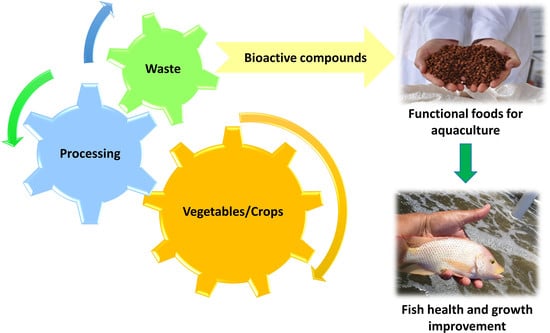
Exploitation of Agro-Industrial Waste as Potential Source of Bioactive Compounds for Aquaculture
Abstract
:1. Introduction
2. Bioactive Compounds from Agro-Industrial Waste
2.1. Phenolic Compounds
2.2. Terpenes
2.3. Dietary Fiber (β-glucans)
2.4. Glucosinolates
2.5. Saponins
3. Biological Properties and Mode of Action of Bioactive Compounds
3.1. Antioxidant Activity
3.2. Immunostimulant Activity
- (1)
- The increase in the enzymatic activity of lysozyme and myeloperoxidase (MPO). Lysozyme exerts its microbicidal action by lysis of peptidoglycans, components of the cell wall of Gram-positive bacteria [68], while MPO catalyzes the formation of hypochlorous, hypobromous and hypothiocyanite acids [69].
- (2)
- Increase in respiratory burst. When phagocytic cells, such as neutrophils and macrophages, respond to the presence of a pathogen, they trigger the action of NADPH oxidase. This generates superoxide anion (O2−). The measurement of this radical by the nitroblue tetrazolium (NBT) reduction method has been considered as an indicator of the phagocytic capacity of the cells of the immune system [70].
- (3)
- Increase in the number of red and white cells. The cell count is a measure used to evaluate the effect of some possible immunostimulants on the health of organisms. A reduction in the count of red cells (erythrocytes) implies that the substance is exerting collateral damage (anemia) in the body. An increase in the number of white cells (leukocytes) indicates a greater response of the immune system to a possible infectious agent. Other blood cell indicators are neutrophils count, hematocrit, level of hemoglobin, etc. [66].
- (4)
- Other immunological parameters evaluated are complement components, such as soluble proteins, enzymes, and receptors that act in signaling processes, opsonization of pathogenic microbes, phagocytosis and microbial destruction [71]. The concentration of immunoglobulins (Ig) and the level of protein are also frequently evaluated as immunological parameters [66]. Melanomacrophage centers (MMCs), pigmented phagocytic cells (melanin) that act as a rapid response to the presence of an infection, and cytokine levels, such as interleukin-1 (IL-1), IL-6, and interferon-gamma (IFN-γ) are also considered markers of the immune response in fish [72].
3.3. Intestinal Microbiota Modulation
4. Use of Bioactive Compounds from Agro-Industrial Waste in Aquaculture
4.1. Bioactive Compounds as Antioxidants in Aquaculture
4.2. Bioactive Compounds as Modulators of the Immune System and Resistance to Infections
4.3. Bioactive Compounds as Modulators of the Intestinal Microbiota
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nasrin, T.A.A.; Matin, M.A. Valorization of vegetable wastes. In Food Processing By-Products and Their Utilization; Anal, A.K., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 53–88. [Google Scholar]
- Guaadaoui, A.; Benaicha, S.; Elmajdoub, N.; Bellaoui, M.; Hamal, A. What is a bioactive compound? A combined definition for a preliminary consensus. Int. J. Nutr. Food Sci. 2014, 3, 174–179. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [Green Version]
- González-Sánchez, M.E.; Pérez-Fabiel, S.; Wong-Villarreal, A.; Bello-Mendoza, R.; Yañez-Ocampo, G. Residuos agroindustriales con potencial para la producción de metano mediante la digestión anaerobia. Rev. Argent. Microbiol. 2015, 47, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Gemede, H.F.; Ratta, N. Antinutritional factors in plant foods: Potential health benefits and adverse effects. Int. J. Nutr. Food Sci. 2014, 3, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Nour-Eldin, H.H.; Madsen, S.R.; Engelen, S.; Jorgensen, M.E.; Olsen, C.E.; Andersen, J.S.; Seynnaeve, D.; Verhoye, T.; Fulawka, R.; Denolf, P.; et al. Reduction of antinutritional glucosinolates in Brassica oilseeds by mutation of genes encoding transporters. Nat. Biotechnol. 2017, 35, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Veneziani, G.; Novelli, E.; Esposto, S.; Taticchi, A.; Servili, M. Chapter 11—Applications of recovered bioactive compounds in food products. In Olive Mill Waste; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 231–253. [Google Scholar]
