Comparison and Discrimination of Two Major Monocultivar Extra Virgin Olive Oils in the Southern Region of Peloponnese, According to Specific Compositional/Traceability Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geographical Distribution, Sampling, and Sample Maintenance
2.2. Chemicals and Standards
2.3. Determination of the Physicochemical Quality Parameters
2.4. Determination of Sterols and Triterpene Dialcohols
2.5. Determination of Fatty Acid Composition
2.6. Statistical and Chemometric Analysis
3. Results and Discussion
3.1. Physico-Chemical Parameter of the Two Major Olive Cultivars of Southern Peloponnese
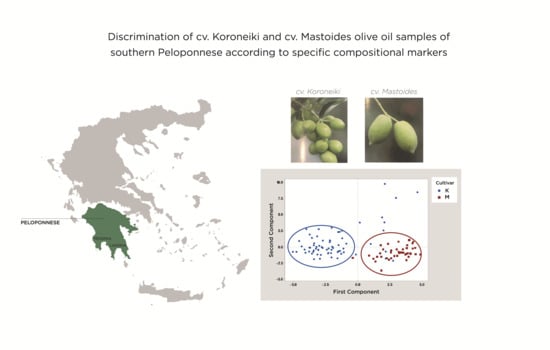
3.2. Evaluation and Discrimination of the Two Examined Cultivars of Southern Peloponnese According to Their Fatty Acid Composition
3.3. Evaluation and Discrimination of the Two Examined Cultivars From the Southern Region of Peloponnese According to Their Sterolic Profile
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Olive Council Database. Available online: http://www.internationaloliveoil.org/estaticos/view/131-world-olive-oil-figures (accessed on 17 November 2019).
- Prospects for the Olive Oil Sector in Spain, Italy and Greece 2012–2020. Available online: https://ec.europa.eu/agriculture/markets-and-prices/market-briefs_en (accessed on 10 November 2019).
- Zampounis, V. Olive oil in the World Market. In Olive Oil: Chemistry and Technology, 2nd ed.; Boskou, D., Ed.; AOCS Press: Campaign, IL, USA, 2006; pp. 21–40. [Google Scholar]
- Montet, D.; Ray, R. (Eds.) Food Traceability and Authenticity; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar] [CrossRef]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Lacoste, F.; Brereton, P.; Garcia-Gonzalez, D.L.; Moreda, W.; et al. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 2019, in press. [Google Scholar] [CrossRef]
- Council Regulation (EC). No. 2081/92 of 14 July 1992 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Union 1992, L208, 1–8. [Google Scholar]
- Council Regulation (EC). No. 2082/92 of 14 July 1992 on certificates of specific character for agricultural products and foodstuffs. Off. J. Eur. Union 1992, L208, 9–14. [Google Scholar]
- Council Regulation (EC). No. 510/2006 of 20 March 2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Union 2006, L93, 12–25. [Google Scholar]
- Council Regulation (EC). No. 1898/2006 of 14 December 2006 laying down detailed rules of implementation of Council Regulation (EC) no. 510/2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs. Off. J. Eur. Union 2006, L369, 1–23. [Google Scholar]
- Council Regulation (EC). No. 182/2009 of 6 March 2009 amending Regulation (EC) no. 1019/2002 on marketing standards for olive oil. Off. J. Eur. Union 2009, L63, 6–8. [Google Scholar]
- Montealegre, C.; Alegre, M.; García-Ruiz, C. Traceability Markers to the Botanical Origin in Olive Oils. J. Agric. Food Chem. 2009, 58, 28–38. [Google Scholar] [CrossRef]
- Aparicio, R.; Luna, G. Characterization of monovarietal virgin olive oil. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Olive Oil: Establishing the Greek Brand, National Bank of Greece. Available online: https://www.nbg.gr/greek/the-group/press-o_ce/e-spot/reports/Documents/Olive%20Oil_2015.pdf (accessed on 23 October 2019).
