Metabolism of Black Carrot Polyphenols during In Vitro Fermentation Is Not Affected by Cellulose or Cell Wall Association
Abstract
:- Black carrot fractions (puree, supernatant, and pellet) differed in fermentability
- Polyphenol fermentations were comparable between carrots and a cellulose model
- Juice-soaked cellulose can be a model for vegetable polyphenol fermentation
1. Introduction
2. Materials and Methods
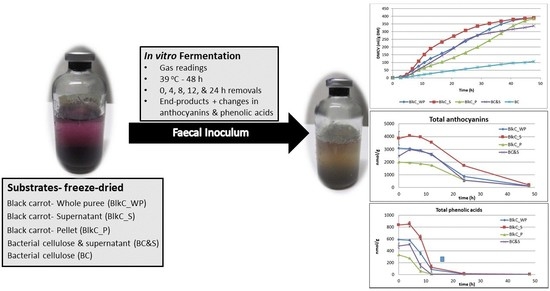
2.1. Materials
2.2. Preparation of Substrates
2.3. Collection and Preparation of Inoculum (Faecal)
2.4. Cumulative Gas Production Technique
2.5. Short Chain Fatty Acid (SCFA) and Ammonium Analyses
2.6. Sugar Analysis
2.7. Analysis of Anthocyanins and Phenolic Acids
2.8. Data Handling and Statistical Analysis
3. Results and Discussion
3.1. Concentration of Anthocyanins and Phenolic Acids in Pre-Fermented Substrates
3.2. Fermentability of Black Carrot vs. BCell Model System
3.3. Polyphenol Metabolism/Degradation during Fermentation
3.3.1. Anthocyanins
3.3.2. Phenolic Acids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BlkC | Black carrot |
| BCell | Bacterial cellulose |
| BCell&S | Bacterial cellulose soaked in supernatant |
| BlkC_P | Black carrot pellet |
| BlkC_S | Black carrot supernatant |
| BlkC_WP | Black carrot whole puree |
| cy | cyanidin |
| cy-3-O-xylglcgal | cyanidin-3-O-xylosyl(glucosyl)galactoside |
| cy-3-O-xylgal | cyanidin-3-O-xylosylgalactoside |
| DM | Dry matter |
| GIT | Gastro-intestinal tract |
| h | Hour(s) |
| min | Minutes |
| mv | malvidin |
| PC | Plant cell |
| PCW | Plant cell walls |
| pg | pelargonidin |
| pn | peonidin |
| SCFA | Short-chain fatty acids |
References
- Costa, C.; Tsatsakis, A.; Mamoulakis, C.; Teodoro, M.; Briguglio, G.; Caruso, E.; Tsoukalas, D.; Margina, D.; Dardiotis, E.; Kouretas, D.; et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem. Toxicol. 2017, 110, 286–299. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, I.; Morales, M.A.; Rojas, A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res. Int. 2020, 129, e108843. [Google Scholar] [CrossRef] [PubMed]
- Pojer, E.; Mattivi, F.; Johnson, D.; Stockley, C.S. The case for anthocyanin consumption to promote human health: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 483–508. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Zhang, J.J.; Manna, P.P.; Daglia, M.; Atanasov, A.G.; et al. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol. Adv. 2020, 38, e107322. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Current understanding of dietary polyphenols and their role in health and disease. Curr. Nutr. Food Sci. 2009, 5, 249–263. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Kundur, A.R.; Sabapathy, S.; Stanley, R.; Singh, I. The potential of anthocyanin-rich Queen Garnet plum juice supplementation in alleviating thrombotic risk under induced oxidative stress conditions. J. Funct. Foods 2015, 14, 747–757. [Google Scholar] [CrossRef]
- Wallace, T.C.; Murray, R.; Zelman, K.M. The nutritional value and health benefits of chickpeas and hummus. Nutrients 2016, 8, e766. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Du, M.Z.; Wei, Y.; Wang, C.T.; Shen, L.Q. A review on the structure-activity relationship of dietary flavonoids for protecting vascular endothelial function: Current understanding and future issues. J. Food Biochem. 2018, 42, e12557. [Google Scholar] [CrossRef]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M.J. Binding of polyphenols to plant cell wall analogues—Part 2: Phenolic acids. Food Chem. 2012, 135, 2287–2292. [Google Scholar] [CrossRef]