- Vermerris, W.; Nicholson, R. Families of phenolic compounds and means of classification. In Phenolic Compound Biochemistry; Springer: Dordrecht, The Netherlands, 2006; pp. 1–34. [Google Scholar]
- Ferreira, I.C.F.R.; Martins, N.; Barros, L. Chapter One—Phenolic compounds and its bioavailability: In vitro bioactive compounds or health promoters? In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 82, pp. 1–44. [Google Scholar]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Soidrou, S.H.; Bousta, D.; Lachkar, M.; Hassane, S.O.S.; Youbi-Hamsas, A.E.; Mansouri, L.E.; Benjilali, J.; El-Hajaji, H.; Farah, A. Immunomodulatory Activity of Phenolic Fraction from Piper Borbonense and Cassytha Filiformis Growing in Comoros Islands; Springer: Dordrecht, The Netherlands, 2014; pp. 105–112. [Google Scholar]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef]
- Lu, J.; Fu, X.; Liu, T.; Zheng, Y.; Chen, J.; Luo, F. Phenolic composition, antioxidant, antibacterial and anti-inflammatory activities of leaf and stem extracts from Cryptotaenia japonica Hassk. Ind. Crop. Prod. 2018, 122, 522–532. [Google Scholar] [CrossRef]
- Morais-Braga, M.F.B.; Souza, T.M.; Santos, K.K.A.; Guedes, G.M.M.; Andrade, J.C.; Vega, C.; Rolón, M.; Costa, J.G.M.; Saraiva, A.A.F.; Coutinho, H.D.M. Phenol composition, cytotoxic and anti-kinetoplastidae activities of Lygodium venustum SW. (Lygodiaceae). Exp. Parasitol. 2013, 134, 178–182. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-L.; Guo, Y.-S.; Wang, C.-H.; Li, G.-Q.; Xu, J.-J.; Chung, H.Y.; Ye, W.-C.; Li, Y.-L.; Wang, G.-C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Gabr, S.; Nikles, S.; Pferschy Wenzig, E.M.; Ardjomand-Woelkart, K.; Hathout, R.M.; El-Ahmady, S.; Motaal, A.A.; Singab, A.; Bauer, R. Characterization and optimization of phenolics extracts from Acacia species in relevance to their anti-inflammatory activity. Biochem. Syst. Ecol. 2018, 78, 21–30. [Google Scholar] [CrossRef]
- Kumar, S.S.; Patra, A. Evaluation of phenolic composition, antioxidant, anti-inflammatory and anticancer activities of Polygonatum verticillatum (L.). J. Integr. Med. 2018, 16, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Yousefian, M.; Shakour, N.; Hosseinzadeh, H.; Hayes, A.W.; Hadizadeh, F.; Karimi, G. The natural phenolic compounds as modulators of NADPH oxidases in hypertension. Phytomedicine 2018, 55, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Leicach, S.R.; Chludil, H.D. Chapter 9—Plant secondary metabolites: Structure–activity relationships in human health prevention and treatment of common diseases. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 42, pp. 267–304. [Google Scholar]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Tarahovsky, Y.S.; Kim, Y.A.; Yagolnik, E.A.; Muzafarov, E.N. Flavonoid–membrane interactions: Involvement of flavonoid–metal complexes in raft signaling. Biochim. Biophys. Acta 2014, 1838, 1235–1246. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Liu, G.; Murtaza, G.; Rahu, N.; Saleem, M.; Yin, Y. Modulatory mechanism of polyphenols and Nrf2 signaling pathway in LPS challenged pregnancy disorders. Oxid. Med. Cell. Longev. 2017, 2017, 8254289. [Google Scholar] [CrossRef] [Green Version]
- Kirby, J.; Keasling, J.D. Biosynthesis of plant isoprenoids: Perspectives for microbial engineering. Annu. Rev. Plant Biol. 2009, 60, 335–355. [Google Scholar] [CrossRef]
- Babahmad, R.A.; Aghraz, A.; Boutafda, A.; Papazoglou, E.G.; Tarantilis, P.A.; Kanakis, C.; Hafidi, M.; Ouhdouch, Y.; Outzourhit, A.; Ouhammou, A. Chemical composition of essential oil of Jatropha curcas L. leaves and its antioxidant and antimicrobial activities. Ind. Crop. Prod. 2018, 121, 405–410. [Google Scholar] [CrossRef]
