Quantification of Polyphenols and Metals in Chinese Tea Infusions by Mass Spectrometry
Abstract
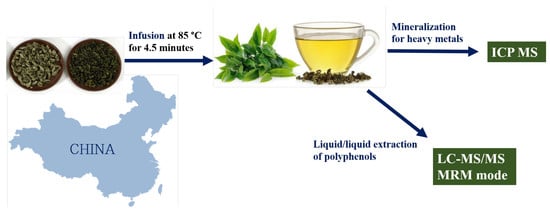
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples
2.3. Metal Extraction of Tea Leaves
2.4. Metal Extraction of Tea Infusion
2.5. ICP-MS Analysis
2.6. Sample Preparation for LC-MS/MS Analysis
2.6.1. Sample Preparation
2.6.2. Quantitative Analysis: External Standard Method
2.7. LC-MS/MS Analysis
2.8. PCA Analysis
3. Results and Discussion
3.1. Optimization of LC-MS/MS Method for Polyphenols Quantification
3.2. Extraction of Polyphenols from Tea Infusions
3.3. PCA Analysis
3.4. Metal Quantification by ICP-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic profiles and antioxidant activities of 30 tea infusions from green, black, oolong, white, yellow and dark teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Brandt, P.A. Coffee or Tea? A prospective cohort study on the associations of coffee and tea intake with overall and cause-specific mortality in men versus women. Eur. J. Epidemiol. 2018, 33, 183–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Kim, Y.; Goodner, K.L.; Park, J.-D.; Choi, J.; Talcott, S.T. Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chem. 2011, 129, 1331–1342. [Google Scholar] [CrossRef]
- Tanaka, T.; Kouno, I. Oxidation of tea catechins: Chemical structures and reaction mechanism. Food Sci. Technol. Res. 2003, 9, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, S.K.; Bergamo, B.M.; Vyalil, P.K.; Elmets, C.A. Green tea polyphenols: DNA photodamage and photoimmunology. J. Photochem. Photobiol. B Biol. 2001, 65, 109–114. [Google Scholar] [CrossRef]
- Leung, L.K.; Su, Y.; Chen, R.; Zhang, Z.; Huang, Y.; Chen, Z.-Y. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J. Nutr. 2001, 131, 2248–2251. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of Green Tea Catechins in the Brain: Epigallocatechin Gallate and its Metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef] [Green Version]
- Zuo, Y.; Chen, H.; Deng, Y. Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 2002, 57, 307–316. [Google Scholar] [CrossRef]
- Baranowska, M.; Suliborska, K.; Chrzanowski, W.; Kusznierewicz, B.; Namieśnik, J.; Bartoszek, A. The relationship between standard reduction potentials of catechins and biological activities involved in redox control. Redox Biol. 2018, 17, 355–366. [Google Scholar] [CrossRef]
- Idrees, M.; Jan, F.A.; Hussain, S.; Salam, A. Heavy metals level, health risk assessment associated with contamination of black tea; a case study from Khyber Pakhtunkhwa (KPK), Pakistan. Biol. Trace Elem. Res. 2020, 1–6. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Chen, R.; Peng, Y.; Wen, X.; Gao, L. Accumulation of heavy metals in tea leaves and potential health risk assessment: A case study from Puan County, Guizhou Province, China. Int. J. Environ. Res. Public Health 2018, 15, 133. [Google Scholar] [CrossRef] [Green Version]
- Carpentieri, A.; Sebastianelli, A.; Melchiorre, C.; Pinto, G.; Staropoli, A.; Trifuoggi, M.; Amoresano, A. Mass spectrometry based proteomics for the molecular fingerprinting of Fiano, Greco and Falanghina cultivars. Food Res. Int. 2019, 120, 26–32. [Google Scholar] [CrossRef]