- Dufour, C.; Loonis, M.; Delosiere, M.; Buffiere, C.; Hafnaoui, N.; Sante-Lhoutellier, V.; Remond, D. The matrix of fruit & vegetables modulates the gastrointestinal bioaccessibility of polyphenols and their impact on dietary protein digestibility. Food Chem. 2018, 240, 314–322. [Google Scholar]
- Hedren, E.; Diaz, V.; Svanberg, U. Estimation of carotenoid accessibility from carrots determined by an in vitro digestion method. Eur. J. Clin. Nutr. 2002, 56, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Wink, M. Compartmentation of secondary metabolites and xenobiotics in plant vacuoles. In Advances in Botanical Research Incorporating Advances in Plant Pathology, Vol 25: The Plant Vacuole; Leigh, R.A., Sanders, D., Eds.; Academic Press: Cambridge, MA, USA, 1997; Volume 25, pp. 141–169. [Google Scholar]
- Le Bourvellec, C.; Guyot, S.; Renard, C. Non-covalent interaction between procyanidins and apple cell wall material Part I. Effect of some environmental parameters. Biochim. Biophys. Acta Gen. Subj. 2004, 1672, 192–202. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C. Non-covalent interaction between procyanidins and apple cell wall material. Part II: Quantification and impact of cell wall drying. Biochim. Biophys. Acta Gen. Subj. 2005, 1725, 1–9. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Zabaras, D.; Mikkelsen, D.; Gidley, M. Binding of polyphenols to plant cell wall analogues—Part 1: Anthocyanins. Food Chem. 2012, 134, 155–161. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Ayala-Zavala, J.F.; Gonzalez-Aguilar, G.A. The role of dietary fiber in the bioaccessibility and bioavailability of fruit and vegetable antioxidants. J. Food Sci. 2011, 76, R6–R15. [Google Scholar] [CrossRef] [Green Version]
- Phan, A.D.T.; Flanagan, B.M.; D’Arcy, B.R.; Gidley, M.J. Binding selectivity of dietary polyphenols to different plant cell wall components: Quantification and mechanism. Food Chem. 2017, 233, 216–227. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Baron, A.; Guyot, S.; Drilleau, J.F. Interactions between apple cell walls and native apple polyphenols: Quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef] [Green Version]
- Bresciani, L.; Favari, C.; Calani, L.; Francinelli, V.; Riva, A.; Petrangolini, G.; Allegrini, P.; Mena, P.; Del Rio, D. The effect of formulation of curcuminoids on their metabolism by human colonic microbiota. Molecules 2020, 25, 940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desjardins, Y. Plant polyphenols bioavailability and modulation of the gut microbiota consortium: A paradigm shift in understanding their effects on diseases. In XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes; O’Hare, T.J., Netzel, M.E., Eds.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2015; Volume 1106, pp. 199–210. [Google Scholar]
- Cardona, F.; Andres-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.J.; Williams, B.A.; Ferruzzi, M.G.; D’Arcy, B.R. Microbial metabolites, but not other phenolics derived from grape seed phenolic extract, are transported through differentiated Caco-2 cell monolayers. Food Chem. 2013, 138, 1564–1573. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Chalet, C.; Rubbens, J.; Tack, J.; Duchateau, G.S.; Augustijns, P. Intestinal disposition of quercetin and its phase-II metabolites after oral administration in healthy volunteers. J. Pharm. Pharmacol. 2018, 70, 1002–1008. [Google Scholar] [CrossRef]
