Foods. 2020; 9(12):1895. https://doi.org/10.3390/foods9121895
Chicago/Turabian StyleHadi, Joshua, Shuyan Wu, and Gale Brightwell. 2020. "Antimicrobial Blue Light versus Pathogenic Bacteria: Mechanism, Application in the Food Industry, Hurdle Technologies and Potential Resistance" Foods 9, no. 12: 1895. https://doi.org/10.3390/foods9121895
APA StyleHadi, J., Wu, S., & Brightwell, G. (2020). Antimicrobial Blue Light versus Pathogenic Bacteria: Mechanism, Application in the Food Industry, Hurdle Technologies and Potential Resistance. Foods, 9(12), 1895. https://doi.org/10.3390/foods9121895