- Hassoun, A.; Emir Çoban, Ö. Essential oils for antimicrobial and antioxidant applications in fish and other seafood products. Trends Food Sci. Technol. 2017, 68, 26–36. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant Activity of Essential Oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Deehan, E.C.; Duar, R.M.; Armet, A.M.; Perez-Muñoz, M.E.; Jin, M.; Walter, J. Modulation of the gastrointestinal microbiome with nondigestible fermentable carbohydrates to improve human health. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Medina-Gali, R.M.; Ortega-Villaizan, M.D.M.; Mercado, L.; Novoa, B.; Coll, J.; Perez, L. Beta-glucan enhances the response to SVCV infection in zebrafish. Dev. Comp. Immunol. 2018, 84, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Gidley, M.J.; Nishinari, K. Chapter 2.2—Physico-chemistry of (1,3)-β-Glucans. In Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides; Bacic, A., Fincher, G.B., Stone, B.A., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 47–118. [Google Scholar]
- Stone, B.A. Chapter 2.1—Chemistry of β-Glucans. In Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides; Bacic, A., Fincher, G.B., Stone, B.A., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 5–46. [Google Scholar]
- Jin, Y.; Li, P.; Wang, F. β-glucans as potential immunoadjuvants: A review on the adjuvanticity, structure-activity relationship and receptor recognition properties. Vaccine 2018, 36, 5235–5244. [Google Scholar] [CrossRef]
- Bischoff, K.L. Chapter 40—Glucosinolates. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 551–554. [Google Scholar]
- Goncharov, N.; Orekhov, A.N.; Voitenko, N.; Ukolov, A.; Jenkins, R.; Avdonin, P. Chapter 41—Organosulfur compounds as nutraceuticals. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2016; pp. 555–568. [Google Scholar]
- Yalcin, H.; Çapar, T.D. Bioactive compounds of fruits and vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: Boston, MA, USA, 2017; pp. 723–745. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampal, G.; Thind, T.S.; Arora, S. Bio-protective effects of glucosinolates—A review. LWT Food Sci. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Kojima, M.; Oawa, K. Studies on the effect of isothyocianates and their analogues on microorganisms. I. Effect of isothiocyanates on the oxygen uptake of yeasts. J. Ferment. Technol. 1971, 49, 740–746. [Google Scholar]
- Zsolnai, T. Antimicrobial effect of thiocyanates and isothiocyanates. Arztl. Forsch. 1966, 16, 870–876. [Google Scholar]
- Yang, Y.; Laval, S.; Yu, B. Chapter 2—Chemical synthesis of saponins. In Advances in Carbohydrate Chemistry and Biochemistry; Horton, D., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 137–226. [Google Scholar]
- Sidana, J.; Singh, B.; Sharma, O.P. Saponins of agave: Chemistry and bioactivity. Phytochemistry 2016, 130, 22–46. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Singh, N.; Kaur, A. Saponins in pulses and their health promoting activities: A review. Food Chem. 2017, 233, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Sarikahya, N.B.; Nalbantsoy, A.; Top, H.; Gokturk, R.S.; Sumbul, H.; Kirmizigul, S. Immunomodulatory, hemolytic and cytotoxic activity potentials of triterpenoid saponins from eight Cephalaria species. Phytomedicine 2018, 38, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Press, J.B.; Reynolds, R.C.; May, R.D.; Marciani, D.J. Structure/function relationships of immunostimulating saponins. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 24, pp. 131–174. [Google Scholar]
- Jacob, M.C.; Favre, M.; Bensa, J.-C. Membrane cell permeabilisation with saponin and multiparametric analysis by flow cytometry. Cytometry 1991, 12, 550–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabski, M.; Wegierek-Ciuk, A.; Czerwonka, G.; Lankoff, A.; Kaca, W. Effects of saponins against clinical E. coli strains and eukaryotic cell line. J. Biomed. Biotechnol. 2012, 2012, 6. [Google Scholar] [CrossRef] [Green Version]
- Francis, G.; Makkar, H.P.; Becker, K. Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 2001, 193, 197–227. [Google Scholar] [CrossRef]
- Omnes, M.-H.; Le Goasduff, J.; Le Delliou, H.; Le Bayon, N.; Quazuguel, P.; Robin, J.H. Effects of dietary tannin on growth, feed utilization and digestibility, and carcass composition in juvenile European seabass (Dicentrarchus labrax L.). Aquacult. Rep. 2017, 6, 21–27. [Google Scholar] [CrossRef]