- Fleschhut, J.; Kratzer, F.; Rechkemmer, G.; Kulling, S.E. Stability and biotransformation of various dietary anthocyanins in vitro. Eur. J. Nutr. 2006, 45, 7–18. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo, C.; Porrini, M.; Ciappellano, S.; Riso, P. Role of polyphenols and polyphenol-rich foods in the modulation of PON1 activity and expression. J. Nutr. Biochem. 2017, 48, 1–8. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef] [Green Version]
- Phan, A.D.T.; Williams, B.A.; Netzel, G.; Mikkelsen, D.; D’Arcy, B.R.; Gidley, M.J. Independent fermentation and metabolism of dietary polyphenols associated with a plant cell wall model. Food Funct. 2020, 11, 2218–2230. [Google Scholar] [CrossRef]
- Mikkelsen, D.; Lopez-Sanchez, P.; Wang, D.; Gidley, M.J. Formation of cellulose-based composites with hemicelluloses and pectins using Komagataeibacter fermentation. In The Plant Cell Wall: Methods and Protocols; Popper, Z.A., Ed.; Humana Press: New York, NY, USA, 2020; Volume 2149, pp. 73–87. [Google Scholar]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Gras, C.C.; Carle, R.; Schweiggert, R.M. Determination of anthocyanins from black carrots by UHPLC-PDA after ultrasound-assisted extraction. J. Food Compos. Anal. 2015, 44, 170–177. [Google Scholar] [CrossRef]
- Montilla, E.C.; Arzaba, M.R.; Hillebrand, S.; Winterhalter, P. Anthocyanin composition of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) cultivars Antonina, Beta Sweet, Deep Purple, and Purple Haze. J. Agric. Food Chem. 2011, 59, 3385–3390. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Simon, P.W.; Tanumihardjo, S.A. Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J. Agric. Food Chem. 2009, 57, 4142–4147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hassan, Y.I.; Renaud, J.; Liu, R.H.; Yang, C.; Sun, Y.; Tsao, R. Bioaccessibility, bioavailability, and anti-inflammatory effects of anthocyanins from purple root vegetables using mono- and co-culture cell models. Mol. Nutr. Food Res. 2017, 61, 1600928. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Ozkan, G.; Isik, H.; Horoz, O.; Van Camp, J.; Capanoglu, E. Black carrot pomace as a source of polyphenols for enhancing the nutritional value of cake: An in vitro digestion study with a standardized static model. LWT Food Sci. Technol. 2016, 77, 475–481. [Google Scholar] [CrossRef]
- Padayachee, A.; Netzel, G.; Netzel, M.; Day, L.; Mikkelsen, D.; Gidley, M.J. Lack of release of bound anthocyanins and phenolic acids from carrot plant cell walls and model composites during simulated gastric and small intestinal digestion. Food Funct. 2013, 4, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by acetobacter-xylinum 2. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.A.; Bosch, M.W.; Boer, H.; Verstegen, M.W.A.; Tamminga, S. An in vitro batch culture method to assess potential fermentability of feed ingredients for monogastric diets. Anim. Feed Sci. Technol. 2005, 123, 445–462. [Google Scholar] [CrossRef]
