Identification of Key Off-Flavor Compounds in Thermally Treated Watermelon Juice via Gas Chromatography–Olfactometry–Mass Spectrometry, Aroma Recombination, and Omission Experiments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
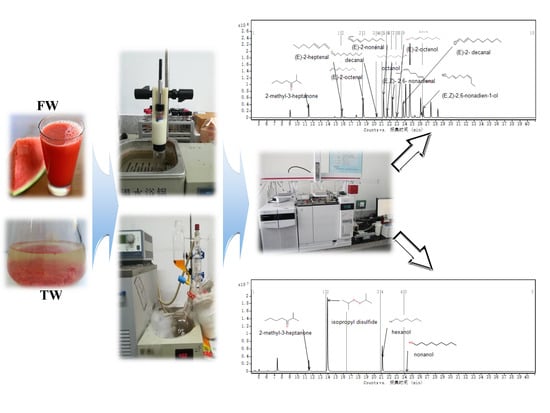
2.2. Preparation of Samples
2.3. Extraction of Flavor Compounds From Watermelon Juice by Solid-Phase Microextraction (SPME)
2.4. Extraction of Flavor Compounds From Watermelon Juice by Solvent-Assisted Flavor Evaporation (SAFE)
2.5. GC–O–MS Analysis
2.6. Identification of Key Flavor Compounds
2.7. Qualitative Analysis of Flavor Compounds
2.8. Quantitative Analysis of Flavor Compounds
2.9. Odor Activity Value (OAV)
2.10. Sensory Evaluation
2.11. Aroma Recombination of TW
2.12. Omission Experiments
2.13. Statistical Analysis
3. Results and Discussion
3.1. Identification of Aroma-Active Compounds in FW and TW
3.1.1. Aldehydes
3.1.2. Alcohols
3.1.3. Ketones
3.1.4. Sulfides
3.2. Identification of Key Off-Flavor Compounds in TW
3.3. OAV of Aroma-Active and Off-Flavor Compounds
3.4. Aroma Recombination of TW
3.5. Omission Experiments
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). STAT 2017. Available online: http://www.fao.org/faostat/zh/#data/QC (accessed on 3 February 2019).
- Tarazona-díaz, M.; Viegas, J.; Moldao-martins, M.; Aguayo, E. Bioactive compounds from flesh and by-product of fresh-cut watermelon varieties. J. Sci. Food Agric. 2011, 91, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.C.; Dudenbostel, T.; Crowe-White, K. Watermelon juice: A novel functional food to increase circulating lycopene in older adult women. Plant Food Hum. Nutr. 2019, 74, 200–203. [Google Scholar] [CrossRef]
- Giovannucci, E. A review of studies of tomatoes, lycopene, and prostate cancer. Exp. Biol. Med. 2002, 227, 852–859. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.S.; Zhao, X.Y.; Song, H.L. Combined effect of high pressure carbon dioxide and mild heat treatment on overall quality parameters of watermelon juice. Innov. Food Sci. Emerg. 2012, 13, 112–119. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Montero-Calderón, M.; Soliva-Fortuny, R.; Martín-Belloso, O. Changes on flavor compounds throughout cold storage of watermelon juice processed by high-intensity pulsed electric fields or heat. J. Food Eng. 2010, 100, 43–49. [Google Scholar]
- Xisto, A.L.R.P.; Boas, E.V.d.B.V.; Nunes, E.E.; Federal, B.M.V.B.; Guerreiro, M.C. Volatile profile and physical, chemical, and biochemical changes in fresh cut watermelon during storage. Food Sci. Technol. Campinas 2012, 32, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Fredes, A.; Sales, C.; Barreda, M.; Valcárcel, M.; Roselló, S.; Beltrán, J. Quantification of prominent volatile compounds responsible for muskmelon and watermelon aroma by purge and trap extraction followed by gas chromatography-mass spectrometry determination. Food Chem. 2016, 190, 689–700. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.S.; Pang, X.L.; Xu, X.X.; Bi, S.; Zhang, W.T.; Wu, J.H. Identification of cooked off-flavor components and analysis of their formation mechanisms in melon juice during thermal processing. J. Agric. Food Chem. 2018, 66, 5612–5620. [Google Scholar] [CrossRef]