- Carpentieri, A.; Sebastianelli, A.; Melchiorre, C.; Pinto, G.; Trifuoggi, M.; Lettera, V.; Amoresano, A. Fiano, Greco and Falanghina grape cultivars differentiation by volatiles fingerprinting, a case study. Heliyon 2019, 5, e02287. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhao, J.; Kim, E.M.; Kim, K.H.; Kang, S.; Lee, H.; Lee, J. Comprehensive investigation of the effects of brewing conditions in sample preparation of green tea infusions. Molecules 2019, 24, 1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganguly, S.; Kumar, T.; Mantha, S.; Panda, K. Simultaneous determination of black tea-derived catechins and theaflavins in tissues of tea consuming animals using ultra-performance liquid-chromatography tandem mass spectrometry. PLoS ONE 2016, 11, e0163498. [Google Scholar] [CrossRef]
- Illiano, A.; Arpino, V.; Pinto, G.; Berti, A.; Verdoliva, V.; Peluso, G.; Pucci, P.; Amoresano, A. Multiple Reaction Monitoring tandem mass spectrometry approach for the identification of biological fluids at crime scene investigations. Anal. Chem. 2018, 90, 5627–5636. [Google Scholar] [CrossRef]
- Fontanarosa, C.; Pane, F.; Sepe, N.; Pinto, G.; Trifuoggi, M.; Squillace, M.; Errico, F.; Usiello, A.; Pucci, P.; Amoresano, A. Quantitative determination of free D-Asp, L-Asp and N-methyl-D-aspartate in mouse brain tissues by chiral separation and Multiple Reaction Monitoring tandem mass spectrometry. PLoS ONE 2017, 12, e0179748. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, A.; Angelo, S.; Chiara, M.; Gabriella, P.; Marco, T.; Vincenzo, L.; Angela, A. Molecular characterization of olive cultivars from Italy (Campania): A mass spectrometry Approach. J. Food Sci. Nutr. 2020, 6. [Google Scholar] [CrossRef]
- Li, Z.-H.; Guo, H.; Xu, W.-B.; Ge, J.; Li, X.; Alimu, M.; He, D.-J. Rapid identification of flavonoid constituents directly from PTP1B inhibitive extract of raspberry (Rubus idaeus L.) leaves by HPLC–ESI–QTOF–MS-MS. J. Chromatogr. Sci. 2016, 54, 805–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Casals, I.; Andrés-Lacueva, C.; Izquierdo-Pulido, M.; Lamuela-Raventós, R.M. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J. Mass Spectrom. 2003, 38, 35–42. [Google Scholar] [CrossRef]
- Cuadros-Rodríguez, L.; Bagur-González, M.G.; Sánchez-Vinas, M.; González-Casado, A.; Gómez-Sáez, A.M. Principles of analytical calibration/quantification for the separation sciences. J. Chromatogr. A 2007, 1158, 33–46. [Google Scholar] [CrossRef]
- Normandin, L.; Hazell, A.S. Manganese neurotoxicity: An update of pathophysiologic mechanisms. Metab. Brain Dis. 2002, 17, 375–387. [Google Scholar] [CrossRef]
- Wang, L.-F.; Lee, J.-Y.; Chung, J.-O.; Baik, J.-H.; So, S.; Park, S.-K. Discrimination of teas with different degrees of fermentation by SPME–GC analysis of the characteristic volatile flavour compounds. Food Chem. 2008, 109, 196–206. [Google Scholar] [CrossRef]
- Das, P.R.; Eun, J.-B. Phenolic acids in tea and coffee and their health benefits. In Phenolic Acids: Properties, Food Sources and Health Effects; Nova Science Publisher: Hauppauge, NY, USA, 2016. [Google Scholar]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A. Analysis of antioxidant activity, chlorogenic acid, and rutin content of Camellia sinensis infusions using response surface methodology optimization. Food Anal. Methods 2014, 7, 2033–2041. [Google Scholar] [CrossRef] [Green Version]
- Pękal, A.; Dróżdż, P.; Biesaga, M.; Pyrzynska, K. Evaluation of the antioxidant properties of fruit and flavoured black teas. Eur. J. Nutr. 2011, 50, 681–688. [Google Scholar] [CrossRef] [Green Version]
- Bhagwat, S.; Beecher, G.; Haytowitz, D.; Holden, J.; Dwyer, J.; Peterson, J.; Gebhardt, S.; Eldridge, A.; Agarwal, S.; Balentine, D. Flavonoid composition of tea: Comparison of black and green teas. Available online: https://vitamor.com/IFT2003_TeaFlav.pdf (accessed on 6 May 2020).