- Kostalenos, G. Greek olive varieties. In Elaiokomia Egkiklopedia-The Olive Oil, 1st ed.; Zambounis, V., Ed.; Axion Ekdotiki: Athens, Greece, 2017; pp. 39–53. (In Greek) [Google Scholar]
- Kostalenos, G. Olive Fruit Data, History, Description and Geographical Distribution of Olive Varieties in Greece, 1st ed.; Kostalenos: Poros Trizinias, Greece, 2011; pp. 1–436. (In Greek) [Google Scholar]
- Stefanoudaki, E.; Kotsifaki, F.; Koutsaftakis, A. The potential of HPLC triglyceride profiles for the classification of Cretan olive oils. Food Chem. 1997, 60, 425–432. [Google Scholar] [CrossRef]
- Stefanoudaki, E.; Kotsifaki, F.; Koutsaftakis, A. Classification of virgin olive oils of the two major Cretan cultivars based on their fatty acid composition. J. Am. Oil Chem. Soc. 1999, 76, 623–626. [Google Scholar] [CrossRef]
- Commission Regulation (EEC). No. 2568/91 of 14 July 1991 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Union 1991, L208, 1–8. [Google Scholar]
- Kiritsakis, A.; Christie, W. Analysis of edible oil. In Handbook of Olive Oil; Harwood, J., Aparicio, R., Eds.; Springer: Aspen, CO, USA; Gaithersburg, MD, USA, 2000; pp. 129–158. [Google Scholar]
- Boskou, D. Olive oil. In More on Mediterranean Diets; Simopoulos, A.P., Visioli, F., Eds.; Karger: Basel, Switzerland, 2007; pp. 180–210. [Google Scholar]
- D’Imperio, M.; Dugo, G.; Alfa, M.; Mannina, L.; Segre, A.L. Statistical analysis on Sicilian olive oils. Food Chem. 2007, 102, 956–965. [Google Scholar] [CrossRef]
- Sanchez Casas, J.J.; Osorio Bueno, E.; Montano Garcıa, A.M.; Martınez Cano, M. Estudio del contenido en acidos grasos de aceites monovarietales elaborados a partir de aceitunas producidas en la region extremena. Grasas y Aceites 2003, 54, 371–377. [Google Scholar]
- Mannina, L.; Dugo, G.; Salvo, F.; Cicero, L.; Ansanelli, G.; Calcagni, C.; Segre, A. Study of the cultivar-composition relationship in Sicilian olive oils by GC, NMR, and statistical methods. J. Agric. Food Chem. 2003, 51, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Mailer, R.J.; Ayton, J.; Graham, K. The Influence of growing region, cultivar and harvest timing on the diversity of Australian olive oil. J. Am. Oil Chem. Soc. 2010, 87, 877–884. [Google Scholar] [CrossRef]
- Longobardi, F.; Ventrella, A.; Casielloa, G.; Sacco, D.; Tasioula-Margari, A.K.; Kiritsakis, A.K.; Kontominas, M.G. Characterisation of the geographical origin of Western Greek virgin olive oils based on instrumental and multivariate statistical analysis. Food Chem. 2012, 133, 1169–1175. [Google Scholar] [CrossRef]
- Di Bella, G.; Maisano, R.; La Pera, L.; Lo Turco, V.; Salvo, F.; Dugo, G. Statistical characterization of Sicilian olive oils from the Peloritana and Maghrebian zones according to the fatty acid profile. J. Agric. Food Chem. 2007, 55, 6568–6574. [Google Scholar] [CrossRef]
- Ollivier, D.; Artaud, J.; Pinatel, C.; Durbec, J.P.; Guerere, M. Triacylglycerol and fatty acid compositions of French virgin olive oils. Characterization by chemometrics. J. Agric. Food Chem. 2003, 51, 5723–5731. [Google Scholar] [CrossRef]