- Sun, X.X.; Baldwin, E.A.; Plotto, A.; Manthey, J.A.; Duan, Y.P.; Bai, J.H. Effects of thermal processing and pulp filtration on physical, chemical and sensory properties of winter melon juice. J. Sci. Food Agric. 2017, 97, 543–550. [Google Scholar] [CrossRef]
- Averbeck, M.; Schieberle, P. Influence of different storage conditions on changes in the key aroma compounds of orange juice reconstituted from concentrate. Eur. Food Res. Technol. 2011, 232, 129–142. [Google Scholar] [CrossRef]
- Al-Attabi, Z.; D’Arcy, B.R.; Deeth, H.C. Volatile sulfur compounds in pasteurised and UHT milk during storage. Dairy Sci. Technol. 2014, 94, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.F.; Ni, H.; Chen, F.; Cai, H.N.; Yang, Y.F.; Xiao, A.F. Effects of pasteurization on the volatiles and aroma of Guanxi Pummelo juice. J. Chin. Inst. Food Sci. Technol. 2015, 15, 225–232. [Google Scholar]
- Liu, Y.; He, C.C.; Song, H.L. Comparison of fresh watermelon juice aroma characteristics of five varieties based on gas chromatography-olfactometry-mass spectrometry. Food Res. Int. 2018, 107, 119–129. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.P.; Yang, W.X.; Huang, J.; Liu, Y.Q.; Huang, M.Q.; Sun, B.G.; Li, C.L. Characterization of potent aroma compounds in preserved egg yolk by gas chromatography−olfactometry, quantitative measurements, and odor activity value. J. Agric. Food Chem. 2018, 66, 6132–6141. [Google Scholar] [CrossRef]
- Liang, J.J.; Xie, J.C.; Hou, L.; Zhao, M.Y.; Zhao, J.; Cheng, J.; Wang, S.; Sun, B.G. Aroma constituents in Shanxi aged vinegar before and after aging. J. Agric. Food Chem. 2016, 64, 7597–7605. [Google Scholar] [CrossRef]
- Sgorbini, B.; Cagliero, C.; Liberto, E.; Rubiolo, P.; Bicchi, C.; Cordero, C. Strategies for accurate quantitation of volatiles from foods and plant-origin materials: A challenging task. J. Agric. Food Chem. 2019, 67, 1619–1630. [Google Scholar] [CrossRef]
- Zhou, Q.; Jia, X.; Yao, Y.Z.; Wang, B.; Wei, C.Q.; Zhang, M. Characterization of the aroma-active compounds in commercial fragrant rapeseed oils via monolithic material sorptive extraction. J. Agric. Food Chem. 2019, 67, 11454–11463. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, B.G.; Zhao, M.M.; Zheng, F.P.; Huang, M.Q.; Sun, J.Y.; Sun, X.T.; Li, H.H. Characterization of the key odorants in Chinese zhima aroma-type Baijiu by gas chromatography-olfactometry, quantitative measurements, aroma recombination, and omission studies. J. Agric. Food Chem. 2016, 64, 5367–5374. [Google Scholar] [CrossRef]
- Dima, G.; Tripodi, G.; Condurso, C.; Verzera, A. Volatile constituents of mini-watermelon fruits. J. Essent. Oil Res. 2014, 26, 323–327. [Google Scholar] [CrossRef]
- Vandamme, J.; Nikiforov, A.; Dujardin, K.; Leys, C.; Cooman, L.D.; Durme, J.V. Critical evaluation of non-thermal plasma as an innovative accelerated lipid oxidation technique in fish oil. Food Res. Int. 2015, 72, 115–125. [Google Scholar] [CrossRef]