- Fernandes, R.D.P.P.; Trindade, M.A.; de Melo, M.P. Chapter 2—Natural antioxidants and food applications: Healthy perspectives. In Alternative and Replacement Foods; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 31–64. [Google Scholar]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Pillon, N.J.; Croze, M.L.; Vella, R.E.; Soulère, L.; Lagarde, M.; Soulage, C.O. The lipid peroxidation by-product 4-hydroxy-2-nonenal (4-HNE) induces insulin resistance in skeletal muscle through both carbonyl and oxidative stress. Endocrinology 2012, 153, 2099–2111. [Google Scholar] [CrossRef]
- Kumar, S.; Krishna, C.R.; Preedy, V.R. Chapter 20—Assessment of antioxidant potential of dietary components. In HIV/AIDS; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 239–253. [Google Scholar]
- Kim, K.M.; Ki, S.H. Chapter 28—Nrf2: A key regulator of redox signaling in liver diseases. In Liver Pathophysiology; Muriel, P., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 355–374. [Google Scholar]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Z.; Lu, H.; Xu, Z.; Tong, R.; Shi, J.; Jia, G. Recent advances of natural polyphenols activators for Keap1-Nrf2 signaling pathway. Chem. Biodivers. 2019, 16, e1900400. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Ohta, K.; Naruse, T.; Kato, H.; Fukui, A.; Shigeishi, H.; Nishi, H.; Tobiume, K.; Takechi, M. Candida albicans β-glucan-containing particles increase HO-1 expression in oral keratinocytes via a reactive oxygen species/p38 mitogen-activated protein kinase/Nrf2 pathway. Infect. Immun. 2018, 86, e00575-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eggler, A.L.; Savinov, S.N. Chemical and biological mechanisms of phytochemical activation of Nrf2 and importance in disease prevention. Recent. Adv. Phytochem. 2013, 43, 121–155. [Google Scholar] [CrossRef] [Green Version]
- Inoue, Y.; Shimazawa, M.; Nagano, R.; Kuse, Y.; Takahashi, K.; Tsuruma, K.; Hayashi, M.; Ishibashi, T.; Maoka, T.; Hara, H. Astaxanthin analogs, adonixanthin and lycopene, activate Nrf2 to prevent light-induced photoreceptor degeneration. J. Pharmacol. Sci. 2017, 134, 147–157. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surh, Y.-J.; Na, H.-K. NF-kappaB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes Nutr. 2008, 2, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Ren, B.; Guo, R.; Zhang, W.; Ma, S.; Yao, Y.; Yuan, T.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem. Toxicol. 2017, 109, 505–516. [Google Scholar] [CrossRef]
- Garcia, Y.J.; Rodríguez-Malaver, A.J.; Peñaloza, N. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J. Neurosci. Methods 2005, 144, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Vallejos-Vidal, E.; Reyes-López, F.; Teles, M.; MacKenzie, S. The response of fish to immunostimulant diets. Fish Shellfish Immunol. 2016, 56, 34–69. [Google Scholar] [CrossRef]
- Caipang, C.M.A.; Lazado, C.C. 9—Nutritional impacts on fish mucosa: Immunostimulants, pre- and probiotics. In Mucosal Health in Aquaculture; Beck, B.H., Peatman, E., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 211–272. [Google Scholar]
- Syngai, G.G.; Ahmed, G. Chapter 11—Lysozyme: A natural antimicrobial enzyme of interest in food applications. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 169–179. [Google Scholar]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.C. The phagocyte respiratory burst: Historical perspectives and recent advances. Immunol. Lett. 2017, 192, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Clos, T.W.D.; Mold, C. 20—Complement and complement deficiencies. In Clinical Immunology, 3rd ed.; Rich, R.R., Fleisher, T.A., Shearer, W.T., Schroeder, H.W., Frew, A.J., Weyand, C.M., Eds.; Mosby: Edinburgh, UK, 2008; pp. 305–325. [Google Scholar]
- Agius, C.; Roberts, R.J. Melano-macrophage centres and their role in fish pathology. J. Fish Dis. 2003, 26, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [Green Version]