- Williams, B.A.; Mikkelsen, D.; le Paih, L.; Gidley, M.J. In vitro fermentation kinetics and end-products of cereal arabinoxylans and (1,3;1,4)-beta-glucans by porcine faeces. J. Cereal Sci. 2011, 53, 53–58. [Google Scholar] [CrossRef]
- Baethgen, W.E.; Alley, M.M. A manual colorimetric procedure for measuring ammonium nitrogen in the soil and plant kjeldahl digests. Commun. Soil Sci. Plant Anal. 1989, 20, 961–969. [Google Scholar] [CrossRef]
- Gross, G.; Jacobs, D.M.; Peters, S.; Possemiers, S.; van Duynhoven, J.; Vaughan, E.E.; van de Wiele, T. In vitro bioconversion of polyphenols from black tea and red wine/grape juice by human intestinal microbiota displays strong inter-individual variability. J. Agric. Food Chem. 2010, 58, 10236–10246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groot, J.C.J.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.A.; Lantinga, E.A. Multiphasic analysis of gas production kinetics for in vitro fermentation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Williams, B.A.; Bosch, M.W.; Awati, A.; Konstantinov, S.R.; Smidt, H.; Akkermans, A.D.L.; Verstegen, M.W.A.; Tamminga, S. In vitro assessment of gastrointestinal tract (GIT) fermentation in pigs: Fermentable substrates and microbial activity. Anim. Res. 2005, 54, 191. [Google Scholar] [CrossRef]
- Kammerer, D.; Carle, R.; Schieber, A. Characterization of phenolic acids in black carrots (Daucus carota ssp sativus var. atrorubens Alef.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 1331–1340. [Google Scholar] [CrossRef]
- Alasalvar, C.; Grigor, J.M.; Zhang, D.L.; Quantick, P.C.; Shahidi, F. Comparison of volatiles, phenolics, sugars, antioxidant vitamins, and sensory quality of different colored carrot varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Forester, S.C.; Waterhouse, A.L. Identification and toxicity of microfloral anthocyanin metabolites. Am. J. Enol. Vitic. 2008, 59, 351A. [Google Scholar]
- Keppler, K.; Humpf, H.U. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorganic Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef]
- Flores, G.; del Castillo, M.L.R.; Costabile, A.; Klee, A.; Guergoletto, K.B.; Gibson, G.R. In vitro fermentation of anthocyanins encapsulated with cyclodextrins: Release, metabolism and influence on gut microbiota growth. J. Funct. Foods 2015, 16, 50–57. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Remesy, C.; Scalbert, A.; Cheynier, V.; Souquet, J.M.; Poutanen, K.; Aura, A.M. Microbial metabolism of caffeic acid and its esters chlorogenic and caftaric acids by human faecal microbiota in vitro. Biomed. Pharmacother. 2006, 60, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hein, E.M.; Rose, K.; Van’t Slot, G.; Friedrich, A.W.; Humpf, H.U. Deconjugation and degradation of flavonol glycosides by pig cecal microbiota characterized by fluorescence in situ hybridization (FISH). J. Agric. Food Chem. 2008, 56, 2281–2290. [Google Scholar] [CrossRef] [PubMed]



| Substrates * | % Sucrose | % Glucose | % Fructose | % Total Sugars ** |
|---|---|---|---|---|
| BCell&S | 7.3 ± 0.6 | 5.9 ± 0.1 | 3.7 ± 0.2 | 16.8 ± 0.9 |
| BlkC_WP | 39.8 ± 3.1 | 3.4 ± 0.3 | 20.5 ± 0.7 | 63.6 ± 4.1 |
| BlkC_S | 59.8 ± 3.2 | 5.7 ± 0.3 | 31.9 ± 1.9 | 97.4 ± 5.4 |
| BlkC_P | 10.8 ± 0.6 | 1.2 ± 0.1 | 5.9 ± 0.1 | 17.8 ± 0.8 |
| BlkC_WP | BlkC_S | BlkC_P | BCell&S | |
|---|---|---|---|---|
| Anthocyanins | ||||
| Cy-3-O-xylglcgal | 319.4 * | 1584 | 655.0 | 587.3 |
| Cy-3-O-xylgal | 1109 | 5568 | 2660 | 2118 |