- Krichene, D.; Taamalli, W.; Daoud, D.; Salvador, M.; Fregapane, G.; Zarrouk, M. Phenolic compounds, tocopherols and other minor components in virgin olive oils of some Tunisian varieties. J. Food Biochem. 2007, 31, 179–194. [Google Scholar] [CrossRef]
- Boskou, D. Olive Oil: Minor. Constituents and Health; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Leontopoulos, S.; Skenderidis, P.; Kalorizou, H.; Petrotos, K. Bioactivity Potential of Polyphenolic Compounds in Human Health and their Effectiveness Against Various Food Borne and Plant Pathogens. A Review. Int. J. Food Biol. Eng. 2017, 7, 1–19. [Google Scholar]
- Lukic, M.; Lukic, I.; Krapac, M.; Sladonja, B.; Pilizota, V. Sterols and triterpene diols in olive oil as indicators of variety and degree of ripening. Food Chem. 2013, 136, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Bucci, R.; Magri, A.D.; Magri, A.L.; Marini, D.; Marini, F. Chemical authentication of extra virgin olive oil varieties by supervised chemometric procedures. J. Agric. Food Chem. 2002, 50, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.C.; Cunha, S.C.; Amaral, J.S.; Pereira, J.A.; Andrade, P.B.; Seabra, R.M.; Oliveira, B. Chemometric characterization of three varietal olive oils (cvs. Cobrancosa, Madural and Verdeal Transmontana) extracted from olives with different maturation indices. Food Chem. 2007, 102, 406–414. [Google Scholar] [CrossRef]
- Brescia, M.A.; Alviti, G.; Liuzzi, V.; Sacco, A. Chemometrics classification of olive cultivars based on compositional data of oils. J. Am. Oil Chem. Soc. 2003, 80, 945–950. [Google Scholar] [CrossRef]
- Nagy, K.; Bongiorno, D.; Avellone, G.; Agozzino, P.; Ceraulo, L.; Vekey, K. High performance liquid chromatography-mass spectrometry based chemometric characterization of olive oils. J. Chromatogr. A 2005, 1078, 90–97. [Google Scholar] [CrossRef]
- Lorenzo, I.M.; Perez Pavon, J.L.; Fernandez Laespada, M.E.; Garcıa Pinto, C.; Moreno Cordero, B.; Henriques, L.R.; Peres, M.F.; Simoes, M.P.; Lopes, P.S. Application of headspace-mass spectrometry for differentiating sources of olive oils. Anal. Bioanal. Chem. 2002, 374, 1205–1211. [Google Scholar] [CrossRef]
- Vekiari, S.A.; Oreopoulou, V.; Kourkoutas, Y.; Kamoun, N.; Msallem, M.; Psimouli, V.; Arapoglou, D. Characterization and seasonal variation of the quality of virgin olive oil of the Throumbolia and Koroneiki varieties from Southern Greece. Grasas y Aceites 2010, 61, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Agiomirgianaki, A.; Petrakis, P.; Dais, P. Influence of harvest year, cultivar and geographical origin on Greek extra virgin olive oils composition: A study by NMR spectroscopy and biometric analysis. Food Chem. 2012, 135, 2561–2568. [Google Scholar] [CrossRef]