- Nair, M.P.N.; Kandaswami, C.; Mahajan, S.; Chadha, K.C.; Chawda, R.; Nair, H.; Kumar, N.; Nair, R.E.; Schwartz, S.A. The flavonoid, quercetin, differentially regulates Th-1 (IFNγ) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim. Biophys. Acta 2002, 1593, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Magrone, T.; Fontana, S.; Laforgia, F.; Dragone, T.; Jirillo, E.; Passantino, L. Administration of a polyphenol-enriched feed to farmed sea bass (Dicentrarchus labrax L.) modulates intestinal and spleen immune responses. Oxid. Med. Cell. Longev. 2016, 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Chi, G.; Wei, M.; Xie, X.; Soromou, L.W.; Liu, F.; Zhao, S. Suppression of MAPK and NF-κB pathways by limonene contributes to attenuation of lipopolysaccharide-induced inflammatory responses in acute lung injury. Inflammation 2013, 36, 501–511. [Google Scholar] [CrossRef]
- Petit, J.; Bailey, E.C.; Wheeler, R.T.; de Oliveira, C.A.F.; Forlenza, M.; Wiegertjes, G.F. Studies into β-glucan recognition in fish suggests a key role for the C-Type lectin pathway. Front. Immunol. 2019, 10, 280. [Google Scholar] [CrossRef]
- Ringø, E.; Zhou, Z.; Vecino, J.L.G.; Wadsworth, S.; Romero, J.; Krogdahl, Å.; Olsen, R.E.; Dimitroglou, A.; Foey, A.; Davies, S.; et al. Effect of dietary components on the gut microbiota of aquatic animals. A never-ending story? Aquacult. Nutr. 2016, 22, 219–282. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Volman, J.J.; Ramakers, J.D.; Plat, J. Dietary modulation of immune function by β-glucans. Physiol. Behav. 2008, 94, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, G.; Sowrirajan, S.; Joseph, B. β-Glucan, a dietary fiber in effective prevention of lifestyle diseases—An insight. Bioact. Carbohydr. Diet. Fibre 2019, 19, 100187. [Google Scholar] [CrossRef]
- Alminger, M.; Eklund-Jonsson, C. Whole-grain cereal products based on a high-fibre barley or oat genotype lower post-prandial glucose and insulin responses in healthy humans. Eur. J. Nutr. 2008, 47, 294. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Chen, J.; Chung, S.S.M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and metabolism of three mulberry anthocyanin monomers by rat gut microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals; License CC BY-NC-SA 3.0 IGO; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; p. 210. [Google Scholar]
- Catap, E.S.; Jimenez, M.R.R.; Tumbali, M.P.B. Immunostimulatory and anti-oxidative properties of corn silk from Zea mays L. in Nile tilapia, Oreochromis niloticus. Int. J. Fish. Aquac. 2015, 7, 30–36. [Google Scholar] [CrossRef]
- Žilić, S.; Janković, M.; Basić, Z.; Vančetović, J.; Maksimović, V. Antioxidant activity, phenolic profile, chlorophyll and mineral matter content of corn silk (Zea mays L.): Comparison with medicinal herbs. J. Cereal Sci. 2016, 69, 363–370. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Cheng, H.L.; Pan, B.S. LDL oxidation, antioxidant capacity and growth of cultured grey mullet (Mugil cephalus) fed dietary sorghum distillery residue pretreated with polyethylene glycol. J. Agric. Food Chem. 2009, 57, 7877–7882. [Google Scholar] [CrossRef]
- Wu, G.; Bennett, S.J.; Bornman, J.F.; Clarke, M.W.; Fang, Z.; Johnson, S.K. Phenolic profile and content of sorghum grains under different irrigation managements. Food Res. Int. 2017, 97, 347–355. [Google Scholar] [CrossRef] [Green Version]
- Giri, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Dietary Administration of banana (Musa acuminata) peel flour affects the growth, antioxidant status, cytokine responses, and disease susceptibility of rohu, Labeo rohita. J. Immunol. Res. 2016, 2016, 4086591. [Google Scholar] [CrossRef] [Green Version]
- Vicente, I.S.T.; Fleuri, L.F.; Carvalho, P.L.P.F.; Guimarães, M.G.; Naliato, R.F.; Müller, H.d.C.; Sartori, M.M.P.; Pezzato, L.E.; Barros, M.M. Orange peel fragment improves antioxidant capacity and haematological profile of Nile tilapia subjected to heat/dissolved oxygen-induced stress. Aquacult. Res. 2019, 50, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Lizárraga-Velázquez, C.E.; Hernández, C.; González-Aguilar, G.A.; Heredia, J.B. Effect of dietary intake of phenolic compounds from mango peel extract on growth, lipid peroxidation and antioxidant enzyme activities in zebrafish (Danio rerio). Lat. Am. J. Aquat. Res. 2019, 47, 602–611. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Abdullah, N.; Yusof, H.M.; Shuib, A.S.; Razak, S.A. Improvement of growth and antioxidant status in Nile tilapia, Oreochromis niloticus, fed diets supplemented with mushroom stalk waste hot water extract. Aquacult. Res. 2017, 48, 1146–1157. [Google Scholar] [CrossRef]