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar]
- Wallace, C.H.; Baczkó, I.; Jones, L.; Fercho, M.; Light, P.E. Inhibition of cardiac voltage-gated sodium channels by grape polyphenols. Br. J. Pharmacol. 2006, 149, 657–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larson, A.J.; Symons, J.D.; Jalili, T. Quercetin: A treatment for hypertension?—A review of efficacy and mechanisms. Pharmaceuticals 2010, 3, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Engelhardt, U.H.; Thräne, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Montano, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar]
- Rothenberg, D.O.N.; Zhou, C.; Zhang, L. A review on the weight-loss effects of oxidized tea polyphenols. Molecules 2018, 23, 1176. [Google Scholar] [CrossRef] [Green Version]
- Castiglione, D.; Platania, A.; Conti, A.; Falla, M.; D’Urso, M.; Marranzano, M. Dietary micronutrient and mineral intake in the mediterranean healthy eating, ageing, and lifestyle (MEAL) study. Antioxidants 2018, 7, 79. [Google Scholar] [CrossRef] [Green Version]
- Gallaher, R.; Gallaher, K.; Marshall, A.; Marshall, A. Mineral analysis of ten types of commercially available tea. J. Food Compos. Anal. 2006, 19, S53–S57. [Google Scholar] [CrossRef]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [Green Version]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green tea catechins during food processing and storage: A review on stability and detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Troisi, J.; Richards, S.; Symes, S.; Ferretti, V.; Di Maio, A.; Amoresano, A.; Daniele, B.; Aliberti, F.; Guida, M.; Trifuoggi, M. A comparative assessment of metals and phthalates in commercial tea infusions: A starting point to evaluate their tolerance limits. Food Chem. 2019, 288, 193–200. [Google Scholar] [CrossRef]




| STANDARD | Quantifier m/z | m | q | R2 | LOD (pg/µL) | LOQ (pg/µL) | Upper Working Range [pg/µL] | %RSD | %Accuracy | %Recovery | Black Infusion (µg/g) | Blue Infusion (µg/g) | Green Infusion (µg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gallic Acid | 124.90 | 2996.5 | 76531 | 0.9935 | 15.16 | 45.95 | 500 | 17.1 | 85.20–126.14 | 87.2 | 582.43 | 22.95 | 159.16 |
| Vanillic Acid | 107.80 | 2314.6 | 15272 | 0.9983 | 7.69 | 23.31 | 500 | 3.69 | 91.70–98.8 | 92.3 | 4.31 | - | - |
| Trans-p-Coumaric Acid | 119.00 | 32776 | 291492 | 0.9986 | 1.41 | 4.3 | 500 | 3.19 | 97.13–103.72 | 95.5 | 10.15 | - | 3.48 |
| Caffeic Acid | 134.90 | 15148 | 137495 | 0.9993 | 4.14 | 12.55 | 500 | 3.18 | 87.73–94.60 | 89.4 | 1.01 | - | 1.01 |
| (-)-Epicatechin | 244.80 | 1200.2 | 1080.6 | 0.9982 | 3.25 | 9.84 | 500 | 14.6 | 75.96–101.62 | 85.2 | 32.62 | 38.52 | 83.95 |
| Average Black Tea | Average Oolong Tea | Average Green Tea | ||||
|---|---|---|---|---|---|---|
| BT Leaves | BT Infusion | BL Leaves | BL Tea | GT Leaves | GT Infusion | |