- Galvao, M.S.; Nunes, M.L.; Cdnstant, P.B.L.; Narain, N. Identification of volatile compounds in cultivars barker, collinson, fortuna and geada of avocado (Persea americana, Mill.) fruit. Food Sci. Technol. 2016, 36, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Condurso, C.; Verzera, A.; Dima, G.; Tripodi, G.; Crinò, P.; Paratore, A.; Romano, D. Effects of different rootstocks on aroma volatile compounds and carotenoid content of melon fruits. Sci. Hortic. 2012, 148, 9–16. [Google Scholar] [CrossRef]
- Pang, X.L.; Hu, X.S.; Liao, X.J.; Sun, Z.J.; Zhang, M.W.; Wu, J.H. Study on Two Evaluation methods of odor-active compounds in Hami melon: Frequency detection-gas chromatography-olfactometry method and odor activity value analysis. J. Chin. Inst. Food Sci. Technol. 2012, 12, 174–182. [Google Scholar]
- Pang, X.L.; Guo, X.F.; Qin, Z.H.; Yao, Y.B.; Hu, X.S.; Wu, J.H. Identification of Aroma-Active Compounds in Jiashi Muskmelon Juice by GC-O-MS and OAV Calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, W.; Lin, D.R.; Shen, Q.; Saleh, A.S.M. The changes in the volatile aldehydes formed during the deep-fat frying process. J. Food Sci. Technol. 2015, 52, 7683–7696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zepka, L.Q.; Garruti, D.S.; Sampaio, K.L.; Mercadante, A.Z.; Da Silva, M.A.A.P. Aroma compounds derived from the thermal degradation of carotenoids in a cashew apple juice model. Food Res. Int. 2014, 56, 108–114. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; He, C.C.; Song, H.L. Comparison of SPME versus SAFE processes for the analysis of flavor compounds in watermelon juice. Food Anal. Method 2018, 11, 1677–1689. [Google Scholar] [CrossRef]
- Dash, P.; Ghosh, G. Fractionation, amino acid profiles, antimicrobial and free radical scavenging activities of Citrullus lanatus seed protein. Nat. Prod. Res. 2017, 31, 2945–2947. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, H.L.; Li, P.; Yao, J.; Xiong, J. Determination of potential off-flavour in yeast extract. LWT-Food Sci. Technol. 2017, 82, 184–191. [Google Scholar] [CrossRef]
- Pang, X.L.; Zhang, Y.Z.; Qiu, J.; Cao, J.M.; Sun, Y.Q.; Li, H.H.; Kong, F.Y. Coupled multidimensional GC and odor activity value calculation to identify off-odors in thermally processed muskmelon juice. Food Chem. 2019, 301, 125307–125317. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Rouseff, R.; Li, G.J.; Wu, H.J. Methanethiol, an off-flavor produced from the thermal treatment of mandarin juices: A study of citrus sulfur volatiles. J. Agric. Food Chem. 2020, 68, 1030–1037. [Google Scholar] [CrossRef]
- Gassenmeier, K.; Schieberle, P. Formation of the intense flavor compound trans-4,5-epoxy-(E)-2-decenal in thermally treated fats. J. Oil Fat Ind. 1994, 71, 1315–1319. [Google Scholar]
- Josephson, D.B.; Lindsay, R.C.; Stuiber, D.A. Biogenesis of lipid-derived volatile aroma compounds in the emerald shiner (Notropis atherinoides). J. Agric. Food Chem. 1985, 32, 1347–1352. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Ferrari, F.; Giannakou, I.; Menkissoglu-Spiroudi, U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011, 67, 341–351. [Google Scholar] [CrossRef]