- Ahmed, M.; Abdullah, N.; Shuib, A.S.; Razak, S.A. Influence of raw polysaccharide extract from mushroom stalk waste on growth and pH perturbation induced-stress in Nile tilapia, Oreochromis niloticus. Aquaculture 2017, 468, 60–70. [Google Scholar] [CrossRef]
- Vaseeharan, B.; Thaya, R. Medicinal plant derivatives as immunostimulants: An alternative to chemotherapeutics and antibiotics in aquaculture. Aquacult. Int. 2014, 22, 1079–1091. [Google Scholar] [CrossRef]
- Romier, B.; Van De Walle, J.; During, A.; Larondelle, Y.; Schneider, Y.-J. Modulation of signalling nuclear factor-κB activation pathway by polyphenols in human intestinal Caco-2 cells. Br. J. Nutr. 2008, 100, 542–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arciuli, M.; Fiocco, D.; Fontana, S.; Arena, M.P.; Frassanito, M.A.; Gallone, A. Administration of a polyphenol-enriched feed to farmed sea bass (Dicentrarchus labrax L.): Kidney melanomacrophages response. Fish Shellfish Immunol. 2017, 68, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Shakouri, M.; Yousefi, S.; Van Doan, H.; Shafiei, S.; Yousefi, M.; Mazandarani, M.; Torfi Mozanzadeh, M.; Tulino, M.G.; Faggio, C. Humoral and skin mucosal immune parameters, intestinal immune related genes expression and antioxidant defense in rainbow trout (Oncorhynchus mykiss) fed olive (Olea europea L.) waste. Fish Shellfish Immunol. 2020, 100, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Khalili, F.; Taghian Dinani, S. Extraction of phenolic compounds from olive-waste cake using ultrasonic process. J. Food Meas. Charact. 2018, 12, 974–981. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Jahazi, M.A.; Nikdehghan, N.; Van Doan, H.; Volpe, M.G.; Paolucci, M. Effects of dietary polyphenols from agricultural by-products on mucosal and humoral immune and antioxidant responses of convict cichlid (Amatitlania nigrofasciata). Aquaculture 2020, 517, 734790. [Google Scholar] [CrossRef]
- Jahazi, M.A.; Hoseinifar, S.H.; Jafari, V.; Hajimoradloo, A.; Van Doan, H.; Paolucci, M. Dietary supplementation of polyphenols positively affects the innate immune response, oxidative status, and growth performance of common carp, Cyprinus carpio L. Aquaculture 2020, 517, 734709. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of fourier transform infrared (FTIR) spectroscopy in the characterization of tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Da Cunha, J.A.; de Ávila Scheeren, C.; Fausto, V.P.; de Melo, L.D.W.; Henneman, B.; Frizzo, C.P.; de Almeida Vaucher, R.; Castagna de Vargas, A.; Baldisserotto, B. The antibacterial and physiological effects of pure and nanoencapsulated Origanum majorana essential oil on fish infected with Aeromonas hydrophila. Microb. Pathog. 2018, 124, 116–121. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting citrus wastes into value-added products: Economic and environmently friendly approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Acar, Ü.; Kesbiç, O.S.; Yılmaz, S.; Gültepe, N.; Türker, A. Evaluation of the effects of essential oil extracted from sweet orange peel (Citrus sinensis) on growth rate of tilapia (Oreochromis mossambicus) and possible disease resistance against Streptococcus iniae. Aquaculture 2015, 437, 282–286. [Google Scholar] [CrossRef]
- Baba, E.; Acar, Ü.; Öntaş, C.; Kesbiç, O.S.; Yılmaz, S. Evaluation of Citrus limon peels essential oil on growth performance, immune response of Mozambique tilapia Oreochromis mossambicus challenged with Edwardsiella tarda. Aquaculture 2016, 465, 13–18. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Salah, A.S.; El Nahas, A.F.; Mahmoud, S. Modulatory effect of different doses of β-1,3/1,6-glucan on the expression of antioxidant, inflammatory, stress and immune-related genes of Oreochromis niloticus challenged with Streptococcus iniae. Fish Shellfish Immunol. 2017, 70, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-S.; Liau, S.-Y.; Huang, C.-T.; Nan, F.-H. Beta 1,3/1,6-glucan and vitamin C immunostimulate the non-specific immune response of white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 2016, 57, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Chirapongsatonkul, N.; Mueangkan, N.; Wattitum, S.; U-taynapun, K. Comparative evaluation of the immune responses and disease resistance of Nile tilapia (Oreochromis niloticus) induced by yeast β-glucan and crude glucan derived from mycelium in the spent mushroom substrate of Schizophyllum commune. Aquacult. Rep. 2019, 15, 100205. [Google Scholar] [CrossRef]