- Karabagias, I.; Michos, C.; Badeka, A.; Kontakos, S.; Stratis, I.; Kontominas, M.G. Classification of Western Greek virgin olive oils according to geographical origin based on chromatographic, spectroscopic, conventional and chemometric analyses. Food Res. Int. 2013, 54, 1950–1958. [Google Scholar] [CrossRef]
- Kandylis, P.; Vekiari, A.S.; Kanellaki, M.; Grati Kamoun, N.; Msallem, M.; Kourkoutas, Y. Comparative study of extra virgin olive oil flavor profile of Koroneiki variety (Olea europaea var. Microcarpa alba) cultivated in Greece and Tunisia during one period of harvesting. LWT J. Food Sci. Technol. 2011, 44, 1333–1341. [Google Scholar] [CrossRef]
- Skiada, V.; Tsarouhas, P.; Varzakas, T. Preliminary study and observation of “Kalamata PDO” extra virgin olive oil, in the Messinia region, southwest of Peloponnese (Greece). Foods 2019, 8, 610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouliarekou, E.; Badeka, A.; Tasioula-Margaria, M.; Kontakos, S.; Longobardic, F.; Kontominas, M.G. Characterization and classification of Western Greek olive oils according to cultivar and geographical origin based on volatile compounds. J. Chromatogr. A 2011, 1218, 7534–7542. [Google Scholar] [CrossRef] [PubMed]
- Aranda, F.; Gomez-Alonso, S.; Rivera del _Alamo, R.M.; Salvador, M.D.; Fregapane, G. Triglyceride, total and 2-position fatty acid composition of Cornicabra virgin olive oil: Comparison with other Spanish cultivars. Food Chem. 2004, 86, 485–492. [Google Scholar] [CrossRef]
- Lerma-Garcıa, M.J.; Herrero-Martınez, J.M.; Ramis-Ramos, G.; Simo-Alfonso, E.F. Prediction of the genetic variety of Spanish extra virgin olive oils using fatty acid and phenolic compound profiles established by direct infusion mass spectrometry. Food Chem. 2008, 108, 1142–1148. [Google Scholar] [CrossRef] [PubMed]



| cv. Koroneiki (N = 69) | cv. Mastoides (N = 43) | EEC Limit for EVOO Category | |||
|---|---|---|---|---|---|
| Parameter | Mean ± SD | Min–Max | Mean ± SD | Min–Max | |
| Free acidity (%) | 0.34 ± 0.13 | 0.17–0.76 | 0.39 ± 0.13 | 0.15–0.77 | ≤0.80 |
| Peroxide value (meqO2/kg) | 7.24 ± 1.88 | 3.64–11.96 | 6.96 ± 2.31 | 2.88–14.70 | ≤20 |
| K232 | 1.55 ± 0.14 | 1.33–2.14 | 1.63 ± 0.11 | 1.33–2.02 | ≤2.50 |
| K268 | 0.13 ± 0.01 | 0.08–0.21 | 0.12 ± 0.02 | 0.08–0.17 | ≤0.22 |
| cv. Koroneiki (N = 69) | cv. Mastoidis (N = 58) | Calculated p-Value | EEC Limit for EVOO Category | |||
|---|---|---|---|---|---|---|
| Parameter | Mean ± SD | Min–Max | Mean ± SD | Min–Max | ||
| Myristic C14:0 (%) | 0.01 ± 0.00 | 0.00–0.02 | 0.01 ± 0.00 | 0.00–0.02 | n.s | ≤0.03 |
| Palmitic C16:0 (%) | 12.02 ± 0.74 | 9.54–13.56 | 12.29 ± 0.77 | 9.96–13.28 | n.s | 7.50–20.00 |
| Palmitoleic C16:1 (%) | 0.92 ± 0.13 | 0.64–1.43 | 0.92 ± 0.10 | 0.64–1.08 | n.s | 0.30–3.50 |
| Heptadecanoic C17:0 (%) | 0.05 ± 0.02 | 0.03–0.15 | 0.14 ± 0.02 | 0.08–0.17 | 0.00 | ≤0.40 |
| Heptadecenoic C17:1 (%) | 0.08 ± 0.04 | 0.06–0.24 | 0.25 ± 0.03 | 0.16–0.29 | 0.00 | ≤0.60 |
| Stearic C18:0 (%) | 2.53 ± 0.19 | 1.98–3.12 | 2.64 ± 0.16 | 2.35–2.99 | 0.001 | 0.50–5.00 |