- Baldovini, N.; Filippi, J.J. Identification of odor active constituents in natural raw materials. In Springer Handbook of Odor; Büttner, A., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 39–62. [Google Scholar]
- Fors, S. Sensory properties of volatile Maillard reaction products and related compounds. In ACS Symposium Series; Waller, G.R., Feather, M.S., Eds.; American Chemical Society: Washington, DC, USA, 1983; Volume 215, pp. 303–338. [Google Scholar]
- Ullrich, F.; Grosch, W. Identification of the most intense odor compounds formed during autoxidation of methyl linolenate at room temperature. J. Am. Oil Chem. Soc. 1988, 65, 1313–1317. [Google Scholar] [CrossRef]
- Butter, R.G.; Teranishi, R.; Ling, L.C.; Turnbaugh, J.G. Quantitative and sensory studies on tomato paste volatiles. J. Agr. Food Chem. 1990, 38, 336–340. [Google Scholar] [CrossRef]
- Karahadian, C.; Johnson, K.A. Analysis of headspace volatiles and sensory characteristics of fresh corn tortillas made from fresh masa dough and spray-dried masa flour. J. Agr. Food Chem. 1993, 41, 791–799. [Google Scholar] [CrossRef]
- Maarse, H. Volatile Compounds in Foods and Beverages; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Josephson, D.B.; Lindsay, R.C.; Stuiber, D.A. Volatile carotenoid-related oxidation compounds contributing to cooked salmon flavor. LWT-Food Sci. Tech. 1991, 24, 425–432. [Google Scholar]
- Steinhaus, M.; Sinuco, D.; Polster, J.; Osorio, C.; Schieberle, P. Characterization of the key aroma compounds in pink guava (Psidium guajava L.) by means of aroma re-engineering experiments and omission tests. J. Agric. Food Chem. 2009, 57, 2882–2888. [Google Scholar] [CrossRef]
- Javidipour, I.; Qian, M.C. Volatile component change in whey protein concentrate during storage investigated by headspace solid-phase microextraction gas chromatography. Dairy Sci. Technol. 2008, 88, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Vera, P.; Canellas, E.; Nerín, C. Compounds responsible for off-odors in several samples composed by poly propylene, polyethylene, paper and cardboard used as food packaging materials. Food Chem. 2020, 309, 125792–125800. [Google Scholar] [CrossRef]
- Xu, X.R.; Zheng, Y.Y.; Song, H.L.; Gong, L.; Pan, W.Q. The effects of enzymatic hydrolysis degree of bovine bone marrow extract on flavor generation via the Maillard reaction. J. Food Meas. Charact. 2019, 13, 521–535. [Google Scholar] [CrossRef]
- Antonis, K.; Pilar, H.M.; Frank, C.; Susan, S. Oxidation derived flavor compounds as quality indicators for package olive oil. J. Am. Oil Chem. Soc. 2004, 81, 251–257. [Google Scholar]
- Chen, H.J.; Wang, Y.; Cao, P.R.; Liu, Y.F. Thermal oxidation rate of oleic acid increased dramatically at 140 °C studied using electron spin resonance and GC-MS/MS. J. Am. Oil Chem. Soc. 2019, 96, 937–944. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.W.; Cheng, Y.J.; Liu, Y.F. Effect of frying oils fatty acid profile on quality, free radical and volatiles over deep-frying process: A comparative study using chemometrics. LWT Food Sci. Technol. 2019, 101, 331–341. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Zhao, J.; Xie, J.C.; Xiao, Q.F.; Fan, M.D.; Wang, T.Z.; Du, W.B.; Wang, M.; Guo, X.Y. Characterization of aroma compounds in two meat flavorings prepared from thermal reaction of enzymatic hydrolysates of black pig and common white pig meat proteinss with oxidized lard. Food Sci. 2017, 38, 40–47. [Google Scholar]