- Nie, S.; Cui, S.W.; Xie, M. Chapter 9—Cereal beta-glucan. In Bioactive Polysaccharides; Nie, S., Cui, S.W., Xie, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 445–482. [Google Scholar]
- Udayangani, R.M.C.; Dananjaya, S.H.S.; Fronte, B.; Kim, C.-H.; Lee, J.; De Zoysa, M. Feeding of nano scale oats β-glucan enhances the host resistance against Edwardsiella tarda and protective immune modulation in zebrafish larvae. Fish Shellfish Immunol. 2017, 60, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Ringø, E.; Olsen, R.E.; Gifstad, T.Ø.; Dalmo, R.A.; Amlund, H.; Hemre, G.-I.; Bakke, A.M. Prebiotics in aquaculture: A review. Aquacult. Nutr. 2010, 16, 117–136. [Google Scholar] [CrossRef]
- Reza, A.; Abdolmajid, H.; Abbas, M.; Abdolmohammad, A.K. Effect of dietary prebiotic inulin on growth performance, intestinal microflora, body composition and hematological parameters of juvenile beluga, Huso huso (Linnaeus, 1758). J. World Aquacult. Soc. 2009, 40, 771–779. [Google Scholar] [CrossRef]
- Mouriño, J.L.P.; Do Nascimento Vieira, F.; Jatobá, A.B.; Da Silva, B.C.; Jesus, G.F.A.; Seiffert, W.Q.; Martins, M.L. Effect of dietary supplementation of inulin and W. cibaria on haemato-immunological parameters of hybrid surubim (Pseudoplatystoma sp.). Aquacult. Nutr. 2012, 18, 73–80. [Google Scholar] [CrossRef]
- Mahious, A.S.; Gatesoupe, F.J.; Hervi, M.; Metailler, R.; Ollevier, F. Effect of dietary inulin and oligosaccharides as prebiotics for weaning turbot, Psetta maxima (Linnaeus, C. 1758). Aquacult. Int. 2006, 14, 219. [Google Scholar] [CrossRef] [Green Version]
- Geraylou, Z.; Souffreau, C.; Rurangwa, E.; Maes, G.E.; Spanier, K.I.; Courtin, C.M.; Delcour, J.A.; Buyse, J.; Ollevier, F. Prebiotic effects of arabinoxylan oligosaccharides on juvenile Siberian sturgeon (Acipenser baerii) with emphasis on the modulation of the gut microbiota using 454 pyrosequencing. FEMS Microbiol. Ecol. 2013, 86, 357–371. [Google Scholar] [CrossRef] [Green Version]
- Geraylou, Z.; Souffreau, C.; Rurangwa, E.; D’Hondt, S.; Callewaert, L.; Courtin, C.M.; Delcour, J.A.; Buyse, J.; Ollevier, F. Effects of arabinoxylan-oligosaccharides (AXOS) on juvenile Siberian sturgeon (Acipenser baerii) performance, immune responses and gastrointestinal microbial community. Fish Shellfish Immunol. 2012, 33, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, C.; Delcour, J.A.; Courtin, C.M.; Broekaert, W.F.; Verstraete, W.; Van de Wiele, T. Microbial metabolism and prebiotic potency of arabinoxylan oligosaccharides in the human intestine. Trends Food Sci. Technol. 2007, 18, 64–71. [Google Scholar] [CrossRef]
- Parrillo, L.; Coccia, E.; Volpe, M.G.; Siano, F.; Pagliarulo, C.; Scioscia, E.; Varricchio, E.; Safari, O.; Eroldogan, T.; Paolucci, M. Olive mill wastewater-enriched diet positively affects growth, oxidative and immune status and intestinal microbiota in the crayfish, Astacus leptodactylus. Aquaculture 2017, 473, 161–168. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Biological activities of phenolic compounds present in virgin olive oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef] [Green Version]
- Ngugi, C.C.; Oyoo-Okoth, E.; Muchiri, M. Effects of dietary levels of essential oil (EO) extract from bitter lemon (Citrus limon) fruit peels on growth, biochemical, haemato-immunological parameters and disease resistance in Juvenile Labeo victorianus fingerlings challenged with Aeromonas hydrophila. Aquacult. Res. 2017, 48, 2253–2265. [Google Scholar] [CrossRef]



| Bioactive Compound. | Source (Doses) | Species | Biological Properties | Effect |
|---|---|---|---|---|
| Phenolic compounds | Corn silk extract (3.5 g/kg) | Oreochromis niloticus | Antioxidant, Immunostimulant | ↓MDA ↑Lysozyme, against Aeromonas hydrophila [89] |