| Oleic C18:1 (%) | 76.70 ± 1.96 | 70.67–81.40 | 75.93 ± 1.27 | 73.15–79.44 | 0.024 | 55.00–83.00 |
| Linoleic C18:2 (%) | 6.09 ± 1.60 | 4.20–12.01 | 6.44 ± 0.69 | 5.11–8.13 | n.s | 2.50–21.00 |
| Linolenic C18:3 (%) | 0.68 ± 0.07 | 0.51–0.86 | 0.55 ± 0.04 | 0.49–0.66 | 0.00 | ≤1.00 |
| Arachidic C20:0 (%) | 0.44 ± 0.03 | 0.33–0.50 | 0.39 ± 0.02 | 0.35–0.44 | 0.00 | ≤0.60 |
| Eicosenoic C20:1 (%) | 0.31 ± 0.02 | 0.27–0.35 | 0.27 ± 0.02 | 0.23–0.32 | 0.00 | ≤0.50 |
| Behenic C22:0 (%) | 0.14 ± 0.01 | 0.09–0.17 | 0.10 ± 0.01 | 0.07–0.12 | 0.00 | ≤0.20 |
| Lignoceric C24:0 (%) | 0.05 ± 0.00 | 0.03–0.08 | 0.04 ± 0.007 | 0.03–0.06 | 0.00 | ≤0.20 |
| cv. Koroneiki (N = 69) | cv. Mastoidis (N = 43) | Calculating p-Value | EEC Limit for EVOO Category | |
|---|---|---|---|---|
| Sterols and Triterpene Diols | Mean ± SD | Mean ± SD | ||
| Cholesterol (%) | 0.11 ± 0.03 | 0.12 ± 0.03 | 0.017 | ≤0.5 |
| 24-methylene-cholesterol % | 0.32 ± 0.09 | 0.19 ± 0.05 | 0.00 | |
| Campesterol % | 3.71 ± 0.38 | 3.14 ± 0.16 | 0.00 | ≤4.0 |
| Campestanol % | 0.05 ± 0.03 | 0.04 ± 0.02 | n.s | <campesterol |
| Stigmasterol % | 0.74 ± 0.19 | 0.64 ± 0.18 | 0.01 | |
| Chlerosterol % | 0.85 ± 0.07 | 0.94 ± 0.07 | 0.00 | |
| β-Sitosterol % | 80.73 ± 3.73 | 84.12 ± 2.69 | 0.00 | |
| Sitostanol % | 0.37 ± 0.30 | 0.31 ± 0.08 | n.s | |
| Δ-5-avenasterol % | 12.28 ± 3.96 | 9.85 ± 2.66 | 0.001 | |
| Δ-5, 24-stigm/dienol % | 0.29 ± 0.10 | 0.22 ± 0.06 | 0.00 | |
| Δ-7-stigmastenol % | 0.19 ± 0.09 | 0.18 ± 0.09 | n.s | ≤0.5 |
| Δ-7-avenasterol % | 0.28 ± 0.11 | 0.22 ± 0.06 | 0.001 | |
| Apparent b-Sitosterol % | 94.63 ± 1.07 | 95.45 ± 0.29 | 0.00 | ≥93.0 |
| Total Erythrodiol % | 2.85 ± 1.25 | 1.40 ± 0.52 | 0.00 | ≤4.5 |
| Total sterols (mg/kg) | 1033.3 ± 150.1 | 1219.6 ± 109.2 | 0.00 | ≥1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skiada, V.; Tsarouhas, P.; Varzakas, T. Comparison and Discrimination of Two Major Monocultivar Extra Virgin Olive Oils in the Southern Region of Peloponnese, According to Specific Compositional/Traceability Markers. Foods 2020, 9, 155. https://doi.org/10.3390/foods9020155
Skiada V, Tsarouhas P, Varzakas T. Comparison and Discrimination of Two Major Monocultivar Extra Virgin Olive Oils in the Southern Region of Peloponnese, According to Specific Compositional/Traceability Markers. Foods. 2020; 9(2):155. https://doi.org/10.3390/foods9020155
Chicago/Turabian StyleSkiada, Vasiliki, Panagiotis Tsarouhas, and Theodoros Varzakas. 2020. "Comparison and Discrimination of Two Major Monocultivar Extra Virgin Olive Oils in the Southern Region of Peloponnese, According to Specific Compositional/Traceability Markers" Foods 9, no. 2: 155. https://doi.org/10.3390/foods9020155
APA StyleSkiada, V., Tsarouhas, P., & Varzakas, T. (2020). Comparison and Discrimination of Two Major Monocultivar Extra Virgin Olive Oils in the Southern Region of Peloponnese, According to Specific Compositional/Traceability Markers. Foods, 9(2), 155. https://doi.org/10.3390/foods9020155