- Mitchell, M.; Brunton, N.P.; Wilkinson, M.G. Impact of salt reduction on the instrumental and sensory flavor profile of vegetable soup. Food Res. Int. 2011, 44, 1036–1043. [Google Scholar] [CrossRef]



| Flavor Attributes | Characteristic |
|---|---|
| cucumber | fresh cucumber |
| grass | chopped freshly grass |
| fruity | mixed aroma associated with fresh fruit |
| floral | light aroma associated with fresh flowers |
| fatty | oily aroma like plant oils or animal fats |
| cooking | cooking smell with high temperature |
| green | pleasant aroma of fresh plant |
| Categories | Compounds a | CAS | Odor Property | RI b | Identification Methods c | FD d | Extraction Methods | ||
|---|---|---|---|---|---|---|---|---|---|
| DB-WAX | DB-5 | FW | TW | ||||||
| Aldehydes (26) | |||||||||
| 1 | 2-methylbutanal | 96-17-3 | cocoa, almond | 964 | 692 | MS,RI,S | - | - | SPME, SAFE |
| 2 | hexanal | 66-25-1 | grass | 1068 | 794 | MS,RI,S | - | - | SPME, SAFE |
| 3 | (E)-2-pentenal # | 1576-87-0 | strawberry, fruity | 1117 | 746 | MS,RI | - | - | SPME |
| 4 | heptanal | 111-71-7 | fatty, putrid | 1174 | 897 | MS,RI,S | - | - | SPME |
| 5 | (E)-2-hexenal # | 6728-26-3 | green, fruity | 1207 | 847 | MS,RI,S | - | - | SPME |
| 6 | octanal | 124-13-0 | pungent, soapy | 1280 | 998 | MS,RI,S | - | - | SPME, SAFE |
| 7 | (E)-2-heptenal * | 18829-55-5 | fatty, fruity, green | 1314 | 952 | MS,RI,O,S | 27 | 81 | SPME |
| 8 | nonanal | 124-19-6 | green, fatty | 1383 | 1100 | MS,RI,S | - | - | SPME, SAFE |
| 9 | (E)-2-octenal * | 2548-87-0 | fatty, nut | 1420 | 1054 | MS,RI,O,S | 27 | >81 | SPME, SAFE |
| 10 | (E,E)-2,4-heptadienal * | 4313-03-5 | nut, fatty | 1482 | - | MS,RI,O,S | 3 | 3 | SPME |
| 11 | decanal | 112-31-2 | pungent, soapy | 1490 | 1202 | MS,RI,O,S | 81 | 81 | SPME |
| 12 | benzaldehyde | 100-52-7 | almond, caramel | 1509 | 957 | MS,RI,S | - | - | SPME, SAFE |
| 13 | (E)-2-nonenal * | 18829-56-6 | cucumber, green | 1527 | - | MS,RI,O,S | 27 | 27 | SPME, SAFE |
| 14 | (E,Z)-2,6-nonadienal * | 557-48-2 | cucumber, green | 1576 | - | MS,RI,O,S | >81 | >81 | SPME |
| 15 | β-cyclocitral | 432-25-7 | mint | 1614 | 1222 | MS,RI | - | - | SPME |
| 16 | (E)-2-decenal * | 3913-81-3 | mechanical, soapy | 1635 | 1259 | MS,RI,O,S | - | 27 | SPME |
| 17 | (E,E)-2,4-nonadienal | 5910-87-2 | fatty, green | 1691 | 1192 | MS,RI,O,S | 9 | 9 | SPME |
| 18 | citral | 5392-40-5 | lemon | 1726 | 1268 | MS,RI | - | - | SPME |
| 19 | 2,6-dimethyl-5-heptenal | 106-72-9 | fruity | - | 1050 | MS,RI | - | - | SPME |
| 20 | (Z)-4-heptenal | 6728-31-0 | cream | - | 892 | MS,RI | - | - | SPME |
| 21 | 2-undecenal | 2463-77-6 | sweet | - | 1346 | MS,RI | - | - | SPME |
| 22 | undecanal | 112-44-7 | fatty, sweet | - | 1303 | MS,RI | - | - | SPME, SAFE |
| 23 | (E,E)-2,4-nonadienal | 25152-84-5 | fried | 1802 | 1314 | MS,RI,S | - | - | SPME |
| 24 | acetal | 105-57-7 | fruity | 900 | 722 | MS,RI | - | - | SAFE |