| Phenolic compounds | Sorghum distillery residue (200 g/kg) | Mugil cephalus | Antioxidant | ↓LDL oxidation ↑TAC [92] |
| Phenolic compounds | Banana peel (10, 30, 50, 70 g/kg) | Labeo rohita | Antioxidant, Immunostimulant | ↓MDA ↑SOD, CAT, GPx ↑IL-1 β, TNF- α ↑Survival rate against Aeromonas hydrophila [94] |
| Phenolic compounds | Orange peel (2, 4, 6, 8 g/kg) | O. Oreochromis niloticus | Antioxidant | ↑SOD, CAT, GPx [95] |
| Phenolic compounds: gallic, 2-hydroxycinnamic and protocatechuic acids, quercetin, mangiferine, methyl gallate, ethyl gallate | Mango peel extract (50, 100, 150, 200 mg/kg) | Danio rerio | Antioxidant | ↑CAT ↓MDA [98] |
| β-glucans | Mushroom stalk waste extract (5, 10 g/kg) | Oreochromis niloticus | Antioxidant | ↑SOD, CAT [99,100] |
| Phenolic compounds: proanthocyanidin, catechins, epicatechins | Grape seeds (0.1, 0.2 g/kg) | Dicentrarchus labrax L. | Immunostimulant | ↓IL-1β, IL-6. ↑IFN-γ ↑MMCs [75] |
| Phenolic compounds: catechins, epigallocatechins | Grape seeds (0.1, 0.2 g/kg) | Dicentrarchus labrax L. | Immunostimulant | ↑Peroxidase ↑Dopa-oxidase ↑MMCs [103] |
| Phenolic compounds | Olive waste cake (0.5, 2.5, 5 g/kg) | Oncorhynchus mykiss | Antioxidant, Immunostimulant | ↑SOD, GPx ↑Lysozyme, Ig ↑IL-8 ↓TGF-β [104] |
| Phenolic compounds | Mixture of chestnut wood and olive mill wastewater extract (0.5, 1, 2 g/kg) | Amatitlania nigrofasciata | Antioxidant, Immunostimulant | ↑Growth performance ↑Ig ↑Lysozyme, peroxidase ↑CAT [106] |
| Phenolic compounds | Mixture of chestnut wood and olive mill wastewater extract (0.5, 1, 2 g/kg) | Cyprinus carpio L. | Immunostimulant | ↑Growth performance ↑Ig, lysozyme ↑CAT, peroxidase [107] |
| Essential oils | Orange peel (1, 3, 5 g/kg) | Oreochromis mossambicus | Immunostimulant | ↑Lysozyme, MPO ↑Survival rate against Streptococcus iniae [111] |
| Essential oils | Lemon peel (5, 7.5, 10 g/kg) | Oreochromis mossambicus | Immunostimulant | ↑NBT ↑Lysozyme, MPO ↓Mortality against Edwardsiella tarda [112] |
| Essential oils | Lemon peel (10, 20, 50, 80 g/kg) | Labeo victorianus | Immunostimulant | ↑Red blood cells, leucocytes, hematocrits, neutrophils ↑Ig, lysozyme ↓Mortality against Aeromonas hydrophila [128] |
| Glucans | Split gill mushroom cultivation waste extract (100 µg/mL) | Oreochromis niloticus | Immunostimulant | ↑Ig, lysozyme ↑TNF-α, IL-1β, NF-κB ↑Survival rate against Aeronomas veronii [116] |
| Phenolic compounds | Olive mill waste water (0.5, 5 g/kg) | Astacus leptodactylus | Antioxidant, Immunostimulant, Microbiota modulation | ↑Growth performance ↑CAT, GR ↑Haemocytes ↓Total intestinal bacteria [126] |
| Arabinoxylans oligosaccharides | Wheat bran (20, 40 g/kg) | Acipenser baerii | Microbiota modulation | ↑Eubacteriaceae, Clostridiaceae, Streptococcaceae, Lactobacillaceae, Bacillaceae [123] |
| Arabinoxylans oligosaccharides | Wheat bran (20 g/kg) | Acipenser baerii | Immunostimulant, Microbiota modulation | ↑Peroxidase, phagocytic activity ↓Aeromonas sp., Citrobacter freundii, Escherichia coli ↑Short-chain fatty acids [124] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-López, N.; Lizárraga-Velázquez, C.E.; Hernández, C.; Sánchez-Gutiérrez, E.Y. Exploitation of Agro-Industrial Waste as Potential Source of Bioactive Compounds for Aquaculture. Foods 2020, 9, 843. https://doi.org/10.3390/foods9070843
Leyva-López N, Lizárraga-Velázquez CE, Hernández C, Sánchez-Gutiérrez EY. Exploitation of Agro-Industrial Waste as Potential Source of Bioactive Compounds for Aquaculture. Foods. 2020; 9(7):843. https://doi.org/10.3390/foods9070843
Chicago/Turabian StyleLeyva-López, Nayely, Cynthia E. Lizárraga-Velázquez, Crisantema Hernández, and Erika Y. Sánchez-Gutiérrez. 2020. "Exploitation of Agro-Industrial Waste as Potential Source of Bioactive Compounds for Aquaculture" Foods 9, no. 7: 843. https://doi.org/10.3390/foods9070843
APA StyleLeyva-López, N., Lizárraga-Velázquez, C. E., Hernández, C., & Sánchez-Gutiérrez, E. Y. (2020). Exploitation of Agro-Industrial Waste as Potential Source of Bioactive Compounds for Aquaculture. Foods, 9(7), 843. https://doi.org/10.3390/foods9070843