| Sum (non-acylated) | 1428 | 7152 | 3315 | 2705 |
| Caffeic acid derivative of cy-3-O-xylglcgal | 96.5 | 129.0 | 70.9 | 66.5 |
| Sinapic acid derivative of cy-3-O-xylglcgal | 479.6 | 948.6 | 471.8 | 633.8 |
| Ferulic acid derivative of cy-3-O-xylglcgal | 5859 | 13380 | 6259. | 8059 |
| p-coumaric acid derivative of cy-3-O-xylglcgal | 663.0 | 1496 | 778.3 | 860.6 |
| Sum (acylated) | 7098 | 15,954 | 7580 | 9620 |
| Total (acylated + non-acylated) | 8526 | 23,106 | 10,895 | 12,325 |
| Phenolic acids | ||||
| Chlorogenic acids | 27009 | 51052 | 16075 | 25597 |
| Non-esterified hydroxycinnamates | 1832 | 3283 | 1271 | 1720 |
| Total | 28,841 | 54,336 | 17,346 | 27,317 |
| Dry matter (%) | 6.7 | 3.8 | 12.3 | 31.5 |
| Substrates | n | DMCV (mL) | ½ Time (h) | TRmax (h) | Rmax (Lm/h) | pH | NH4 mmol/bottle |
|---|---|---|---|---|---|---|---|
| BCell | 3 | 111 c | 23.97 b | 12.09 b | 0.413 d | 6.50 a | 2.50 a |
| BCell&S | 4 | 331 b | 18.04 c | 12.35 b | 1.455 b | 6.35 b | 2.22 a |
| BlkC_WP | 4 | 377 a | 23.10 b | 12.96 b | 1.445 b | 6.37 ab | 1.16 a |
| BlkC_S | 4 | 396 a | 15.3 c | 9.23 b | 1.965 a | 6.34 b | 2.61 a |
| BlkC_P | 4 | 394 a | 31.9 a | 18.43 a | 1.205 c | 6.37 ab | 2.00 a |
| Probability | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.023 | 0.58 | |
| MSD | 32.3 | 2.84 | 3.76 | 0.165 | 0.138 | 1.34 | |
| Blank | 2 | 8.7 | 20.3 | 10.29 | 0.310 | 6.47 | 0.30 |
| Substrates | n | Acetic | Propionic | Butyric | Total SCFA | %Acet | %Prop | %But | BCR |
|---|---|---|---|---|---|---|---|---|---|
| mmol/gDM | |||||||||
| BCell | 3 | 2.60 b | 0.83 d | 0.32 c | 4.6 c | 56.4 b | 18.0 e | 7.03 a | 0.452 a |
| BCell&S | 3 | 4.71 a | 2.07 c | 0.40 bc | 7.79 b | 60.5 a | 26.6 c | 5.18 c | 0.165 b |
| BlkC_WP | 3 | 5.79 a | 3.33 ab | 0.62 ab | 10.4 ab | 55.5 b | 31.9 b | 5.94 b | 0.137 b |
| BlkC_S | 3 | 5.63 a | 4.22 a | 0.67 a | 11.3 a | 49.9 c | 37.4 a | 5.90 b | 0.139 b |
| BlkC_P | 3 | 6.17 a | 2.42 bc | 0.70 a | 9.9 ab | 62.1 a | 24.4 d | 7.04 a | 0.138 b |
| Probability | <0.0001 | <0.0001 | 0.0006 | 0.0002 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| MSD * | 1.49 | 1.06 | 0.22 | 3.01 | 1.77 | 1.26 | 0.61 | 0.032 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netzel, G.; Mikkelsen, D.; Flanagan, B.M.; Netzel, M.E.; Gidley, M.J.; Williams, B.A. Metabolism of Black Carrot Polyphenols during In Vitro Fermentation Is Not Affected by Cellulose or Cell Wall Association. Foods 2020, 9, 1911. https://doi.org/10.3390/foods9121911
Netzel G, Mikkelsen D, Flanagan BM, Netzel ME, Gidley MJ, Williams BA. Metabolism of Black Carrot Polyphenols during In Vitro Fermentation Is Not Affected by Cellulose or Cell Wall Association. Foods. 2020; 9(12):1911. https://doi.org/10.3390/foods9121911
Chicago/Turabian StyleNetzel, Gabriele, Deirdre Mikkelsen, Bernadine M. Flanagan, Michael E. Netzel, Michael J. Gidley, and Barbara A. Williams. 2020. "Metabolism of Black Carrot Polyphenols during In Vitro Fermentation Is Not Affected by Cellulose or Cell Wall Association" Foods 9, no. 12: 1911. https://doi.org/10.3390/foods9121911
APA StyleNetzel, G., Mikkelsen, D., Flanagan, B. M., Netzel, M. E., Gidley, M. J., & Williams, B. A. (2020). Metabolism of Black Carrot Polyphenols during In Vitro Fermentation Is Not Affected by Cellulose or Cell Wall Association. Foods, 9(12), 1911. https://doi.org/10.3390/foods9121911