| 26 | dodecanal | 112-54-9 | floral | - | 1405 | MS,RI | - | - | SAFE |
| Alcohols (11) | |||||||||
| 27 | hexanol | 111-27-3 | bitter, floral | 1346 | 865 | MS,RI,O,S | 81 | 81 | SPME, SAFE |
| 28 | (Z)-3-hexenol | 928-96-1 | grass | 1376 | 852 | MS,RI | - | - | SPME, SAFE |
| 29 | Octanol * | 111-87-5 | metal, burnt | 1548 | 1068 | MS,RI,O,S | 81 | 81 | SPME |
| 30 | heptanol | 111-70-6 | green | - | 967 | MS,RI,S | - | - | SPME |
| 31 | 1-octene-3-ol | 3391-86-4 | mushroom | 1394 | 977 | MS,RI,S | - | - | SPME |
| 32 | benzyl alcohol | 100-51-6 | floral | 1865 | 1037 | MS,RI | - | - | SPME, SAFE |
| 33 | (E)-2-octenol * | 18409-17-1 | plastic, soapy | 1601 | 1167 | MS,RI,O,S | - | 3 | SPME |
| 34 | nonanol | 143-08-8 | fatty, green | 1650 | 1170 | MS,RI,O,S | 27 | 27 | SPME, SAFE |
| 35 | (E,Z)-3,6-nonadienol * | 56805-23-3 | fishy | 1738 | - | MS,RI,O,S | - | 3 | SPME, SAFE |
| 36 | (E,Z)-2,6-nonadienol * | 28069-72-9 | cucumber | 1754 | - | MS,RI,O,S | 27 | 81 | SPME, SAFE |
| 37 | 2-methylbutanol | 137-32-6 | wine | - | 745 | MS,RI | - | - | SAFE |
| Ketones (7) | |||||||||
| 38 | 6-methyl-5-hepten-2-one | 110-93-0 | rubbery | 1327 | 983 | MS,RI | - | - | SPME, SAFE |
| 39 | geranyl acetone * | 3796-70-1 | floral, green | 1844 | 1450 | MS,RI,O,S | 3 | 9 | SPME, SAFE |
| 40 | (Z)-β-ionone * | 79-77-6 | oat, floral | 1931 | - | MS,RI,O,S | SPME | ||
| 41 | 3-octanone | 106-68-3 | medicine, fatty | 1248 | - | MS,RI | - | - | SPME |
| 42 | 2-butanone | 78-93-3 | floral | 894 | - | MS,RI | - | - | SAFE |
| 43 | 2-pentanone | 107-87-9 | fruity | - | 684 | MS,RI,S | - | - | SAFE |
| 44 | 2-hexanone | 591-78-6 | ether | - | 729 | MS,RI | - | - | SAFE |
| Sulfides (5) | |||||||||
| 45 | diethyl disulfide | 110-81-6 | pungent, garlic | 1206 | - | MS,RI | - | - | SAFE |
| 46 | ethyl propyl disulfide | 30453-31-7 | garlic | 1231 | 970 | MS,RI | - | - | SAFE |
| 47 | diisopropyl disulfide * | 4253–89-8 | garlic, sulfur | 1249 | 1016 | MS,RI,O,S | 81 | 81 | SAFE |
| 48 | dipropyl trisulfide | 6028-61-1 | vegetable, garlic | 1527 | 1231 | MS,RI,O,S | - | 3 | SAFE |
| 49 | methyl propyl disulfide | 2179-60-4 | sulfur, garlic | 1112 | - | MS,RI | - | - | SAFE |
| Others (8) | |||||||||
| 50 | 2-n-pentylfuran | 3777-69-3 | fatty | 1220 | 988 | MS,RI,S | - | - | SPME |
| 51 | ethyl acetate | 141-78-6 | fruity | 884 | - | MS,RI | - | - | SAFE |
| 52 | o-xylene | 95-47-6 | floral | 1126 | 865 | MS,RI | - | - | SPME, SAFE |
| 53 | meta-xylene | 108-38-3 | plastic | 1119 | 856 | MS,RI | - | - | SPME, SAFE |
| 54 | limonene | 5989-27-5 | green, fruity | 1194 | - | MS,RI | - | - | SAFE |
| 55 | naphthalene | 91-20-3 | wax | 1733 | 1185 | MS,RI | - | - | SAFE |
| 56 | styrene | 100-42-5 | gasoline | - | 886 | MS,RI | - | - | SAFE |
| 57 | 3-methylbutyric acid | 503-74-2 | sweat | - | 909 | MS,RI,S | - | - | SAFE |
| No. | Key Flavor Compounds a | FD | Quantitative Ion (m/z) | Standard Curve | R2 b | Concentration (μg/L) c | Recovery (%) | RSD (%) d |
|---|---|---|---|---|---|---|---|---|
| 1 | (E)-2-octenal * | >81 | 108.2, 83.1, 70.1 | y = 0.6813x + 0.1175 | 0.9995 | 654.47 | 126 | 4.94 |