| Metals | Conc. (µg/g) | Conc. (µg/g) | Conc. (µg/g) | Conc. (µg/g) | Conc. (µg/g) | Conc. (µg/g) |
| K | 8996.102 | 11535.429 | 2655.626 | 3946.741 | 7264.210 | 11260.935 |
| P | 1644.388 | 1944.327 | 304.034 | 220.817 | 2165.274 | 1533.009 |
| Mg | 1216.800 | 515.621 | 544.392 | 170.475 | 1275.141 | 598.120 |
| Ca | 670.392 | 55.530 | 542.526 | 77.562 | 983.943 | 26.662 |
| Mn | 357.941 | 124.278 | 140.959 | 46.335 | 244.590 | 141.309 |
| Al | 287.661 | 49.306 | 483.618 | 182.910 | 115.614 | 34.697 |
| Na | 159.809 | 105.778 | 139.745 | 72.741 | 252.535 | 136.846 |
| Fe | 72.556 | 7.749 | 18.575 | <0.001 | 33.254 | <0.001 |
| Rb | 33.233 | 37.755 | 11.932 | 21.611 | 15.155 | 24.513 |
| Zn | 24.552 | 3.489 | 17.818 | 1.856 | 29.256 | 9.958 |
| Ba | 12.433 | 2.027 | 6.912 | 0.743 | 16.633 | 0.729 |
| Sr | 10.471 | 2.176 | 7.181 | 1.002 | 14.670 | 0.788 |
| Cu | 7.859 | 1.349 | 1.500 | 0.307 | 7.153 | 1.673 |
| Ni | 4.381 | 1.577 | 0.881 | 0.467 | 8.894 | 11.800 |
| Cr | 3.978 | <0.001 | 0.487 | <0.001 | 0.833 | <0.001 |
| Ti | 2.125 | 0.201 | 0.503 | 0.033 | 1.518 | 0.097 |
| Pb | 0.281 | 0.069 | 0.138 | 0.009 | 0.679 | 0.027 |
| Cs | 0.219 | 0.203 | 0.035 | 0.112 | 0.071 | 0.088 |
| Mo | 0.165 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| V | 0.130 | 0.008 | 0.029 | 0.001 | 0.071 | 0.002 |
| Co | 0.121 | 0.041 | 0.017 | 0.026 | 0.213 | 0.373 |
| LI | 0.116 | 0.051 | 0.070 | 0.071 | 0.139 | 0.034 |
| As | 0.096 | 0.035 | 0.015 | 0.005 | 0.046 | 0.025 |
| Sn | 0.053 | 0.005 | 0.053 | 0.004 | 0.091 | 0.006 |
| Sb | 0.045 | 0.002 | 0.012 | <0.001 | 0.035 | 0.008 |
| Se | 0.031 | 0.007 | 0.025 | 0.010 | 0.025 | 0.008 |
| Cd | 0.023 | 0.003 | 0.015 | 0.001 | 0.035 | 0.002 |
| Be | 0.004 | 0.002 | <0.001 | 0.009 | 0.003 | 0.001 |
| Te | 0.001 | 0.000 | 0.003 | 0.001 | 0.001 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, G.; Illiano, A.; Carpentieri, A.; Spinelli, M.; Melchiorre, C.; Fontanarosa, C.; di Serio, M.; Amoresano, A. Quantification of Polyphenols and Metals in Chinese Tea Infusions by Mass Spectrometry. Foods 2020, 9, 835. https://doi.org/10.3390/foods9060835
Pinto G, Illiano A, Carpentieri A, Spinelli M, Melchiorre C, Fontanarosa C, di Serio M, Amoresano A. Quantification of Polyphenols and Metals in Chinese Tea Infusions by Mass Spectrometry. Foods. 2020; 9(6):835. https://doi.org/10.3390/foods9060835
Chicago/Turabian StylePinto, Gabriella, Anna Illiano, Andrea Carpentieri, Michele Spinelli, Chiara Melchiorre, Carolina Fontanarosa, Martino di Serio, and Angela Amoresano. 2020. "Quantification of Polyphenols and Metals in Chinese Tea Infusions by Mass Spectrometry" Foods 9, no. 6: 835. https://doi.org/10.3390/foods9060835
APA StylePinto, G., Illiano, A., Carpentieri, A., Spinelli, M., Melchiorre, C., Fontanarosa, C., di Serio, M., & Amoresano, A. (2020). Quantification of Polyphenols and Metals in Chinese Tea Infusions by Mass Spectrometry. Foods, 9(6), 835. https://doi.org/10.3390/foods9060835