| 2 | (E,Z)-2,6-nonadienal * | >81 | 109.1, 70.1, 67.1 | y = 0.8418x + 0.0274 | 0.9934 | 459.54 | 112 | 4.86 |
| 3 | (E)-2-heptenal # | 81 | 112.0, 83.1, 70.1 | y = 0.2955x + 0.0183 | 0.9959 | 1090.23 | 111 | 6.08 |
| 4 | Decanal # | 81 | 209.0, 193.0, 70.1 | y = 0.2869x - 0.0088 | 0.9921 | 93.37 | 99 | 0.23 |
| 5 | Octanol # | 81 | 96.9, 84.1, 69.0 | y = 0.5663x + 0.0091 | 0.9992 | 259.1 | 82 | 6.04 |
| 6 | (E,Z)-2,6-nonadienol * | 81 | 122.2, 81.0, 69.1 | y = 0.4322x - 0.0065 | 0.9928 | 825.18 | 87 | 7.83 |
| 7 | diisopropyl disulfide # | 81 | 150.1, 108.0, 66.0 | y = 2.2337x + 0.6887 | 0.9974 | 2122.17 | 72 | 0.99 |
| 8 | Hexanol # | 81 | 134.1, 119.0, 56.2 | y = 0.8208x + 0.0072 | 0.9985 | 18.68 | 98 | 8.97 |
| 9 | (E)-2-nonenal * | 27 | 122.0, 83.1, 70.1 | y = 0.8483x + 0.1171 | 0.9919 | 597.69 | 114 | 5.79 |
| 10 | (E)-2-decenal # | 27 | 136.0, 83.1, 70.1 | y = 0.6988x + 0.1383 | 0.9929 | 293.72 | 121 | 7.34 |
| 11 | Nonanol * | 27 | 182.0, 70.1, 50.1 | y = 1.2630x + 0.0130 | 0.9983 | 17.77 | 77 | 8.71 |
| 12 | (E)-2-octenol # | 3 | 146.8, 81.1, 57.2 | y = 0.2583x + 0.0231 | 0.9939 | 16.73 | 109 | 0 |
| No. | Key Flavor Compounds | Threshold (μg/L) a | OAV |
|---|---|---|---|
| 7 | diisopropyl disulfide | 0.1 [37] | 21,222 |
| 9 | (E)-2-nonenal | 0.19 [38] | 3146 |
| 2 | (E,Z)-2,6-nonadienal | 0.7 [38] | 656 |
| 6 | (E,Z)-2,6-nonadienol | 1.3 [38] | 635 |
| 10 | (E)-2-decenal | 1 [39] | 294 |
| 1 | (E)-2-octenal | 3 [39] | 218 |
| 4 | decanal | 0.9 [40] | 104 |
| 3 | (E)-2-heptenal | 13 [41] | 84 |
| 11 | nonanol | 2 [40] | 9 |
| 5 | octanol | 110 [39] | 2 |
| 8 | hexanol | 500 [42] | <1 |
| 12 | (E)-2-octenol | 40 [42] | <1 |
| No. | Compounds Omitted from the Complete Recombinate a | nb | Significance c |
|---|---|---|---|
| 1 | (E)-2-heptenal | 4 | |
| 2 | decanal | 5 | |
| 3 | octanol | 10 | *** |
| 4 | diisopropyl disulfide | 8 | * |
| 5 | hexanol | 9 | ** |
| 6 | (E)-2-decenal | 8 | ** |
| 7 | (E)-2-octenol | 8 | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Yang, F.; Liu, Y.; Li, J.; Song, H.-L. Identification of Key Off-Flavor Compounds in Thermally Treated Watermelon Juice via Gas Chromatography–Olfactometry–Mass Spectrometry, Aroma Recombination, and Omission Experiments. Foods 2020, 9, 227. https://doi.org/10.3390/foods9020227
Yang X, Yang F, Liu Y, Li J, Song H-L. Identification of Key Off-Flavor Compounds in Thermally Treated Watermelon Juice via Gas Chromatography–Olfactometry–Mass Spectrometry, Aroma Recombination, and Omission Experiments. Foods. 2020; 9(2):227. https://doi.org/10.3390/foods9020227
Chicago/Turabian StyleYang, Xiao, Fan Yang, Ye Liu, Jian Li, and Huan-Lu Song. 2020. "Identification of Key Off-Flavor Compounds in Thermally Treated Watermelon Juice via Gas Chromatography–Olfactometry–Mass Spectrometry, Aroma Recombination, and Omission Experiments" Foods 9, no. 2: 227. https://doi.org/10.3390/foods9020227
APA StyleYang, X., Yang, F., Liu, Y., Li, J., & Song, H. -L. (2020). Identification of Key Off-Flavor Compounds in Thermally Treated Watermelon Juice via Gas Chromatography–Olfactometry–Mass Spectrometry, Aroma Recombination, and Omission Experiments. Foods, 9(2), 227. https://doi.org/10.3390/foods9020227